Editorial Open Access
Potential Drug Targets in the Death Pathway: Therapeutic Approaches in Apoptosis
| Md. Salahuddin1*, Md. Shariful Islam2, Abu Zaffar Shibly2, Hasibul Haque Rakib2, Muhammad Jahangir Hossen3, Md. Ramim Tanver Rahman4, MA Momin5, Md. Sayfullah Razin6, Jay Prakash Sah7, Md. Abdur Rahman8, Muhammad Aurang Zeb9 and Mizanur Rahman Washim10 | |
| 1Faculty of Medicine, University of Hongkong, Pokfulam Road, Hongkong | |
| 2Department of Biotechnology and Genetic Engineering, Faculty of Life science, Mawlana Bhashani Science and Technology University, Tangail-1902, Bangladesh | |
| 3Department of Animal Science, Patuakhali Science and Technology University, Bangladesh | |
| 4National Engineering research Centre for functional Food, Jiangnan University, Wuxi 214122, PR China | |
| 5Department of Microbiology, Faculty of Veterinary Medicine, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh | |
| 6College of Medicine, Shaheed Ziaur Rahman Medical College, Bogra, University of Rajshahi, Bangladesh | |
| 7Department of Medical Laboratory Science, School of Health and Allied Sciences, Pokhara University, Lekhnath -12 Kaski, Nepal | |
| 8Faculty of veterinary Medicine, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh | |
| 9Department of Biochemistry, Hazara University, Mansehra, Khyber Pakhtunkhwa, Pakistan | |
| 10Bangladesh Fisheries research Institute, Mymensing, Bangladesh | |
| Corresponding Author : | Md. Salahuddin PhD Research Fellow Faculty of Medicine, University of Hongkong Pokfulam Road, Hongkong Tel: +852-5584- 1096 E-mail: ssdin23@gmail.com |
| Received: October 26, 2015; Accepted: October 27, 2015; Published: November 03, 2015 | |
| Citation: Salahuddin M, Shariful Islam M, Shibly AZ, Rakib HH, Hossen MJ, et al. (2015) Potential Drug Targets in the Death Pathway: Therapeutic Approaches in Apoptosis. Biochem Physiol 4:e141. doi:10.4172/2168-9652.1000e141 | |
| Copyright: © 2015 Salahuddin M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
The form of programmed cell death known as Apoptosis has become an intense focus of investigation in various fields including carcinogenesis and cancer therapy. It is a sequentially regulated suicidal programme where cells regulate certain enzymes including caspase 9 activation and cytochrome C release with apoptosis inducing factor (AIF). The Bcl-2 proteins also represent a promising target for modulating tumor cell sensitivity to Apoptosis. Disturbance of this regulatory pathway may lead to various diseases like autoimmune diseases, neurodegenerative diseases and cancers. Therefore, understanding the mechanisms for apoptosis signaling pathway will give us huge knowledge to enlighten the pathogenesis of various diseases including cancer, and will open new horizons to therapeutic approaches in drug designing.
| Keywords |
| Cancer; Apoptosis; Apoptosis inducing factor; Drug designing |
| Editorial |
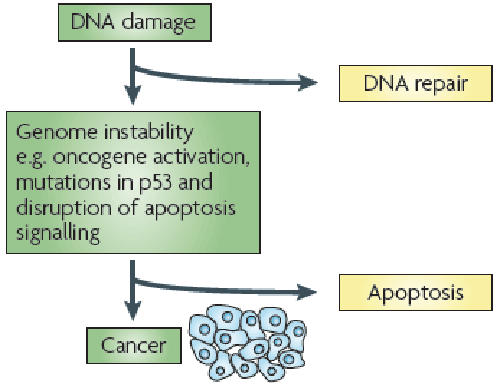
| Apoptosis known as programmed cell death is a self regulatory mechanism that results in the efficient removal of damaged or unnecessary cells during growth and development. There are many factors that contribute to this process, have demonstrating specificity of function, regulation, and meaningful involvement in cascades. Recent progress has extended our understanding of cancer and its underlying etiology to encompass aberrant cellular survival, as a consequence of failing to appropriately induce apoptosis or cell death, as a major contributor to the transformed state. It is associated with the rapid engulfment and removal of cell corpses by phagocytic cells that recognize signals displayed on the outer surface of the apoptotic cell [1] (Figure 1). This consequence of a series of regulated events in apoptotic cell death is frequently altered in malignant cells. And this idea provides the opportunity for selective clinical intervention to bring about the death of the tumor cell without damaging to normal cells. In the same way, another pertinent factor is p53; that can engage apoptosis in response to DNA damage to avert the potential acquisition of lethal mutations; is frequently mutated or deleted in tumor cells. |
| Recent findings demonstrate that proapoptotic signal generates a tumor cell with a distinct survival advantage over normal cells. Can we exploit this to our advantage for the successful treatment of cancer rather than allowing it to hinder our progress with conventional chemotherapeutics? Caspases are synthesized as inactive zymogens, which must be proteolytically cleaved at two aspartate residues to generate the active mature enzyme. These cleavage events remove an NH2-terminal peptide and separate the small and large subunits of the proenzyme to generate a mature hetero-tetrameric caspase comprising two large and two small subunits. The generation of active caspases forms a cascade in which “initiator” caspases interact with specific adapter molecules to facilitate their own auto-processing. Then these active initiator caspases in turn cleave and activate the downstream “executioner” caspases, that later cleave their target substrates to orchestrate the proteolytic dismantling of the cell [2-5]. |
| It has been reported that the sequences of event culminating in the activation of caspases has been broadly categorized into two pathways, the “extrinsic” pathway characterized by the engagement of cell surface “death receptors” and the “intrinsic” pathway involving key mitochondrial events. In both cases, an initiator caspase, via its interaction with a specialized adapter molecule, mediates its selfactivation, and ultimately activation of the downstream effector or executioner caspases. It is the activity of these caspases that ensure destruction of the cell. |
| Several recent studies have defined a form of cell death that proceeds independently of Apaf-1 and caspase activation but major theme is the subject to be regulated by factors typically associated with the apoptotic cascade. Then apoptosis inducing factor (AIF) is released from mitochondria via a mechanism regulated by Bcl-2 [6]. These observations appear to define a regulated form of cell death that retains certain features of apoptosis but cannot be classified as necrosis. Recent descriptions of an alternative Apaf- 1-independent but caspase-dependent form of cell death also imply the existence of a novel mechanism for the activation of caspases [7-9]. These studies describe one or more forms of Apaf-1-independent death engaged by specific stimuli, including endoplasmic reticulum stress [9], serum withdrawal [7] that can be suppressed by Bcl-2 [7], caspase inhibitors, and significantly attenuated by the overexpression of catalytically inactive forms of caspases-9 and -12 [7]. This series of observations indicates a complexity of caspase-dependent cell death that is not currently understood by researchers. |
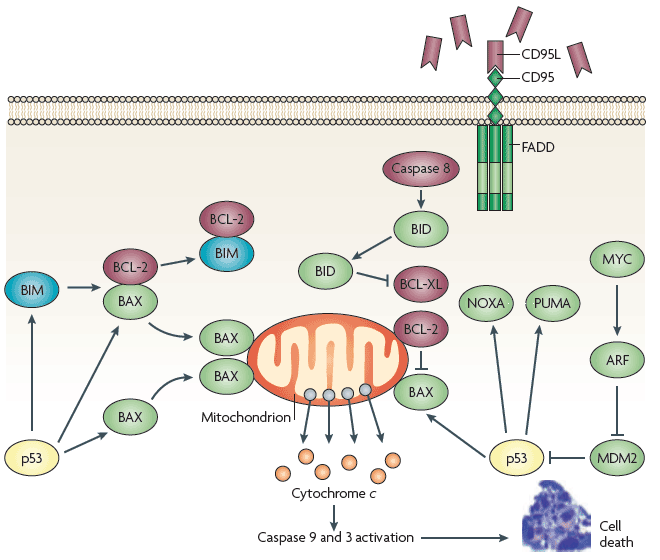
| The development of tumor arises as a consequence of dysregulated proliferation and a suppression of apoptosis, and each of these primary defects provides an obvious opportunity for clinical intervention. However, targeting and overcoming abnormalities in tumor cells that suppress apoptosis could generate a potent proapoptotic stimulus by virtue of the growth-deregulating mutations, for example Myc, which can drive apoptosis. Replacement of wild-type p53 using both viral and nonviral delivery systems, although promising in preclinical studies, has fallen short of expectation in the clinic as a consequence of limited target specificity, inefficient delivery, and adverse immune reaction [10]. Recently a number of small molecule-based approaches designed to modify the function of p53 include peptides and antibodies able to revert the conformation of mutant p53 to that of the wild type and, in doing so, restoring its transcriptional competence [11-13]. The Bcl-2 proteins represent a promising target for modulating tumor cell sensitivity to apoptosis [14] (Figure 2). |
| Over expression of antiapoptotic Bcl-2 proteins is observed in many tumor types, which may contribute to the drug-resistant state and help to mediate the expansion of a transformed population by disrupting normal cell turnover. According to literature reviewed, overcoming a blockade induced by Bcl-2 would restore normal cellular homeostasis, reverse the drug-resistant phenotype, and restore tumor cell sensitivity to conventional chemotherapeutics. Clinical success using antisense oligonucleotides [15] and antibodies against Bcl-2 has been also reported [16,17]. Therefore additional small molecule-based strategies for disrupting the activities of antiapoptotic Bcl-2 proteins have arisen from our extensive understanding of the structures of these proteins. |
| Summary |
| In summary, apoptosis is a complex area involving many protein families, sub cellular compartments, and signal transduction cascades. While much is known, more work is necessary on the multiple pathways in multiple cell types. At present, the precise pathways executed under specific circumstances are unconfirmed and many details of the complex regulatory mechanisms are unknown. The hope for a successful resolution to design specific agents that effectively induce tumor regression; at least in part, in the need to overcome the inherent resistance of many transformed cells to the engagement of apoptosis. We hope that rising insights into the field of apoptosis and cancer will uncover new and effective strategies to tackle the complexity of tumor chemo resistance. |
References
|
--
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 14583
- [From(publication date):
December-2015 - Jul 15, 2025] - Breakdown by view type
- HTML page views : 9910
- PDF downloads : 4673
