Pre-conditioning with Nicotinamide-mononucleotide Enhances Cardioprotective Potentials of Umbilical Cord-derived Mesenchymal Stem Cells in Diabetes: Role of Autophagy Flux
Received: 26-Sep-2021 / Accepted Date: 21-Oct-2021 / Published Date: 28-Oct-2021 DOI: 10.4172/1165-158X.1000216
Abstract
Background: The application of stem cell-based therapies has promising cardioprotective impacts in Ischemia- Reperfusion (IR) injury, especially in a comorbid condition like diabetes. Preconditioning of mesenchymal stem cells (MSCs) in vitro may improve their functions in vivo. Here, we have investigated the effects of preconditioning of human umbilical cord-derived MSCs with Nicotinamide-Mononucleotide (NMN) on myocardial infarct size, and the involvement of autophagy flux in diabetic rats.
Methods: Type 2 diabetes was induced by a high-fat diet and single-dose streptozotocin in Sprague Dawley rats (250 ± 20 g). Myocardial IR injury was applied through ligation of left coronary artery occlusion. NMN-preconditioned or unconditioned-MSCs were injected intra-myocardially at early reperfusion. Myocardial hemodynamics was recorded throughout the experiment. Cardiac injury was assessed using the measurement of infarct size and CK-mB release via planimetry and ELISA methods. Mitochondrial function was evaluated by fluorometric assays. Autophagy-related protein expression was evaluated using immunoblotting.
Results: Administration of NMN or MSCs alone had no significant protective effects. NMN-preconditioned MSCs significantly reduced myocardial infarction and CK-mB levels, restored IR-induced mitochondrial reactive oxygen species, membrane depolarization and ATP production, and improved cardiac hemodynamic following IR injury in diabetic rats. IR-induced overexpression of proteins Beclin-1, P62, and LC3-II and reduction of LC3-II/LC3-I ratio were significantly reversed following treatment with preconditioned-MSCs. The administration of chloroquine, an autophagy flux inhibitor, abolished these cardioprotective effects.
Conclusion: Therefore, NMN serves as a good preconditioning modality to increase the cardioprotective efficacy of MSCs in diabetic rats and the improvement of autophagy flux may play a significant role.
Keywords: GLIPR1, Astroglioma, Proliferation, Migration, Invasion, CD63
Introduction
Diabetes is the main comorbidity increasing the vulnerability of the heart to Ischemia-Reperfusion (IR) injury and severely reduce the efficacy of cardioprotection. Diabetes negatively affects functional and adaptive reserve capacities of cardiomyocytes and leads to 2-3 times more deaths due to myocardial IR injury [1-3]. So, protecting the diabetic IR heart in the clinical situation has great importance and promising outcomes [1]. Mitochondria are the critical end effector of cardioprotection against IR injury and diabetes may gradually decrease the density and activity of myocardial mitochondria, fading the final efficiency of cardioprotective interventions [3,4]. Dysfunctional mitochondria lead to overproduction of reactive oxygen species and reduce the activity of cellular autophagy, resulting in greater infarction and myocardial dysfunction during IR conditions [5,6].
Beclin1, LC3 (microtubule-associated proteins), and its substrate, ubiquitin-binding protein P62 are the main players of the autophagy process which are dysregulated during myocardial IR injury. As a result, the autophagy flux and autophagosome clearance are significantly reduced and misfolded proteins and dysfunctional mitochondria are accumulated in cardiomyocytes [7,8]. Thus, promoting autophagy flux and mitochondrial function following myocardial IR injury during diabetes would be an effective intervention to increase the power of cardioprotection.
Mesenchymal Stem Cells (MSCs) are promising candidates for limiting infarct size in myocardial IR situations due to their multiplex actions [9]. The human umbilical cord is a rich source of MSCs which is easily accessible and can be readily used in such situations [10,11]. Differences of culture medium with in vivo conditions, post-injection shortage of sufficient nutrition and communication with cell-matrix and adjacent cells, activation of various death signals, and low rates of survival have all led to treatment with MSCs not being completely successful [11-14]. Additionally, the cardioprotective potency of MSCs is significantly lost during diabetic conditions. However, preconditioning of these cells before injection can positively enhance their biological activity especially in the presence of comorbid diabetes [14]. Nicotinamide-mononucleotide (NMN) is a precursor of NAD+ in mitochondria and has cardioprotective impacts on the hearts of healthy and nondiabetic rats suffered from myocardial infarction [15]. It can limit IR damages by improving mitochondrial function and restoring the autophagy process via affecting certain signaling mediators and pathways such as PI3K/Akt and Sirt1/PGC1α [15-17].
In addition, NMN boosts the expansion of adult stem cells derived from different tissues like bone marrow, pancreatic cells, intestine, and umbilical cord [18]. It has been reported that this compound promotes cell survival and positively influences the differentiation of MSCs into neuronal and cardiac lineages [18,19]. Also, mitochondrial NAD+ replenishment via NMN supplementation delays stem cell senescence and facilitates their reprogramming [20]. As a result, it is hypothesized that preconditioning of umbilical cord-derived MSCs with NMN may enhance the cardioprotective of these cells in diabetic hearts. Despite the application of NMN in stem cell therapy, the molecular mechanisms of its combination with MSCs are still unclear.
Therefore, the present study aimed to investigate the cardioprotective potentials of NMN preconditioned-MSCs in diabetic rats, by assessing the mechanistic involvement of mitochondrial activity and autophagy flux.
Materials and Methods
Animal selection
In this study, the male Sprague Dawley rats (225 ± 20 g) were provided and transferred to the animal room in which they had free access to water and food. The animal room had a temperature of 22 ± 2ºC, the humidity of 50-55%, and 12-h dark/light cycles. All experiments followed the Guide for the Care and Use of Laboratory Animals published by the National Research Council’s criteria (NIH publication, revised 2011) and confirmed by the local Animal Ethical Committee (SDPT-2021012453).
Human umbilical cords-derived MSCs preparation
After getting patients’ consent, the five birth-associated samples of fresh human umbilical cords were taken and immediately immersed in Hank’s balanced salt solution (Invitrogen, Germany) having 100 U/ml penicillin and 100 U/ml streptomycin (Gibco, Germany). After removing the tissue debris and blood clots via washing the samples with phosphate-buffered saline (PBS), the samples were cut into 2–5 mm3 small pieces in a sterile Petri dish and then treated with 2 mg/ml collagenase IV (Sigma-Aldrich, St. Louis, USA), followed by incubation in a 5% CO2 condition for 24 h at 37ºC. The samples were washed again and the MSCs were passed through a 70 μm filter and seeded in dishes at a density of 1 × 104 cells/cm2 following resuspension in an alphaminimal essential medium containing 10% fetal bovine serum and 2 mmol/l L-glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin. Following removal of non-adherent cells after 72 h the culture medium was refreshed every 2–3 days with fresh media. Then, the cells at 70%- 80% confluence were detached via 0.25% trypsin and 1 ml EDTA and passed to other dishes to be expanded. For preconditioning protocol, freshly prepared 1 × 106 MSCs at the third passage were placed in 12-well plates and incubated with NMN (10−8 mol/l) for 6 h. Trypan blue staining technique was employed to count the viable cells using a hemocytometer. Finally, the fluorescein isothiocyanateconjugated mouse antibodies including anti-CD105, CD44, CD45, and phycoerythrin-conjugated mouse antibodies including anti-CD73, CD- 90, and CD34 (Dako Company, USA) were used for the identification of MSCs phenotype using flow cytometry.
Induction of type 2 diabetes mellitus
Type 2 diabetes mellitus was modeled in rats using the high-fat diet and low-dose streptozotocin method. Briefly, rats fed a 62% high-fat diet having 4.6 kcal/g total calories for six weeks. The composition of the diet was 35% normal rat pellet, 30% lard, 24% casein, 4% sucrose, 1% cholesterol, and 0.3% DL-methionine. Then, the streptozotocin at the concentration of 35 mg/kg was dissolved in pH 4 citrate buffer and injected intraperitoneally to the animals. At the end of the seventh week, a glucometry was done via a small scratch on the tail vein. The cut-off point of blood glucose levels for diabetes confirmation was ≥ 250 mg/dl. The rats with lower glucose levels were excluded from the study and new rats were replaced. The total diabetic period lasted for 14 weeks to simulate chronic type 2 diabetes.
Experimental design
Seventy-two rats were randomly allocated into 6 groups (n=12/ each) (Figure 1): 1. Sham (experiencing chest surgery without LAD ligation), 2. IR (modeling of IR injury), 3. IR + MSC (IR + receiving 1 × 106 MSCs in 150 μl medium at early reperfusion), 4. IR + nico (IR + receiving NMN 500 mg/kg, i.p. at early reperfusion), 5. IR + nico- MSC (IR + receiving 1 × 106 NMN preconditioned-MSCs in 150 μl medium at early reperfusion), and 6. IR + nico-MSC+CQ (IR + nico- MSC-receiving rats pretreated with chloroquine, CQ, as the inhibitor of autophagy flux). CQ was administered in 10 mg/kg intraperitoneally 1 h before surgery and repeated in the reperfusion phase [7]. Sham and IR groups also received 150 μl of vehicle (medium). Intact or preconditioned-MSCs were divided into three volumes (50 μl) and injected into three different borders of risk zones of the hearts. At the end of reperfusion, six hearts in each group were used for infarct size detection and the other six hearts were used for other measurements.
Myocardial IR injury model
After being anesthetized by i.p injection of sodium pentobarbital (50 mg/kg), the animals were intubated and ventilated through an animal ventilator (Harvard Apparatus VentElit, USA). Then, the chest was opened through a lateral incision on the left side, and the left anterior descending (LAD) coronary artery was exposed and occluded by a 6.0 silk ligature. The ischemic period was 40 min. Thereafter, reperfusion was settled following the releasing of LAD ligature and lasted for 24 h. Finally, the chest was sutured with 2.0 silk thread and the lungs were inflated through increasing positive end-expiratory pressure until recovery.
Measurement of infarct size
At the end of the experiment, the LAD was re-ligated and 0.25% Evans blue was infused via the femoral vein for 5 min. Then, the hearts were isolated, weighed, and cut into ≈5 pieces with a thickness of 2 mm. The slices were stained with 2,3,5-triphenyl-tetrazolium-chloride for 15 min at 37ºC. Finally, the total volume of the left ventricle and the percentages of risk zones and infarcted areas were visualized using Image J software (NIH, USA).
Creatine kinase-mB assay
The blood samples were obtained via the tail vein of the animals at the end of reperfusion. The samples were centrifuged at 4500 g for 15 min, at 4ºC, and then the serum was collected and used for the measurement of creatine kinase-mB (CK-mB) employing the colorimetric method with a commercial kit (Bioscience, Germany) following the instructions of the kit. Values were expressed in U/l.
Tissue sampling and mitochondrial isolation
At the end of the experiment, the hearts were immediately removed from the body, and the peri-infarcted regions of left ventricles were sampled and added into the mitochondrial isolation buffer solution (70 mM sucrose, 210 mM mannitol, and one mM EDTA in 50 mM Tris- HCl, pH 7.4) and homogenized. Then, the samples were centrifuged at 1300 g for 10 min at 4ºC, and the supernatant was centrifuged again at 12000 g for 10 min. Finally, the mitochondrial pellet was suspended in a storage buffer (70 mM sucrose and 210 mM mannitol in 50 mM Tris-HCl, pH 7.4 at the final volume of 100 μl). The samples protein concentration was determined using the Nanodrop method.
Mitochondrial reactive oxygen species generation
The dichlorohydro-fluorescein diacetate (DCFDA) fluorometric method was used to measure the mitochondrial reactive oxygen species (mito-ROS) in samples. After incubation of mitochondrial pellets in a storage buffer with 2 μM of DCFDA dye for 30 min, their fluorescence was detected at an excitation wavelength of 480 nm, and emission wavelength of 530 nm. The fluorescence intensities (FI) were normalized to the protein content of samples and the levels of mito- ROS were expressed as FI/mg sample protein.
Mitochondrial membrane potential
The mitochondrial membrane potential changes (ΔΨm) were detected by the JC-1 method using the manufacturer’s instructions (Sigma-Aldrich, St. Louis, USA). Briefly, the isolated mitochondrial pellets were incubated in 100 μl JC-1 assay buffer for 15 min at 25ºC in a dark place. This incubation reduced the loss of JC-1 over time due to substrate consumption and led to the loss of mitochondrial membrane potential. Red and green fluorescence intensities from JC-1-incubated mitochondrial samples were detected fluorometrically at excitation wavelengths of 525 nm and 485 nm, and emission wavelength of 590 nm and 530 nm, respectively. To calculate the mitochondrial membrane potential, the red/green ratio was normalized to the protein content of the samples. The diminished red/green ratio was considered as mitochondrial depolarization.
ATP production levels
The cellular content of ATP was measured through a bioluminescent assay kit (MAK190, Sigma, USA). Ten milligrams of left ventricles from each sample were lysed in a tube containing 100 μl of ATP assay buffer. Then, the developer and ATP probe were added to the solution, and the absorbance was detected at 570 nm. After calculation, the ATP contents were expressed in nmol/mg sample protein.
Western blotting
The isolated left ventricular tissues were washed in normal saline and the peri-infarcted regions of tissues were homogenized in radio immunoprecipitation assay lysis buffer (Sigma-Aldrich; St Louis, USA). The solution was centrifuged at 13,000 × g for 20 min and the supernatant was collected. After electrophoresis in SDS-PAGE, the proteins were transferred to a polyvinylidene difluoride membrane (Sigma-Aldrich; St Louis, USA). After blocking with 5 % non-fat dry milk solution in Tris buffered-saline-Tween 20 (pH 7.4) for 2 h at room temperature, the membranes were separately incubated overnight with the antibodies against Beclin1, p62, LC3, and GAPDH (at 1:1000; Cell Signaling Technology, USA). After removing primary antibodies, an anti-rabbit secondary antibody was pipetted on the blots for one hour at room temperature. Finally, the enhanced chemiluminescent (ECL) substrate was added to detect the immunoreactivity of the membranes through Kodak X-OMAT films and a visualizing machine. After densitometry analysis and normalization with the intensity of loading control GAPDH, the proteins bands were quantified and reported in arbitrary units.
Statistical analysis
All data were presented as mean ± standard errors. One-way analysis of variance and subsequent Tukey post hoc test were employed to compare the differences in parameters of experimental groups. An alpha level less than 0.05 was considered statistically significant.
Results
General characteristics of diabetic animals
The initial body weight (BW) and heart weight (HW) of rats were 233 ± 14 g and 1.46 ± 0.03 g, respectively, and the HW/BW was 0.62 ± 0.01. The final BW, HW, and HW/BW of rats after induction of diabetes were 204 ± 14 g, 1.81 ± 0.06 g, and 0.89 ± 0.04, respectively. Also, the initial fasting blood glucose level was 5.2 ± 0.6 mmol/l, and the insulin level was 6.1 ± 0.4 μmol/l. At the end of the diabetic phase, the final glucose and insulin levels reached 23.4 ± 2.8 mmol/l, and 8.2 ± 0.6 μmol/l, respectively. Finally, the insulin resistance tested by homeostatic model assessment (HOMA) was 8.8 ± 0.6 at the end of the diabetic period in comparison to its initial level of 1.4 ± 0.1. These findings confirmed the successful modeling of type 2 diabetes in rats.
Reduction of myocardial injury by NMN preconditioned- MSCs and its reversal by CQ
Following confirmation of MSCs identity by flow cytometry and cell viability by trypan blue staining, the preconditioned or unconditioned MSCs were injected to the diabetic rats with myocardial injury to measure the outcomes. LAD ligation for 40 min led to the development of similar areas at risk in the diabetic hearts of experimental groups, indicating a similar degree of ischemic injury in all groups (Figures 2a- 2c). Induction of myocardial IR injury considerably led to the formation of infarct size by 57% in diabetic rats as compared with the Sham group (P<0.001) (Figure 2b). Similarly, the levels of CK-mB release in the IR group were significantly higher than sham group (Figure 2c). Administration of NMN alone or injection of intact MSCs to periinfarcted areas in diabetic rats did not reduce myocardial CK-mB release or infarct size in comparison to the IR group. However, preconditioning of MSCs with NMN (nico-MSC) significantly diminished the infarct size (to 32%) (P <0.01) and the levels of CK-mB release (P <0.05) in diabetic rats as compared with those of the IR group. Importantly, the infarct-limiting effect of the NMN preconditioned-MSCs was greater than those of individual treatments, so that infarct size in the IR+nico- MSC group was significantly lower than those of IR+nico or IR+MSC groups (P <0.05) (Figure 2b). Finally, co-administration of autophagy flux inhibitor, CQ, to diabetic rats significantly abolished the effect of NMN preconditioned-MSCs of both infarct size and CK-mB release (P <0.05) (Figures 2b and 2c). It should be noted that the results of the IR+CQ group were not significantly different from the IR group and therefore the results of this group were not included in the study.
Figure 2: Effects of NMN, unconditioned-MSCs, and NMN-preconditioned MSCs on myocardial areas at risk (a) myocardial infarct size, (b) and serum CK-mB levels and (c) in diabetic rats. (n=6), &&P <0.01 and &&P <0.001, and $$$P <0.001 vs. corresponding Sham groups; *P <0.05, and **P <0.01, and #P <0.05 vs. corresponding IR groups; +P <0.05 vs. IR+MSC group. IR: Ischemia- Reperfusion, MSC: Mesenchymal Stem Cell, nico: NMN, CQ: Chloroquine.
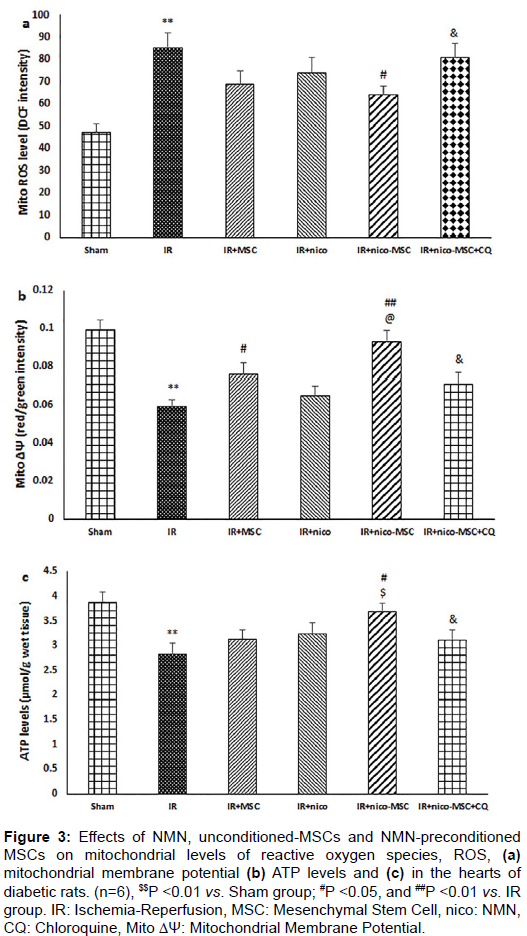
Improvement of mitochondrial function by NMN preconditioned-MSCs and its reversal by CQ
The production of mitochondrial ROS and mitochondrial membrane depolarization were significantly increased while the level of cellular ATP was reduced following IR injury as compared with the Sham group (P <0.01) (Figures 3a-3c). Although alone administration of NMN or MSCs tended to restore these changes, other differences were not significant from the IR group except for changes in mitochondrial membrane potential by MSCs. However, injection of preconditioned- MSCs to IR diabetic hearts significantly reduced mitochondrial ROS (P <0.05) and membrane depolarization (P <0.01), and increased ATP level in comparison to those of the IR group (Figure 3). The effects of preconditioned-MSCs on mitochondrial membrane potential and ATP level were statistically different from IR+nico and IR+MSC groups, respectively (P <0.05). Additionally, inhibition of autophagy flux using CQ co-administration significantly reversed the protective effects of NMN preconditioned-MSCs on mitochondrial function (P <0.05).
Figure 3: Effects of NMN, unconditioned-MSCs and NMN-preconditioned MSCs on mitochondrial levels of reactive oxygen species, ROS, (a) mitochondrial membrane potential (b) ATP levels and (c) in the hearts of diabetic rats. (n=6), $$P <0.01 vs. Sham group; #P <0.05, and ##P <0.01 vs. IR group. IR: Ischemia-Reperfusion, MSC: Mesenchymal Stem Cell, nico: NMN, CQ: Chloroquine, Mito ΔΨ: Mitochondrial Membrane Potential.
Promotion of autophagy by NMN preconditioned-MSCs and its reversal by CQ
The expression of autophagy-related proteins Beclin-1, LC3-I, and LC3-II was significantly increased following myocardial IR injury of diabetic hearts compared to the Sham group (P <0.05 to P <0.001) (Figures 4a-4f). The protein expression of P62, as the LC3 substrate, has also been upregulated significantly, indicating the possible reduction of autophagy flux in diabetic IR hearts. The effects of alone treatment with NMN or MSCs on the expression of these proteins were not statistically significant. Nevertheless, NMN-preconditioned MSCs significantly downregulated the expression of Beclin-1 (P <0.05), LC3-I, LC3-II, and P62 (P <0.01), while increasing the LC3-II/I ration (P <0.05) (Figure 4). Also, the expression of LC3 in the IR+nico-MSC group was significantly lower than those of individual treatments (P <0.05). Administration of CQ, as an inhibitor of autophagy flux, significantly reversed the effects of NMN preconditioned-MSCs on the regulation of Becline-1, LC3, and P62 expressions (P <0.05), confirming the reduction of autophagy flux in IR hearts.
Figure 4: Effects of NMN, unconditioned-MSCs, and NMN-preconditioned MSCs on protein expression of Beclin 1 (a) LC3-I, (b) LC3-II, (c) LC3-II/I ratio, (d) P62, (e) representative immunoblotting bands of proteins and (f) in the myocardium of diabetic rats. Samples were derived from the same experiment and blots were processed in parallel. (n=5), $P <0.05 and $$P <0.01 vs. Sham group; #P <0.05 and ##P <0.01 vs. IR group. IR: Ischemia-Reperfusion, MSC: Mesenchymal Stem Cell, nico: NMN, CQ: Chloroquine.
Discussion
In this study, monotherapy with NMN or umbilical cord-derived MSCs failed to protect the type 2 diabetic heart from IR injury. However, preconditioning of these MSCs with NMN significantly reduced myocardial infarct size and CK-mB release. This treatment improved the cardiac mitochondrial function and positively modulated the autophagy in diabetic cardiac cells. Importantly, blocking autophagy flux through prior administration of CQ significantly abolished the effects of preconditioned-MSCs on myocardial infarction and mitochondrial function.
Although having the higher capability of MSCs transplantation in the treatment of myocardial infarction, some important problems like incomplete homing of MSCs and lack of their sufficient physiological relationship with the host tissue reduce their greater therapeutic features [14,21]. Additionally, diabetic condition (especially in the chronic phase) significantly hinders the effectiveness of monotherapybased protective modalities in cardioprotection. Similar to this, we found here that administration of NMN alone or unconditioned MSCs was not able to produce significant cardioprotection. Diabetes-induced several metabolic, cellular, and molecular alterations can interfere with cardioprotection [3]. For example, the mitochondria in cardiomyocytes which are the crucial end effector in cardioprotection, progressively become dysfunctional, leading to an imbalance in cellular metabolism [5]. Therefore, to enhance this cardioprotective potential during diabetes in the present study, we firstly preconditioned MSCs with NMN in vitro and determined that this action significantly augmented the effectiveness of MSCs in reducing infarct size, CK-mB release, and boosting mitochondrial activity in the diabetic heart.
It has been reported that NMN itself and its and related compounds confer good cardioprotection in healthy and non-diabetic animals through different routes, including attenuating oxidative stress, overwhelming inflammation, and promoting cell survival and antiapoptotic mechanisms [17,22,23]. NMN is also able to activate the AMPK/Sirt1/PGC-1α pathway which is crucial in the prevention of mitochondrial ROS overproduction and mitochondrial dysfunction [24,25]. On the other hand, preconditioning of stem cells with NMN may increase the proliferation and survival of transplanted MSCs as well as their paracrine efficiency [26,27]. Preconditioning with NMN may also enhance the paracrine effects of MSCs and liberating protective mediators-enriched exosomes from MSCs after transplantation. The contribution of this important hypothesis in cardioprotection was not tested in the present study and needs to be appraised in the future.
However, we showed here that the inhibition of autophagy flux was able to abolish the beneficial impacts of NMN preconditioned-MSCs in diabetic rats. Similarly, transplantation of preconditioned-MSCs significantly modified the expression of main proteins involved in the autophagy process including Beclin-1, LC3, and P62. Autophagy is a central pathway regulating cellular metabolism, and its level decreases significantly during diabetes. As a result, preconditioned-MSCs might restore cardioprotection and mitochondrial function in diabetic hearts via reinforcing autophagy activity and promoting autophagy flux. The conversion of LC3-I to LC3-II is accompanied by autophagosome formation and is often used as an indicator of autophagy induction [28,29]. Beclin-1 locates upstream of LC3 and also contributes to autophagosome formation in the reperfusion phase [30]. Another protein, P62, plays as an LC3 substrate to trigger autophagy flux via interactions between LC3 and ubiquitinated proteins [8]. The higher ratio of LC3-II/LC3-I and concomitant downregulation of P62 in the hearts of rats receiving NMN preconditioned-MSCs indicates that autophagy is triggered and the fusion of autophagosome to lysosomes and thereby the autophagosome clearance is promoted. The administration of CQ reversed these actions. This finding demonstrates that treatment of diabetic IR hearts with NMN preconditioned-MSCs corrected IR-induced dysregulation of autophagy and thereby prompted autophagosome degradation and autophagy flux, and ultimately exerted effective cardioprotection. Identifying the contribution of other survival mechanisms in MSCs preconditioning-induced cardioprotection will help us to find the central therapeutic cellular targets.
Conclusion
Preconditioning of human umbilical cord-derived MSCs with NMN significantly restored the cardioprotective effects of MSCs in diabetic hearts subjected to IR injury via restricting infarct size, reducing CK release, improving mitochondrial function, and modifying the expression of the Beclin/LC3/P62 autophagy pathway. Progression of autophagy flux had a positive role in this cardioprotection. Therefore, enhancing MSCs responsiveness with appropriate preconditioning stimuli can overcome the negative impacts of diabetes on cardioprotection.
Conflicts of Interest
The authors have no conflict of interest to declare.
Authors’ Contributions
All authors designed the project, performed the experimentations, analyzed, and interpreted the data. QW was the major contributor in writing the manuscript. All authors read and approved the final manuscript.
References
- Juhaszova, M., Rabuel, C., Zorov, D. B., Lakatta, E. G., & Sollott, S. J. Protection in the diabetic heart: Preventing the heart-break of old age? Cardiovasc Res., 2005;66(2): 233-244
- Boengler, K., Schulz, R., & Heusch, G. Loss of cardioprotection with ageing. Cardiovasc Res., 2009;83(2): 247-261
- Ruiz-Meana, M., Bou-Teen, D., Ferdinandy, P., Gyongyosi, M., Pesce, M., Perrino, C., et al. Cardiomyocyte ageing and cardioprotection: Consensus document from the ESC working groups cell biology of the heart and myocardial function. Cardiovasc Res., 2020;116(11): 1835-1849
- Fernandez-Sanz, C., Ruiz-Meana, M., Castellano, J., Miro-Casas, E., Nuñez, E., & Inserte, J., et al. Altered FoF1 ATP synthase and susceptibility to mitochondrial permeability transition pore during ischaemia and reperfusion in aging cardiomyocytes. Thromb Haemost., 2015;113(3): 441-451
- Wang, Y., Li, Y., He, C., Gou, B., & Song, M. Mitochondrial regulation of cardiac aging. Biochim Biophys Acta Mol Basis Dis., 2019;1865(7): 1853-1864
- Shirakabe A., Ikeda Y., Sciarretta S., Zablocki D. K. & Sadoshima J. Aging and autophagy in the heart. Circ Res., 2016;118(10): 1563-1576
- Hong, L., Sun, Y., An, J. Z., Wang, C., & Qiao, S. G. Sevoflurane preconditioning confers delayed cardioprotection by upregulating amp-activated protein kinase levels to restore autophagic flux in ischemia-reperfusion rat hearts. Med Sci Monit., 2020;26: e922176
- Islam, M. A., Sooro, M. A., & Zhang, P. Autophagic regulation of p62 is critical for cancer therapy. Int J Mol Sci., 2018;19(5): 1405
- Oliva, J. Therapeutic properties of mesenchymal stem cell on organ ischemia-reperfusion injury. Int J Mol Sci., 2019;20(21): 5511
- Alijani-Ghazyani, Z., Sabzevari, R., Roushandeh, A. M., Jahanian-Najafabadi, A., Amiri, F., & Roudkenar, M. H. Transplantation of umbilical cord-derived mesenchymal stem cells overexpressing lipocalin 2 ameliorates ischemia-induced injury and reduces apoptotic death in a rat acute myocardial infarction model. Stem Cell Rev Rep., 2020;16(5): 968-978
- Amiri, F., Halabian, R., Salimian, M., Shokrgozar, M. A., Soleimani, M., Jahanian-Najafabadi, A., et al. Induction of multipotency in umbilical cord-derived mesenchymal stem cells cultivated under suspension conditions. Cell Stress Chaperones., 2014;19(5): 657-666
- Varkouhi, A. K., Monteiro, A. T., Tsoporis, J. N., Mei, S. H. J., Stewart, D. J., & Dos Santos, C. C. Genetically modified mesenchymal stromal/stem cells: application in critical illness. Stem Cell Rev Rep., 2020;16(5): 812-827
- Li, L., Chen, X., Wang, W. E., & Zeng, C. How to Improve the Survival of Transplanted Mesenchymal Stem Cell in Ischemic Heart? Stem Cells Int., 2016;2016: 9682757
- Hu, C., & Li, L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J Cell Mol Med., 2018;22(3): 1428-1442
- Yamamoto, T., Byun, J., Zhai, P., Ikeda, Y., Oka, S. & Sadoshima, J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS One., 2014;9(6): e98972
- Morevati, M., Egstrand, S., Nordholm, A., Mace, M. L., Andersen, C. B., & Salmani, R., et al. Effect of NAD+ boosting on kidney ischemia-reperfusion injury. PLoS One., 2021;16(6): e0252554
- Nadtochiy, S. M., Wang, Y. T., Nehrke, K., Munger, J., & Brookes, P. S. Cardioprotection by nicotinamide mononucleotide (NMN): Involvement of glycolysis and acidic pH. J Mol Cell Cardiol., 2018;121: 155-162
- Meng, Y., Ren, Z., Xu, F., Zhou, X., Song, C., Wang, V. Y., et al. Nicotinamide promotes cell survival and differentiation as kinase inhibitor in human pluripotent stem cells. Stem Cell Rep. 2018;11(6): 1347-1356
- Griffin, S. M., Pickard, M. R., Orme, R. P., Hawkins, C. P., Williams, A. C., & Fricker, R. A., et al. Nicotinamide alone accelerates the conversion of mouse embryonic stem cells into mature neuronal populations. PLoS One., 2017;12(8): e0183358
- Son, M. J., Kwon, Y., Son, T., & Cho Y. S. Restoration of mitochondrial NAD+ levels delays stem cell senescence and facilitates reprogramming of aged somatic cells. Stem Cell., 2016;34(12): 2840-2851
- Li, C., Cheung, M. K. H., Han, S., Zhang, Z., Chen, L., Chen, J., et al. Â Mesenchymal stem cells and their mitochondrial transfer: A double-edged sword. Biosci Rep., 2019;39(5): BSR20182417
- De Castro, J. M., Assumpção, J. A. F., Stein, D. J., Toledo, R. S., da Silva, L. S., Caumo, W., et al. Nicotinamide riboside reduces cardiometabolic risk factors and modulates cardiac oxidative stress in obese Wistar rats under caloric restriction. Life Sci., 2020;263: 118596
- Villeda-González, J. D., Gómez-Olivares, J. L., Baiza-Gutman, L. A., Manuel-Apolinar, L., Damasio-Santana, L., Millán-Pacheco, C., et al. Nicotinamide reduces inflammation and oxidative stress via the cholinergic system in fructose-induced metabolic syndrome in rats. Life Sci., 2020;250: 117585
- Huang, R. X., & Tao, J. Nicotinamide mononucleotide attenuates glucocorticoid‑induced osteogenic inhibition by regulating the SIRT1/PGC‑1α signaling pathway. Mol Med Rep., 2020;22(1): 145-154
- Klimova, N., Long, A., & Kristian, T. Nicotinamide mononucleotide alters mitochondrial dynamics by SIRT3-dependent mechanism in male mice. J Neurosci Res., 2019;97(8): 975-990
- Yang, J., Liu, G. Q., Wei, R., Hou, W. F., Gao, M. J., Zhu, M. X., et al. Nicotinamide promotes differentiation of human embryonic stem cells into cardiomyocytes.nActa Pharmacol Sin., 2011;32: 1239-1245.
- Han, D., Huang, W., Ma, S., Chen, J., Gao, L., Liu T., et al. Nicotinamide improves functional survival of engrafted adipose-derived mesenchymal stem cells in ischemic heart through PI3K/Akt signaling pathway. Biomed Res Int., 2015;2015: 858349
- Fang, T. X., Wei, Y. S., & Jie, Z. Y. Autophagy, dysglycemia and myocardial infarction. IJC Metab Endoc., 2017;14: 40-44
- Ma, S., Wang, Y., Chen, Y., & Cao, F. The role of the autophagy in myocardial ischemia/reperfusion injury. Bioch Biophys Acta., 2015;1852(2): 271-276
- Matsui, Y., Takagi, H., Qu, X., Abdellatif, M., Sakoda, H., Asano, T., et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res., 2007;100(6): 914-922
Citation: Wang Q, Zhang X (2021) Pre-conditioning with Nicotinamidemononucleotide Enhances Cardioprotective Potentials of Umbilical Cord-derived Mesenchymal Stem Cells in Diabetes: Role of Autophagy Flux. Cell Mol Biol 67: 216. DOI: 10.4172/1165-158X.1000216
Copyright: © 2021 Wang Q, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2384
- [From(publication date): 0-2021 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 1679
- PDF downloads: 705