Potential Hematological Biosignatures as Screening Tools for Tuberculosis Co-Infection among People Living with HIV
Received: 28-Apr-2024 / Manuscript No. JIDT-24-133450 / Editor assigned: 30-Apr-2024 / PreQC No. JIDT-24-133450 (PQ) / Reviewed: 15-May-2024 / QC No. JIDT-24-133450 / Revised: 22-May-2024 / Manuscript No. JIDT-24-133450 (R) / Published Date: 29-May-2024 DOI: 10.4173/2332-0877.589
Abstract
Background: A routine laboratory test like complete blood count is a common practise during any hospital visit. It doesn’t provoke social stigma, especially when diagnosis of infectious diseases like tuberculosis and Human Immuno Deficiency Virus (HIV) infection are to be done. Thus if tested for its potential to diagnose or screen tuberculosis co-infection among people living with HIV, it will serve as a valuable laboratory evidence to start treatment in such vulnerable population. We hereby address the plausibility of using haematological parameters as screening tools to identify TB among People Living with HIV.
Methods: Our retrospective analysis included four cohorts of people, 259 healthy volunteers, 299 newly diagnosed tuberculosis patients, 135 PLHIV and 255 HIV-TB co-infected patients, wherein we tried to identify haematological variables with higher potential to distinguish among these groups.
Results: We report for the first time, a three analyte haematological signature (anemic-decreased CD4-increased Monocyte Lymphocyte Ratio (MLR)) which has higher capacity to serve as HIV-TB screening tool among PLHIV population (sensitivity 88.9%, specificity 91.7%, AUC-0.965). Another three analyte signature with increased MLR-neutrophilic- thrombocytotic nature has a sensitivity of 90.1%, specificity of 91.3% and AUC 0.957 in differentiating healthy people from pulmonary tuberculosis patients. Followed by this, lymphocyte percentage and MLR as single haematological markers have sensitivity, specificity, AUC of 90.2%, 83.6%, 0.92% and 88.3%, 88%, 0.931% respectively in differentiating healthy from pulmonary tuberculosis population.
Conclusion: Further studies supporting this three analyte panel as biosignature for easy and effective screening of HIV-TB among the vulnerable PLHIV cohort is warranted, which is the need of the hour to prevent mortality caused by this co-infection.
Keywords: Lymphocyte; Diagnosis; Complete blood count; Biomarkers
Abbreviations
TB: Tuberculosis; PLHIV: People Living with Human Immuno Deficiency Virus Infection; ATT: Anti Tuberculosis Therapy; ART: Anti-Retroviral Therapy; ANOVA: Analysis of Variance; ROC: Receiver Operating Characteristic; AUC: Area Under Curve; MLR: Monocyte Lymphocyte Ratio; NLR: Neutrophil Lymphocyte Ratio; WHO: World Health Organization; Hgb: Hemoglobin; CBC: Complete Blood Count; PTB: Pulmonary Tuberculosis
Introduction
An estimated 10.6 million people (95% uncertainty interval: 9.9- 11 million) fell ill with Tuberculosis (TB) in 2021 [1]. People Living with Human Immuno Deficiency Virus (PLHIV) are more likely than others to become infected with TB [2]. For patients co-infected with HIV and TB, both Anti Tuberculosis Therapy (ATT) and Anti- Retroviral Therapy (ART) are required to prevent deaths occurring due to either of these two diseases. Providing prompt TB treatment and early initiation of ART to PLHIV diagnosed with TB, has averted 74 million deaths between 2000 and 2021 [1]. The provision of ART for PLHIV among notified newly diagnosed TB has been 89% since 2019. However, when compared to the total number of PLHIV estimated to have developed TB in 2021, the coverage reduced to a mere 46%, far below the overall level of coverage of ART for PLHIV, which was 75% [3]. The primary reason for this difference is the big gap between the estimated (703,000) and reported (368,641) number of PLHIV who developed TB in 2021 [1]. In high burden countries like India where the estimated numbers are far higher than the actually diagnosed TB cases, mortality prediction using routine clinical laboratory assays rather than any other confirmatory testing looks lucrative. Although the tests are non-definitive, these tests could still assist TB diagnosis and prognosis at a low cost.
Inflammation in TB is very critical; many inflammatory cells including macrophages, monocytes, neutrophils, primed T cells and B cells are recruited to the site of infection. Following recruitment, several pro and anti-inflammatory cytokines, chemokines and proteins are produced by these cells in an attempt to control the infection [4]. Besides immune cells, platelet activity is also documented to increase in pulmonary TB patients as compared to healthy volunteers [5] and this has been correlated with severity of TB disease [6]. A high neutrophil count has been claimed to be a useful predictor of unfavourable TB treatment outcome [7], with TB causing alterations in the haematological profiles as well.
Abnormalities in blood profiles are well documented among HIV- infected persons. Anemia is reported to be associated with disease advancement and increase in mortality [8,9]. Thrombocytopenia, a condition with decreased platelet count is prevalent in the later stages of disease where the CD4 cell count touches abysmal levels [10]. Leukopenia or decrease in white blood cells is also a common accompaniment especially in advanced HIV disease [11]. Even though it is well established that both HIV and TB cause haematological aberrations, very few studies have explored into the haematological profiles or Complete Blood Count (CBC) of Pulmonary Tuberculosis (PTB) and PLHIV with a view to understand their importance as screening tools to identify HIV-TB co-infection. Few studies that have documented altered hematological profile in co-infected participants, including a study from Northwest Ethiopia which demonstrated higher levels of neutropenia, anemia, and thrombocytopenia among HIV-TB co-infected patients compared to TB group [12]. Another study from Guyana reported decreased haemoglobin and WBC counts in the co- infected cohort compared to PTB group [13].
It has been ascertained in both these studies that haematological abnormalities worsen in HIV-TB coinfection. In a high burden setting, as HIV infection increases the risk of getting TB disease [14], we were interested to evaluate the use of haematological parameters in determining the possibility of detecting TB in PLHIV participants and vice versa. Although there are existing tuberculosis screening tests or tools in PLHIV like symptom screening, CRP, urine LAM, etc., a routine haematology test has an advantage over these specific confirmatory tests wherein it doesn’t require additional tests and does not provoke social stigma, Hence we analysed haematological parameters from four different cohorts of people to identify their significance as screening indices for HIV-TB coinfection among PLHIV.
Materials and Methods
Study population and data collection
This retrospective analysis involved four different group of people namely Healthy Volunteers (HV), PLHIV, PTB and HIV-TB who were recruited from different parts of Tamil Nadu. Their haematological values were compared to understand the possibility of using these parameters in differentiating one group from the other.
The data were extracted from randomised clinical trials conducted at ICMR-National Institute for Research in Tuberculosis in the past 10 years. Participants included adults aged 18-65 years. Moribund patients, pregnant and lactating women were excluded in the trials. The PLHIV group consisted of newly diagnosed HIV ART-naïve patients. The PTB group included newly diagnosed smear positive cases before the start of ATT. The HIV-TB group comprised of PLHIV who were newly diagnosed with PTB started on ART as per prevailing guidelines.
Measurement of heamtological markers
Briefly, in all the studies, 2 ml of EDTA blood was collected from participants and processed in AcT 5diff CP (Cap Pierce) Haematology Analyser (Beckman Coulter) and the results were documented.
Approvals
All trials were conducted with prior approval of the institutional ethical committee and after obtaining informed written consent. Except for the HIV-TB and PLHIV groups, all other participants were HIV negative. These cohorts who had concomitant TB had culture confirmed, rifampicin sensitive pulmonary TB and were not suffering from any other opportunistic infection [15,16].
Statistical analysis
For this analysis, data were extracted from records, checked for completeness and entered into IBM SPSS Statistics applicable to Windows (IBM Corp. Released 2017, Version 25.0. Armonk, NY). Data were analysed using Mann-Whitney U test for comparison between two groups and Analysis Of Variance (ANOVA) for comparison between more than two groups. Receiver Operating Characteristic (ROC) curve analysis was performed to determine the discriminatory power of the hematological parameters i.e. sensitivity and specificity of individual haematological candidate markers to distinguish between the different study groups. Combinations of haematological parameters were analysed through integrative ROC using the freely available web application CombiROC v.1.2 [17].
Outcome definitions
Anaemia was defined as Hgb15]. Lymphocytopenia, thrombocytopenia and neutropenia were defined as lymphocytes <18%, platelets 3 cells/μl and neutrophils <44% and <42% in females and males respectively. On the other hand, lymphocytosis, neutrophilia and thrombocytosis are defined as lymphocytes >45%, neutrophils >75% and >74% and platelets >420 × 103 cells/μ1 and >380 × 103 cells/μl in females and males respectively [15].
Results
A total of 948 participants comprising 135 PLHIV, 259 HV, 255 HIV- TB and 299 PTB patients were included in this analysis. The various haematological parameters studied and their statistical significance among the four study groups are documented in Table 1.
| Characteristic | All | PLHIV (G1) | HV (G2) | HIV-TB (G3) | TB (G4) | P value | (Groups with P value <0.05) |
|---|---|---|---|---|---|---|---|
| (n=948) | (n=135) | (n=259) | (n=255) | (n=299) | |||
| M: F | 625:323 | 66:99 | 134:125 | 196:59:00 | 229:70 | ||
| Age (years)# | 40.0 ± 12.3 | 38.3 ± 8.5 | 47.0 ± 14.0 | 39.1 ± 8.8 | 35.3 ± 12.2 | ||
| Hgb (g/dl)# | 11.7 ± 2.5 | 13.1 ± 2.1 | 12.9 ± 2.1 | 9.7 ± 2.1 | 11.9 ± 2.2 | <0.001 | (G2 and G4; G2 and G3; G1 and G3; G1 and G4; G3 and G4) |
| RBC (106/µL)# | 4.3 ± 0.9 | 4.3 ± 0.7 | 4.7 ± 1.1 | 3.6 ± 0.8 | 4.5 ± 0.6 | <0.001 | (All groups) |
| WBC# | 8067.8 ± 2922.8 | 6722.3 ± 2173.0 | 8086.6 ± 2539.2 | 6938.2 ± 3148.4 | 9622.4 ± 2580.0 | <0.001 | (G2 and G4; G2 and G3; G1 and G2; G1 and G4; G3 and G4) |
| Platelet# ( × 103/µL) | 3.3 ± 1.3 | 2.5 ± 1.0 | 2.8 ± 0.9 | 3.4 ± 1.4 | 4.1 ± 1.3 | <0.001 | (All groups) |
| PCV | 35.6 ± 18.3 | 38.3 ± 5.6 | 39.2 ± 26.7 | 30.7 ± 16.2 | 35.6 ± 13.4 | <0.001 | (G2 and G4; G2 and G3; G1 and G3; G1 and G4; G3 and G4) |
| Neutrophil (%)# | 62.1 ± 11.8 | 51.7 ± 10.7 | 57.2 ± 8.1 | 65.0 ± 13.4 | 68.5 ± 7.9 | <0.001 | (All groups) |
| Lymphocytes (%)# | 25.1 ± 12.6 | 34.9 ± 8.6 | 31.2 ± 7.4 | 22.1 ± 10.1 | 18.1 ± 14.2 | <0.001 | (All groups) |
| Eosinophil (%)# | 4.2 ± 3.9 | 5.5 ± 5.3 | 4.6 ± 3.3 | 3.0 ± 3.1 | 4.3 ± 4.1 | <0.001 | (G2 and G3; G1 and G2; G1 and G3; G1 and G4; G3 and G4) |
| Monocytes (%)# | 8.2 ± 3.4 | 7.3 ± 2.6 | 6.3 ± 2.5 | 9.2 ± 4.5 | 9.3 ± 2.5 | <0.001 | (G2 and G4; G2 and G3; G1 and G2; G1 and G3; G1 and G4) |
| Basophil (%)# | 0.6 ± 0.5 | 0.5 ± 0.5 | 0.7 ± 0.4 | 0.6 ± 0.5 | 0.6 ± 0.7 | 0.006 | (G2 and G3; G1 and G2) |
| CD4# | 316.0 ± 272.3 | 525.3 ± 272 | 204.7 ± 196.8 | <0.001 | (G1 and G3) | ||
| CD8# | 910.7 ± 558.8 | 1160.5 ± 520.3 | 777.9 ± 533.3 | <0.001 | (G1 and G3) | ||
| CD4/CD8# | 0.4 ± 0.3 | 0.6 ± 0.4 | 0.3 ± 0.3 | <0.001 | (G1 and G3) | ||
| NLR# | 3.4 ± 3.1 | 1.7 ± 1.0 | 2.1 ± 1.1 | 4.3 ± 4.8 | 4.6 ± 2.2 | <0.001 | (G2 and G4; G2 and G3; G1 and G2; G1 and G3; G1 and G4) |
| MLR# | 0.42 ± 0.32 | 0.22 ± 0.09 | 0.23 ± 0.14 | 0.51 ± 0.42 | 0.60 ± 0.26 | <0.001 | (G1 and G3; G1 and G4; G3 and G4; G2 and G4; G2 and G3) |
Note: #Plus: Minus values are mean ± SD; Hgb: Haemoglobin; RBC: Red Blood Cell; WBC: White Blood Cell; NLR: Neutrophil Lymphocyte Ratio; n: number, P ≤ 0.05 is considered statistically significant.
Table 1: Comparison of haematological profile among different study groups.
Haematological abnormalities in the study groups
Using the haematological observations, the prevalence of various haematological abnormalities were analysed and their values are given in Table 2. We found that 12% of healthy controls and 43% of TB group had anaemia. The PLHIV population showed only 13% prevalence of anaemia, however upon coinfection with TB, it increased to 84%. Similarly, thrombocytosis (30%) and neutrophilia (26%) were also highly prevalent among HIV-TB cases, whereas their prevalence rates were only 5% and 3% in the PLHIV group. The PTB group had highest prevalence of thrombocytosis (57%) and neutrophilia (27%). We also observed a higher prevalence of lymphocytopenia in the TB (56%) and HIV-TB (38%) groups compared to the PLHIV group (4%). Thus our observations suggest that haematological abnormalities increase manifold during HIV-TB coinfection compared to PLHIV condition.
| Healthy | TB | PLHIV | HIV-TB | |
|---|---|---|---|---|
| (N=259) | (N=299) | (N=135) | (N=255) | |
| Neutropenia (F<44%, M<42%) | 9 | 1 | 29 | 15 |
| Neutrophilia (F>75%, M>74%)* | 7 (3) | 71 (24) | 4 (3) | 67 (26) |
| Neutrophils within range | 243 | 227 | 102 | 173 |
| Thrombocytopenia (<130 × 103 platelets) | 10 | 5 | 6 | 18 |
| Thrombocytosis (F>420 × 103 platelets, M>380 × 103 platelets)* | 17 (7) | 171 (57) | 7 (5) | 76 (30) |
| Platelets within range | 232 | 123 | 122 | 161 |
| Anemia (<12.3g/dl in men, <9.9g/dl in women)* | 32 (12) | 130 (43) | 18 (13) | 214 (84) |
| HgB within range | 227 | 169 | 117 | 41 |
| Lymphocytopenia (<18%)* | 14 (5) | 167 (56) | 5 (4) | 98 (38) |
| Lymphocytosis (>45%) | 7 | 1 | 17 | 6 |
| Lymphocyte count within range | 238 | 131 | 113 | 151 |
Note: *N (%): number (percentage); F: Female, M: Male
Table 2: Prevalence of haematological abnormalities among different study groups.
| Variables | Healthy vs TB | Healthy vs HIV-TB | TB vs HIV-TB | PLHIV vs HIV-TB | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Se% | Sp% | AUC | Se% | Sp% | AUC | Se% | Sp% | AUC | Se% | Sp% | AUC | |
| WBC | 69.02 | 61.89 | 0.69 | 36.76 | 92.21 | 0.62 | 89.56 | 54.55 | 0.749 | 78.26 | 90.22 | 0.516 |
| RBC | 65.32 | 50.41 | 0.58 | 77.87 | 82.38 | 0.841 | 86.53 | 67.19 | 0.82 | 69.17 | 72.93 | 0.753 |
| N% | 79.12 | 79.1 | 0.843 | 64.03 | 72.28 | 0.72 | 87.83 | 30.43 | 0.57 | 64.82 | 85.71 | 0.8 |
| L% | 90.24 | 83.61 | 0.922 | 64.03 | 84.43 | 0.773 | 87.54 | 38.74 | 0.633 | 71.54 | 85.71 | 0.829 |
| M% | 80.47 | 73.77 | 0.834 | 62.45 | 78.28 | 0.74 | 82.49 | 33.6 | 0.55 | 51.68 | 71.43 | 0.647 |
| B% | 69.02 | 51.64 | 0.603 | 73.91 | 51.64 | 0.652 | 53.2 | 60.08 | 0.553 | 77.47 | 43.61 | 0.559 |
| E% | 32.32 | 85.66 | 0.576 | 51.38 | 85.66 | 0.72 | 61.95 | 60.47 | 0.644 | 66.8 | 69.92 | 0.723 |
| Platelet | 64.98 | 86.48 | 0.82 | 44.66 | 81.56 | 0.632 | 63.64 | 64.43 | 0.67 | 68.77 | 65.41 | 0.695 |
| Hgb | 82.49 | 38.11 | 0.639 | 69.57 | 87.7 | 0.863 | 78.79 | 64.03 | 0.78 | 84.58 | 77.44 | 0.877 |
| CD4 | - | - | - | - | - | - | - | - | - | 77.08 | 86.47 | 0.865 |
| CD8 | - | - | - | - | - | - | - | - | - | 50.2 | 84.21 | 0.72 |
| CD4/CD8 | - | - | - | - | - | - | - | - | - | 69.17 | 68.42 | 0.739 |
| NLR | 87 | 84.1 | 0.906 | 66.54 | 80.31 | 0.753 | 41.73 | 84.62 | 0.618 | 66.9 | 87.4 | 0.821 |
| MLR | 88.3 | 88 | 0.931 | 67.8 | 86.1 | 0.808 | 47.8 | 79.6 | 0.633 | 68.6 | 88.9 | 0.811 |
Note: %: Percentages; Se%: Sensitivity; Sp%: Specificity; AUC: Area Under the Curve
Table 3: Sensitivity and specificity of haematological parameters as determined by ROC in differentiating the four study groups.
ROC analysis reveals the best haematological biomarker as lymphocyte percentage to differentiate TB from healthy controls
The ability of haematological parameters to differentiate between the HV, PLHIV, PTB and HIV-TB groups was analysed by determining the sensitivity, specificity and Area Under Curve (AUC) (Table 3). Since, Neutrophil Lymphocyte Ratio (NLR) and Monocyte Lymphocyte Ratio (MLR) have gained importance as predictors of various pathologies; we also calculated NLR and MLR and compared them between the groups. As single analytes, lymphocyte percentage gave a sensitivity of 90.2% and specificity of 83.6% (AUC: 0.92%) and MLR gave a sensitivity of 88.3% and specificity of 88% (AUC: 0.931%) in differentiating healthy from TB group. This was followed by NLR, with a sensitivity of 87.0% and specificity of 84.1% (AUC: 0.90%).
Three analyte signature differentiate PTB from HIV-TB
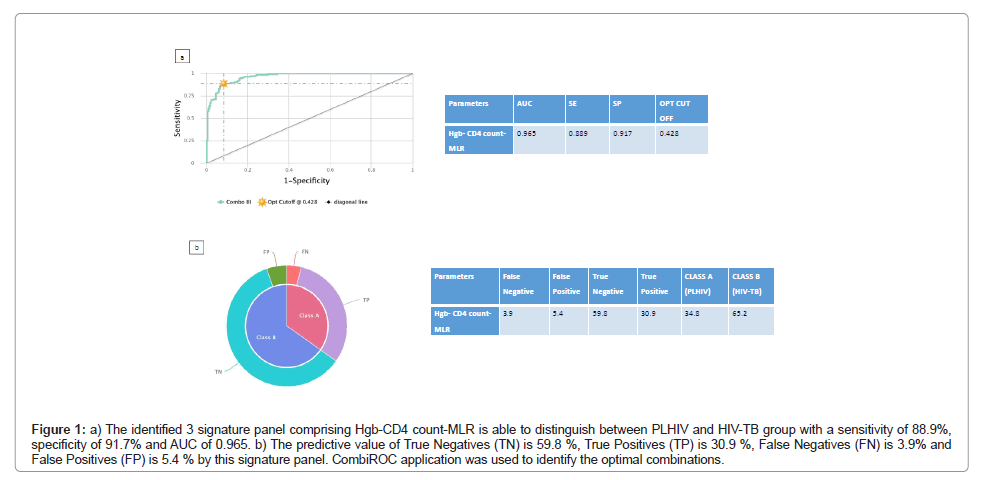
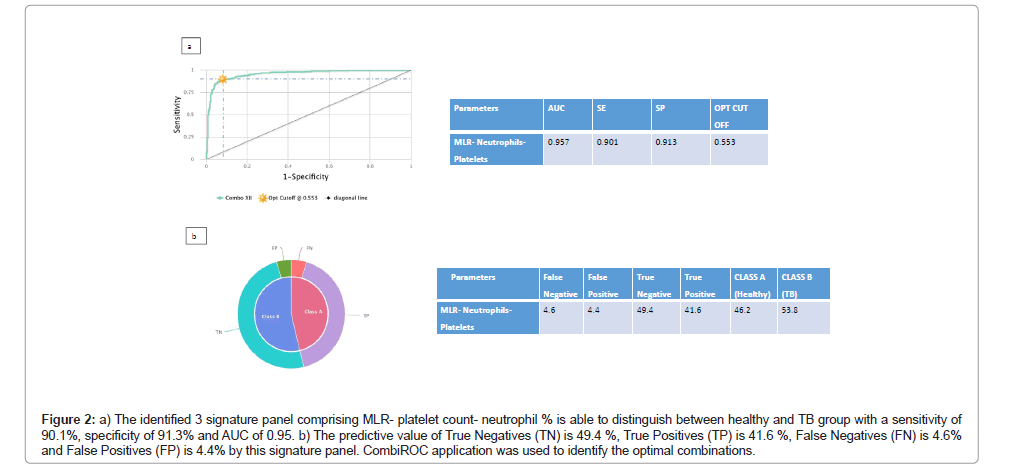
Since, other haematological parameters as individual components had lower AUC in differentiating between other groups, in order to increase their Target Product Profile (TPP) for diagnostic markers according to World Health Organisation (WHO) [16], we tested combinations of haematological parameters to determine their diagnostic/screening potential. This was achieved with the help of CombiROC software [17] which computes sensitivity and specificity for different marker combinations and performs a combined ROC analysis. This analysis gave better sensitivity, specificity and AUC for certain analytes and the best combination of haematological markers and their sensitivity, specificity and AUC are shown in the provided Figures 1 and 2. Using this approach, we hereby report a three signature panel of haematological parameters which hold promise for distinguishing HIV-TB from PLHIV with AUC, sensitivity and specificity meeting the TPP standards of WHO required for a good diagnostic biomarker. As seen in Figure 1, an anaemic decreased CD4 increased MLR signature was associated with HIV-TB co-infection with a sensitivity of 88.9% and specificity of 91.7% compared to HIV infection alone. We also found that a three signature haematology panel, comprising of increased MLR thrombocytotic neutrophilic nature, which gave a sensitivity of 90.1% and specificity of 91.3% in differentiating healthy from TB group (Figure 2) suggesting that, this three analyte panel has the potential to serve as a screening tool for TB among general population.
Figure 1: a) The identified 3 signature panel comprising Hgb-CD4 count-MLR is able to distinguish between PLHIV and HIV-TB group with a sensitivity of 88.9%, specificity of 91.7% and AUC of 0.965. b) The predictive value of True Negatives (TN) is 59.8 %, True Positives (TP) is 30.9 %, False Negatives (FN) is 3.9% and False Positives (FP) is 5.4 % by this signature panel. CombiROC application was used to identify the optimal combinations.
Figure 2: a) The identified 3 signature panel comprising MLR- platelet count- neutrophil % is able to distinguish between healthy and TB group with a sensitivity of 90.1%, specificity of 91.3% and AUC of 0.95. b) The predictive value of True Negatives (TN) is 49.4 %, True Positives (TP) is 41.6 %, False Negatives (FN) is 4.6% and False Positives (FP) is 4.4% by this signature panel. CombiROC application was used to identify the optimal combinations.
Discussion
As WHO recommends TB screening for all PLHIV before the initiation of ART [18] and haematological profile gives a snapshot of the body’s functionality, we questioned the possibility of using haematological parameters for this purpose. In order to identify differences in various haematological parameters and their ability to distinguish between individuals with PTB, PLHIV and HIV-TB coinfection from healthy controls, we analysed the haematological parameters. Since the sensitivity of sputum microscopy falls within 24%-61% [19] in PLHIV population, we ventured in with this novel approach of using the hemogram analysis which is a routine test done during any hospital visit, both in the outpatient and inpatient departments in order to evaluate its usefulness not only in TB diagnosis in the immunocompromised but also treaded one step ahead to see if it could distinguish the sub-groups as well. Haematology, if proven to be a good diagnostic/screening tool may aid in early detection of HIV-TB coinfection, which will greatly benefit the society. Variations in haematological profile is a common phenomenon during any infection/ disease [20]. These changes are well documented across different infectious diseases including TB [21] and HIV [22].
As expected, the haematological parameters in healthy controls differed from the rest of three study groups. Of particular interest, as reported earlier [23,24] we observed increased prevalence of anaemia among PTB participants and the prevalence of anaemia was further exacerbated by the HIV coinfection. This emphasises the fact that anaemia seen in PTB is due to the chest inflammatory conditions and not nutritional anaemia always. Thus, there is a possibility that control of PTB can reverse the anaemia status. This observation also reinforces the fact that special care should be given to TB and HIV-TB people with respect to redeeming their haemoglobin status to normal values for a successful recovery. Similarly, the increased prevalence of neutrophilia and lymphocytopenia in those with HIV-TB coinfection as compared to HIV infection alone, raises our opinion that these cells not only in their functions, but also in their numbers respond uniquely to infections.
In recent times, rather than the hematological analytes by themselves, their ratios have gained importance in various diagnoses. NLR has gained prominence as a prognostic/diagnostic marker for cardiovascular and inflammatory diseases, coronavirus disease and several types of cancer [25-28] as well as respiratory disease like pneumonia and different forms of TB [29,30]. Analogous to the above reports, we also found that NLR has great potential to serve as a screening tool to aid in TB diagnosis with a sensitivity of 87%. In addition to NLR, our analysis confirms the fact that MLR can be considered as a crucial biomarker to identify TB in adults (31).
Even though most of the parameters analysed were statistically different between the study groups, their diagnosing accuracy in differentiating between the groups was not satisfactory. Area under the curve was inadequate to rank them as good diagnostic markers as per the WHO’s, TPP for a diagnostic biomarker for tuberculosis with or without HIV. However, lymphocyte numbers (Table 3) had the greatest discriminative power compared to all other tested haematological parameters, in distinguishing TB from the healthy group. We did a combinatorial analysis of haematological parameters to check their diagnostic ability in differentiating between the four groups. By this method we report two different three signature panels, one for differentiating TB from healthy group and one for differentiating HIV- TB from PLHIV.
Even though it is widely agreed that these parameters are shown to be influenced during co-infections like PTB and HIV to our knowledge [12,13], no study has documented the use of these parameters as a screening criteria in the dually infected. With our observations presented here we provide small leads towards using haematological parameters as screening tools for HIV-TB coinfection among PLHIV group, in a low resource setting. Even though our data are from well characterized dataset, interpretation of haematological data should be made keeping in mind, underlying confounding factors like intake of drugs, diabetes, age and so forth. Also, the range identified here for the biosignatures cannot be generalized for diverse population across the globe. Further validation using multi-centric and multi-national cohorts will add strength to our investigations. Additional biomarkers like C-reactive protein might be added in the future studies, which is a limitation of our analysis. Presumptive TB cases identified by these signatures, may be subjected to further confirmatory tests like Xpert, culture and chest x-ray which will help in early diagnosis of TB among HIV positive individuals and help in rapid ATT initiation among this vulnerable population.
Conclusion
Haematological parameters are indicative of prevailing infections and are cost effective and easily accessible. Our findings reveal that a combination of haematological parameters can be used as screening aid for HIV-TB coinfection. This finding has to be validated in a large multi-centric study before further implementation. Haematological screening will reduce the stigma in patients being asked to get tested for TB, thus increasing TB screening, rapid detection and early initiation of treatment, thereby contributing to TB control in the long run.
Ethics Approval Statement
All the studies from which data has been obtained were done with prior approval of NIRT IEC (TRC IEC No: 2008002/NIRT IEC No: 2011001/NIRT IEC No: 2015023).
Availability of Data
De-identified data may be shared with qualified researchers upon request to the corresponding author.
Acknowledgements
The authors whole heartedly thank the Director, ICMR-NIRT for her constant support throughout the different phases of this analysis. The authors acknowledge and thank all the study participants of earlier NIRT studies who formed the backbone of this analysis. The authors also thank staff of ICMR-NIRT who were involved in study 24 and study 25 for their support and contribution.
Funding Statement
The data used here are from different studies funded by Institutional Intramural grants and USAIDS under the Model Dots project (NCT00933790).
Authorship Contributions
NHJ conceptualised and wrote the manuscript. NG, BR and LEH shared data and proof read the manuscript, PC, TM did the statistical analysis, DN, RKS, ANS and PGD helped with participant recruitment, LK, NC and HB collected the data, KP, MG, M and KC collected samples and captured demographics.
Competing Interests
No financial & non-financial competing interests to declare.
References
- Bagcchi S (2023) WHO's global tuberculosis report 2022. Lancet Microbe 4(1):e20.
- Flynn JL, Chan J, Lin PL (2011) Macrophages and control of granulomatous inflammation in tuberculosis. Mucosal Immunol 4(3):271-278.
- Şahin F, Yazar E, Yıldız P (2012) Prominent features of platelet count, plateletcrit, mean platelet volume and platelet distribution width in pulmonary tuberculosis. Multidiscip Respir Med 7:1-7.
- Tozkoparan E, Deniz O, Ucar E, Bilgic H, Ekiz K (2007) Changes in platelet count and indices in pulmonary tuberculosis. De Gruyter
- Carvalho AC, Amorim G, Melo MG, Silveira AK, Vargas PH, et al. (2021) Pre-treatment neutrophil count as a predictor of antituberculosis therapy outcomes: A multicenter prospective cohort study. Front immunol 12:661934.
- Sullivan PS, Hanson DL, Chu SY, Jones JL, Ward JW, et al. (1998) Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: Results from the multistate adult and adolescent spectrum of HIV disease surveillance project. Am J Hematol 91(1):301-308.
- Semba RD, Shah N, Klein RS, Mayer KH, Schuman P, et al. (2002) Prevalence and cumulative incidence of and risk factors for anemia in a multicenter cohort study of human immunodeficiency virus-infected and uninfected women. Clin Infect Dis 34(2):260-266.
- Enawgaw B, Alem M, Addis Z, Melku M (2014) Determination of hematological and immunological parameters among HIV positive patients taking highly active antiretroviral treatment and treatment naïve in the antiretroviral therapy clinic of Gondar University Hospital, Gondar, Northwest Ethiopia: A comparative cross-sectional study. BMC hematol 14:1-7.
- CDCTB (2022) TB and HIV coinfection [Internet]. Centers for Disease Control and Prevention.
- Global HIV & AIDS statistics - Fact sheet. Unaids.org (2023)
- Evans RH, Scadden DT (2000) Haematological aspects of HIV infection. Best pract res cl ha 13(2):215-30.
- Abay F, Yalew A, Shibabaw A, Enawgaw B (2018) Hematological abnormalities of pulmonary tuberculosis patients with and without HIV at the University of Gondar Hospital, Northwest Ethiopia: A comparative cross-sectional study. Tuberc Res Treat 2018:1-6.
- Kurup R, Flemming K, Daniram S, Marks-James S, Roberts Martin R (2016) Hematological and biochemistry profile and risk factors associated with pulmonary tuberculosis patients in Guyana. Tuberc Res Treat.
- Bruchfeld J, Correia-Neves M, Källenius G (2015) Tuberculosis and HIV coinfection. Cold Spring Harb Perspect Med 5(7):a017871.
- Sairam S, Domalapalli S, Muthu S, Swaminathan J, Ramesh VA, et al. (2014) Hematological and biochemical parameters in apparently healthy Indian population: Defining reference intervals. Indian J Clin Biochem 29:290-297.
- World Health Organization (2014) High priority target product profiles for new tuberculosis diagnostics: Report of a consensus meeting, 28-29 April, Geneva, Switzerland. Google Scholar]
- Mazzara S, Rossi RL, Grifantini R, Donizetti S, Abrignani S, et al. (2017) CombiROC: An interactive web tool for selecting accurate marker combinations of omics data. Sci Rep 7(1):45477.
- World Health Organization (2018) Latent tuberculosis infection: Updated and consolidated guidelines for programmatic management.
- Getahun H, Harrington M, O'Brien R, Nunn P (2007) Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: Informing urgent policy changes. The Lancet 369(9578):2042-2049.
- Quaglino D, Corazza GR (1993) Hematological changes in infectious diseases. Hematological consequences of bacterial, protozoal and spirochetal infections (2). Ann Ital Med Int 8(1):29-34. Google Scholar]
[PubMed]
- Shafee M, Abbas F, Ashraf M, Mengal MA, Kakar N, et al. (2014) Hematological profile and risk factors associated with pulmonary tuberculosis patients in Quetta, Pakistan. Pak J Med Sci 30(1):36.
- Firnhaber C, Smeaton L, Saukila N, Flanigan T, Gangakhedkar R, et al. (2010) Comparisons of anemia, thrombocytopenia, and neutropenia at initiation of HIV antiretroviral therapy in Africa, Asia, and the Americas. Int J Infect Dis. 14(12):e1088-e1092.
- Chhabra S, Kashyap A, Bhagat M, Mahajan R, Sethi S (2021) Anemia and nutritional status in tuberculosis patients. Int J Appl Basic Med Res 11(4):226-230.
- Lowe DM, Bandara AK, Packe GE, Barker RD, Wilkinson RJ, et al. (2013) Neutrophilia independently predicts death in tuberculosis. Eur Respir J 42(6):1752-1757.
- Mentis AF, Kyprianou MA, Xirogianni A, Kesanopoulos K, Tzanakaki G (2016) Neutrophil-to-lymphocyte ratio in the differential diagnosis of acute bacterial meningitis. Eur J Clin Microbiol Infect Dis 35:397-403.
- Karimi A, Shobeiri P, Kulasinghe A, Rezaei N (2021) Novel systemic inflammation markers to predict COVID-19 prognosis. Front Immunol 12:741061.
- Gopalan N, Senthil S, Prabakar NL, Senguttuvan T, Bhaskar A, et al. (2022) Predictors of mortality among hospitalized COVID-19 patients and risk score formulation for prioritizing tertiary care-An experience from South India. PLoS One 17(2):e0263471.
- Forget P, Khalifa C, Defour JP, Latinne D, Van Pel MC, et al. (2017) What is the normal value of the neutrophil-to-lymphocyte ratio?. BMC Res Notes 10:1-4.
- Jeon YL, Lee WI, Kang SY, Kim MH (2019) Neutrophil-to-monocyte-plus-lymphocyte ratio as a potential marker for discriminating pulmonary tuberculosis from nontuberculosis infectious lung diseases. Lab Med 50(3):286-291.
- Liu H, Li Y, Yi J, Zhou W, Zhao S (2022). Neutrophil-lymphocyte ratio as a potential marker for differential diagnosis between spinal tuberculosis and pyogenic spinal infection. J Orthop Surg Res 17(1):357.
- Adane T, Melku M, Ayalew G, Bewket G, Aynalem M (2022) Accuracy of monocyte to lymphocyte ratio for tuberculosis diagnosis and its role in monitoring anti-tuberculosis treatment: Systematic review and meta-analysis. Medicine 101(44):e31539.
Citation: Nancy J, Gopalan N, Rekha B, Hanna LE, Ponnuraja C, et al. (2024) Potential Hematological Biosignatures as Screening Tools for Tuberculosis Co-Infection among People Living with HIV. J Infect Dis Ther 12: 589 DOI: 10.4173/2332-0877.589
Copyright: © 2024 Nancy J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 926
- [From(publication date): 0-2024 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 728
- PDF downloads: 198