Editorial Open Access
Potential Application of Chitin Signaling in Engineering Broad-Spectrum Disease Resistance to Fungal and Bacterial Pathogens in Plants
| Jeanne Andary1,2*, Jaqueline Maalouly3, Rosette Ouaini3, Hanna Chebib3, Marc Beyrouthy4, Douglas N Rutledge1,5 and Naim Ouaini2 | ||
| 1AgroParisTech, Laboratory of Analytical Chemistry, 16 rue Claude Bernard, Bernard, 75005 Paris, France | ||
| 2Department of Chemistry and Life Science, Holy Spirit University of Kaslik, 446 Jounieh, Lebanon | ||
| 3Faculty of Science, Department of Chemistry, Libanese University, 90656, Jdaideth El Maten, Fanar, Lebanon | ||
| 4Faculty of Agronomy, Holy Spirit University of Kaslik, 446 Jounieh, Lebanon | ||
| 5INRA/AgroParisTech UMR1145 Food Process Engineering GENIAL, 16, rue Claude Bernard 75005 Paris, France | ||
| Corresponding Author : | Jeanne Andary Departement of Chemistry and life Science Holy Spirit University of Kaslik, 446 Jounieh Lebanon Tel: +9619600900 E-mail: jeanne_andary@Hotmail.com |
|
| Received January 18, 2013; Accepted February 28, 2013; Published March 12, 2013 | ||
| Citation: Andary J, Maalouly J, Ouaini R, Chebib H, Beyrouthy M, et al. (2013) Phenolic Compounds from Diluted Acid Hydrolysates of Olive Stones: Effect of Overliming. Adv Crop Sci Tech 1:103. doi: 10.4172/2329-8863.1000e103 | ||
| Copyright: © 2013 Andary J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Advances in Crop Science and Technology
Abstract
Chitin, a polymer of N-acetylglucosamine, is an important structural component in the cell walls of fungal pathogens. Plant chitinases are capable of degrading this component to directly inhibit infection by fungal pathogens. Plants can further detect the chitin fragments released by chitinases to trigger additional defense responses. Recent studies have identified chitin receptors in different plant species and a number of downstream signaling components. The chitin-mediated signal transduction pathway appears to be a unique and promising target for engineering plants for broad-spectrum resistance to various fungal as well as bacterial pathogens.
| Keywords | |
| Olive stone; Phenolic compounds; Overliming; GC-MS; HPLC-UV | |
| Abbreviations | |
| PC: Phenolic Compounds; FF: Furfuraldeyde; HMF: 5-hydroxymethyl-2-furaldehyde; HPLC: High-Performance Liquid Chromatography; LLME: Liquid-Liquid Microextraction; DAH: Dilute-Acid Hydrolysates; GC: Gas Chromatography; TA: Tannic Acid; MS: Mass Spectrometer; EI: Electron Energy; BHT: 2,6-bis(1,1- dimethylethyl)-4-methylphenol; BSTFA: N,O-Bis(trimethylsilyl) trifluoroacetamide; UV: Ultraviolet Light; H: Hydrolysate; T: Tyrosol; VA: Vanillic Acid; PA: Protocatechic Acid; V: Vanillin; Pca: p-Coumaric Acid; CA: Caffeic Acid; SYR: Syringaldehyde; PHBA: Parahydroxybenzoic Acid; HCA: Hydroxycinnamic Acid; PHPA: 4-Hydroxyphenylacetic Acid | |
| Introduction | |
| Olives are the most extensively cultivated fruit crop in the world [1]. Olive oil extraction represents an economical and social industrial activity that is highly relevant in the Mediterranean countries [2,3] where olive oil constitutes the main source of nutritional fat and is a very valuable product for exportation; today Mediterranean countries account for around 98% of world’s olive cultivation [4]. The extraction process has a large environmental impact due to production of highly polluting wastewater and/or solid residue [1,2]. In the olive oil industry, only olive stones can be recovered from the filtration of solid waste. Combustion of olive stones as a fuel with high calorific value is the most commonly used procedure to eliminate its harmful effects on the environment; however, greater environmental and economic benefits could result from the conversion of this byproduct to a derivative with higher added value [4,5]. | |
| Olive stone is a lignocellulosic material with hemicellulose, cellulose and lignin as main components [2]. To obtain monomeric sugars from lignocellulose, the hemicellulose and cellulose need to be hydrolyzed [6]. Hydrolysis with dilute sulfuric acid is a simple fast and cheap method [6,7]. A significant drawback of this process is the generation of several byproducts that negatively affect the fermentation efficiency of microorganisms. Based on their origin, the inhibitors are usually divided into three major groups: weak acids, furan derivatives and phenolic compounds [7-9]. When hemicellulose is degraded, xylose, mannose, acetic acid, galactose and glucose are liberated. Cellulose is hydrolysed to glucose. At high temperature, xylose is further degraded to Furfural (FF) while hexoses lead to 5-Hydroxymethylfurfural (HMF). Phenolic Compounds (PC) are generated from the partial breakdown of lignin [7]. | |
| Treatment with lime is widely used for conditioning hydrolysates and Larsson et al. [10] found that overliming is the most cost effective method for detoxifying hydrolysates [10]. Overliming is generally performed by the addition of lime to increase the pH, followed by pH adjustment to a value suitable for micrial growth [11,12]. However, knowledge of the chemistry behind the detoxification process is still limited. The chemical effects of alkali treatment have been taken into consideration by many researches [11,13,14]. Alkali treatment is known to affect the concentration of toxic compounds, but the reason behind the great improvement in fermentability remain to be revealed; in addition, the differences in the effect for various forms of alkali are not yet understood [7]. | |
| In order to evaluate and improve different hydrolysis as well as detoxification methods, it is important to be able to identify and quantify the inhibitors present in the hydrolysate. The wet chemistry analysis of phenolic compounds is very challenging due to the great variety and reactivity of these compounds [15]. New technologies that allow the separation and quantitative determination of individual PCs by either gas chromatography (GC) or liquid chromatography (LC) are preferable [16,17]. In complex matrices such as wood hydrolysates, the key for phenolic determination is the separation of phenolic fraction from the other constituents present in the samples. Liquid-liquid microextraction (LLME) is essentially a simultaneous extraction and concentration process suitable for the analysis of a wide range of organic trace compounds in water. LLME avoids the use of large solvent volumes and minimizes the cost and time needed for the analysis [17]. | |
| The objective of the current work is to identify the phenolic compounds and to study the effect of overliming treatment on the evolution of PCs present in the dilute-acid hydrolysate (DAH) of olive stones. Determination of phenolic compounds is based on two chromatographic methods: GC with LLME as a preliminary step and HPLC with appropriate conditions. The effects of three variables pH, duration and temperature of detoxification on overliming were examined. | |
| Material and Methods | |
| Preparation and detoxification of DAH | |
| The hydrolysis of the olive stones is carried out with a sulphuric acid solution (5% w/v) [18,19]. When cooled, the solid and liquid phases are separated by filtration. The DAH is then stocked at -4°C for further analysis. The titration is carried out with a Ca(OH)2 solution (50 g.L-1) [18]. The samples were stirred under specific conditions and then vacuum filtered. Sulfuric acid was added until a final pH value near 4 to 5, suitable for microbial growth, was reached [10]. | |
| Experimental design | |
| This experimental design was set up to study the effect of Ca(OH)2 on phenolic compounds behaviour. Overliming is carried out under the following conditions: pH 10 and 12, temperature 25 and 60°C and time 15, 30 and 60 min. The experimental design containing 22x31=12 experiments was created using the NemrodW software [20]. Each experiment was repeated three times. Table 1 summarises the different overliming conditions applied to the DAH. | |
| Determination of total phenolic content | |
| The classical method for quantification of total phenols is a colorimetric procedure using the Folin-Ciocalteu reagent [21]. Analyses involve both fresh DAH and synthetic solutions which are used in order to examine if there are substances other than phenolic compounds that might be present in the DAH of olive stones, which may interfere with color development, and therefore distort the results. Synthetic solutions of furans and sugars are prepared at concentrations similar to those found in the acid hydrolysate of olive stones as shown in table 2 [19,22]. | |
| GC-MS | |
| Gas chromatographic analysis was performed using an Agilent 6890 N Series GC System (Agilent Technologies, Wilmintong, DE, USA) gas chromatograph fitted with a splitless injector for a low background. Separation was performed using HP-5MS fused silica capillary column (30 m×0.25 mm i.d.×0.1μm film thickness) supplied by Agilent. A silanized injector liner 7683 B split/splitless (2 mm i.d.) was used. Detection was done with a 5975 mass-selective single quadrupole detector (Agilent Technologies). The GC-MS control and the data processing were carried out using the Chem-Station software package (Agilent Technologies). The injector temperature was 250°C. The oven temperature was held at 90°C for 1 min, then increased to 220°C at a heating rate of 6°C.min-1, then to 290°C at 10°C.min-1 and held for 1.23 min and finally to 310°C at a rate of 40°C.min-1 and the temperature was held for 7.5 min. The total run time was 39 min. The detector temperature was 280°C. The carrier gas was helium (purity 99.999%) at a flow rate of 1 mLmin-1. The samples were injected in the split less mode and the splitter was opened after 5 min (delay time). The sample volume in the direct injection mode was 1 μL. Mass spectrometer was in EI mode (electron energy 70 eV), and data acquisition was in scanning mode from m/Z=50 to m/Z=550. Compounds were identified by comparison of the retention time and mass spectra with library data of mass spectra and authentic compounds. Quantification was performed by the internal standard method using BHT, 2,6-bis(1,1- dimethylethyl)-4-methylphenol. | |
| Liquid-liquid microextraction procedure: Quantitative extraction of phenolic compounds in natural matrices is difficult. Therefore, the LLME procedure was selected as appropriate for analyte extraction. It was observed that the extraction efficiency is maximum for acid pH values [17]. This behavior could be attributed to a drop in the extraction efficiency of compound because under basic conditions, the dissociated form remains in aqueous phase. Organic extracting solvents ethyl ether, n-hexane, dichloromethane, trichloromethane and ethyl acetate were tested. Due to its good recovery, ethyl acetate was selected as the most adequate extracting agent. The volume of organic solvent to be used and the ionic strength of the medium were also optimized. Saturation with sodium chloride and 0.25 mL of solvent bring the optimum values obtained for all compounds. A comparison between different combinations of derivatization agents was carried out. Derivatization volume, temperature and reaction time was tested on the analytical response corresponding to a mixture of selected phenolic compounds. Considering the results, the following optimal values were found: 50 μL of BSTFA (N,O-Bis(trimethylsilyl)trifluoroacetamide) was added and the mixture was manually shacked for 2 min at room-temperature to obtain an adequate derivatization. | |
| Conditioning of samples for GC-MS: Two milliliters of each sample (DAH and the 12 others treated with lime) were transferred to a 5mL vial then 50 μL of BHT were added. The sample was saturated with NaCl and 250 μL of ethyl acetate was added. The mixture was agitated with orbital shaker (Stuart orbital shaker SSL1) at 250 rpm for 10 min at room-temperature and then centrifuged for 15 minutes at 2500 rpm. The organic phase was removed to a 1 mL vial. The extraction was repeated twice and organic phases were mixed together. Fifty microlitres of BSTFA were added and the mixture was manually shacked for 2 min at room-temperature. At this point, the samples were ready to be injected into the gas chromatograph-mass spectrometer. | |
| HPLC-UV | |
| High-performance liquid chromatography (Thermo Electron APSHypersil) was used to analyze samples. The mobile phase is composed of ultra-pure water, methanol and acetic acid (80, 10, 3:v/v/v) with benzoic acid as internal standard. Furfural (FF), hydroxymethylfurfural (HMF) and polyphenolic compounds (PCs) are analyzed on a C18 column with a UV detector at 280 nm (UV 1000-Thermo Finnigan) [19-22]. | |
| The injection volume was 20 μL. The flow-rate is programmed to give maximum separation of constituents: 0.5 mL min-1 from 0 to 10 min, 1 mL min-1 from 10 to 11 min (hold 24 min). | |
| Results and Discussion | |
| Overliming and total phenolic content | |
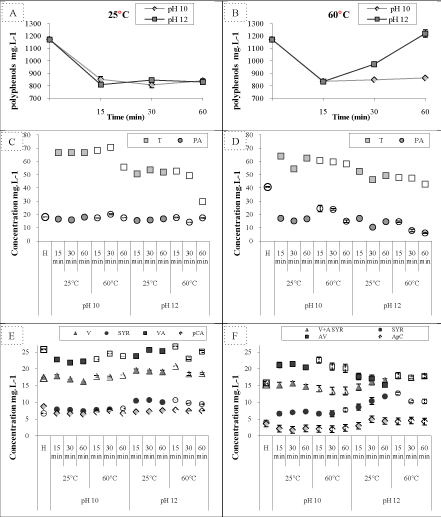
| The influence of overliming on total phenolic compounds found in the DAH of olive stones is shown in figures 1A and 1B. The DAH contained about 1.17 gL-1 of total phenolic compounds which were released from the lignin fraction during hydrolysis with sulfuric acid. A decrease in concentration was observed during the first 15 minutes of overliming regardless pH and temperature values. After 15 minutes of overliming at 25°C, the phenolic compounds were practically stable regardless of the pH or treatment duration. However, overliming at 60°C has affected phenolic compounds and a difference in behavior was observed for the two pH levels. An increase, to a maximum of 1.3 g.L-1 was detected at pH 12 after 30 min of treatment. To better understand this apparent increase, we studied synthetic solutions as indicated in table 2. Analyses showed that solutions of formic, acetic, lactic acid and furanes (FF + HMF) had negative absorbance in all treatments. Conversely, solutions containing xylose and/or glucose treated at pH 12 and 60°C for 0, 30 and 60 minutes reduced the Folin-Ciocalteu reagent and developed a blue coloration. Since that the absorbance of solutions increased with the severity of treatment, it is high probably that the degradation products of sugars may in part be responsible for this coloration. Folin-Ciocalteu reagent is based on a chemical reduction and many substances other than phenolic compounds may interfere and distort the result. Singleton and Rossi [21] showed that heating the sugars present in samples in an alkaline solution interferes with the reaction because of enediol formation. This can explain the high concentration of phenolic compounds in the samples at pH 12 and 60°C, compared to the others. Therefore, it is necessary to use alternative methods, such as chromatography, in order to determine the exact concentration and evolution of each phenolic compound. | |
| GC-MS analysis | |
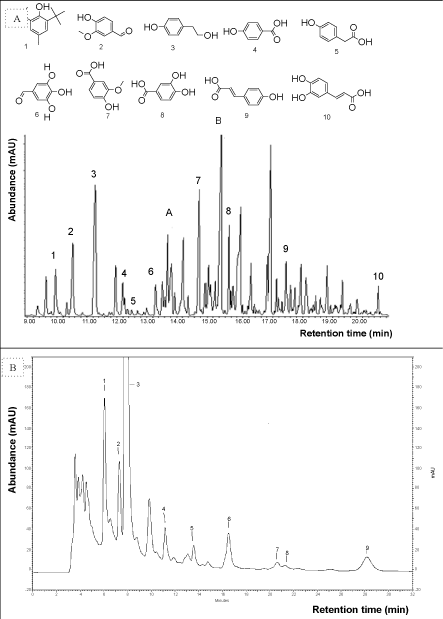
| Phenolic compounds identification: Extraction with ethyl acetate led to the identification of ten PC present in the DAH of olive stones. These compounds were derivatives of benzoic acid, cinnamic acid, simple phenols and aldehydes. A representative chromatogram of a DAH sample is shown in figure 2A. The ratio of tyrosol (T) (peak area) compared to the standard (BHT) is greater than for the other lignin degradation products (vanillic acid (VA), protocatechic acid (PA), vanillin (V), p-coumaric acid (pCA), caffeic acid (CA), syringaldehyde (SYR), parahydroxybenzoic acid (PHBA), hydroxycinnamic acid (HCA) and 4-hydroxyphenylacetic acid (PHPA)). It shows that it’s one of the major components of the olive stones DAH. The results of the current work are in line with previous reports identifying phenolic compounds in diluted acid hydrolysate. A number of phenolic compounds recognized in lignocellulosic hydrolysates include 3-methoxy-4-hydroxybenzaldehyde, 4-hydroxyacetophenone, vanillin, syringaldehyde, acetovanilone, ferulic acid, vanillic acid and 4-hydroxybenzoic acid [7-13,23,24]. These compounds are mainly liberated from lignin degradation in addition to aromatic wood extractives. | |
| Phenolic compounds identification has been carried out on olives, olive oil, waste water and stones. In their review on phenolic compounds in olive, Robards and Ryan [25] report that only the benzoic acids, cinnamic acids flavonoids and iridoids are of major significance in olives. Zafra et al. [17] identify in olive oil waste water, the presence of several phenolic compounds derivatives of low molecular weight such as phenolic hydroxycinnamic and benzoic acid. Conde et al. [26] find that hydroxytyrosol, followed by homovanillyl alcohol and oleuropein are the most abundant compounds after hydrothermal treatments of olive tree pruning. Olive phenolic compounds are eliminated in the olive oil and waste water, therefore, olive stones contain few PCs. Phenolic compounds present in the DAH were similar to those identified by Fernández-Bolañose et al. [27] in the study of olive stones treated by steam explosion. Hydroxytyrosol and syringic acid (4-hydroxy-3,5- dimethoxybenzoic acid) are identified in olive stones treated by steam explosion. However, syringic acid was not identified in the ethyl acetate extract of DAH treated with BSTFA. Conversely, we noted its presence in the ethyl acetate extract of DAH without BSTFA with a retention time of 15.312 min, thus HPLC analysis was used to quantify syringic acid. | |
| Overliming treatment: Phenolic compounds identified in the ethyl acetate extract of DAH were found in the samples treated with Ca(OH)2 as shown in table 3. Two additional PC were noted in the overlimed samples, homovanillic acid (3-methoxy-4hydroxybenzeneacetic acid) (HVA) eluted at 14.625 min and ferulic acid (FA) (4-hydroxy 3-methoxycinnamic acid) eluted at 19.792 min. Caffeic acid (CA) identified in the DAH was only present in two samples: B2 (pH 10, 60°C for 30 min) and D3 (pH 12, 60°C for 60 min). The presence or absence of caffeic acid in the treated samples was probably not correlated with the treatment itself but influenced by the level of phenolic compounds extracted with ethyl acetate. This latter is most effective when the pH was close to 3 as is the case for B2 (final pH of the sample=3.72) and D3 (final pH of the sample=4.17) [17]. | |
| The behavior of each identified PC under various overliming conditions was followed relative to the surface of the internal standard (Spolyphenol/SBHT). Degradation of tyrosol, major phenolic compound, was influenced by the overliming severity. The ratio Styrosol/SBHT decreased approximately 73% in the D3 sample (pH 12, 60°C and 60 min) in comparison to the hydrolysate (H). Concentration of p-coumaric acid (pCA) declined by 53% with the first liming treatment (pH 10, 25°C and 15 min), but remains however, more or less stable during different treatments combinations (from A1 till D3). On the other hand, ferulic acid (FA), initially absent in the DAH, appeared and remained static. Parahydroxybenzoic acid (PHBA), parahydroxyphenylacetic acid (PHA), vanillic acid (V A), homovanillic acid (HVA) and protocatechic acid (PA) remained constant during treatments. Surface area fluctuations seemed more important for vanillic acid (VA) and protocatechic acid (PA). These fluctuations might be due to extraction level of these acids by ethyl acetate, this latter being related to the final pH of the sample [17]. Consequently, for the five PC listed above there was an increase in the B2 sample in which the pH was lower (pH=3.72), and a decrease in the D2 samples with higher pH value (pH=4.57). The two aldehydes PC (vanillin (V) and syringaldehyde (SYR)) evolved in a parallel manner regardless of the treatment type. They were stable at pH 10 (all times and temperatures), while at pH 12 a slight increase was noticed (again for all times and temperatures). This increase was more noticed for the syringaldhehyde (SYR). Conde et al. [26] suggest that part of the phenolic compounds are substituents of the solubilised oligosaccharides that are released and at high treatment temperature the proportion obtained free from sugars increases. This will explain the high concentration in the samples at pH 12 and 60°C, compared to the others. | |
| Based on the results listed above, we noticed that phenolic compounds behaved in different ways according to the treatment. Therefore they can be classified into three groups: acid phenolic compounds were more or less stable regardless of the treatment, aldehydes were stable at pH 10 and slightly increased at pH 12, simple phenols were unstable, and their degradation increased with treatment severity. Among the phenolic compounds identified, we quantified the major six: vanillic acid (VA), p-coumaric acid (pCA), tyrosol (T), vanillin (V), protocatechic acid (PA) and syringaldehyde (SYR) as shown in figures 1C and 1E. Tyrosol (T) presented the highest concentration with 66.7 mg L-1 followed by the vanillic acid (VA) with 25.8 mg L-1 whereas the lowest concentration found was for p-coumaric acid (pCA) and syringaldehyde (SYR) with respectively 8.8 and 6.7 mgL-1. | |
| HPLC-UV analysis | |
| Phenolic compounds identification: A representative chromatogram from the analysis by HPLC-UV of phenolic compounds found in the DAH of olive stones is shown in figure 2B. HPLC-UV analysis identified the following PC: protocatechic acid (PA), tyrosol (T), vanillic acid (VA), vanillin+syringic acid (V+SYRA), syringaldehyde (SYR) and p-coumaric acid (pCA). In addition, we noted the presence of the two furans (HMF) and (FF) generated from sugar degradation during acid hydrolysis. | |
| Overliming treatment: Analysis by HPLC-UV of the hydrolysate and the 12 overlimed samples are represented in figures 1D and F. Results have showed similarities to those obtained by GC-MS. Tyrosol (T) presented the highest concentration with 63.4 mgL-1 followed by protocatechic acid (PA) with 40.7 mgL-1. Moreover, syringaldehyde (SYR) and p-coumaric acid (pCA) were found at lower concentrations than the others, around 3.9 and 3.7 mg.L-1 respectively. HPLC analysis showed that overliming affected the concentration of individual PC in different ways (Figure 1D). Tyrosol (T) and protocatechic acid (PA), identified as major PC, decreased with overliming treatment. Fluctuations were more observed for protocatechic acid (PA). This decrease was influenced by the overliming severity. The most important decrease of Tyrosol (T) and protocatechic acid (PA) were noticed under the harshest treatment (pH12, 60°C and 60min) for approximately 63 and 41%. While that p-coumaric acid (pCA), vanillin+syringic acid (V+ SA) and syringaldehyde (SYR) evolved in parallel manner, remaining were stable under different liming treatments performed as depicted in figure 1F. A small increase in their concentration was noticed for pH 12 (samples ranging from C1 to D3). Concentration of vanillic acid (VA) were higher at pH 10 than those overlimed at pH 12. | |
| These results are in agreement with observations made by Person et al. in their study of the effect of different forms of alkali treatment on fermentation inhibitors including phenolic compounds [24]. The author found that the concentration of 3,4-dihydroxybenzaldehyde, 4-hydroxybenzaldehyde and cinnamic acid decreased after different alkali treatments. However, phenol concentration increased drastically after treatment with calcium hydroxide. The concentration of vanillin was not much affected by any of the alkali treatments applied. Treatment of a synthetic cocktail containing acetic acid, formic acid, HMF, 2-furaldehyde, ferulic acid, and coniferyl aldehyde at pH 10 resulted in less complex effects than the hydrolysate [24]. | |
| Martinez et al. reported that for hemicelluloses hydrolysate most of the phenolic compounds are unchanged by Ca(OH)2 treatment at pH 10. HPLC-UV chromatograms regions corresponding to phenolic compounds remain unchanged for untreated and treated bagasse with overliming. Of the phenolic compounds tested only syringic acid was destroyed or modified [13]. Conde et al. [26] concluded that generally, furan and phenolic compounds were found to decrease after the wood ash or alkaline treatments. | |
| It is often assumed that lignin would be the only important source of aromatics in biomass. However, condensation and cyclisation of selected aldehydes and ketones formed from cellulose degradation is apparently involved in the formation of aromatic compounds. Forssk et al. [28] showed that at high pH, D-glucose yielded to 11 phenols and 2 enols, similar products were also obtained from D-xylose. Alkaline degradation of sugar resulted in a complex mixture of more than 50 compounds, including glycolic, lactic, glyceric, 2-C-methylglyceric, deoxytetronic, and deoxypentonic acids [29]. These observations helped explaining the elevation of phenolic compounds observed at pH 12. Since that at this pH value formation of aromatic compounds can be due to low molecular weight, sugar degradation intermediates. In a previous study concerning the overliming of olive stones, we concluded that components other than furans and xylose found in the DAH were also involved in the overliming process. Chromatograms analysis of the 12 overlimed samples showed that xylose degradation was negatively correlated with sugar degradation products eluted at the beginning of HPLC-UV chromatograms [22]. | |
| Comparison of different quantification methods: In aim to quantify and identify phenolic compounds, three analytical methods were used (spectrophotometric, GC-MS and HPLC-UV). Folin Ciocalteu classical method overestimated the amount of PC due to interference with sugar degradation products present in the matrix of DAH [21]. Chromatographic methods allowed better understanding of each identified phenolic compound in function of different overliming treatments. Analysis by HPLC-UV confirmed the presence of major phenolic compounds identified by the GC-MS (protocatechic acid, tyrosol, vanillic acid, vanillin+syringic acid, syringaldehyde, and p-coumaric acid). Regardless of the small difference in values observed with the GC and the HPLC quantification methods, phenolic compounds seemed to present a well-defined trend when overlimed. | |
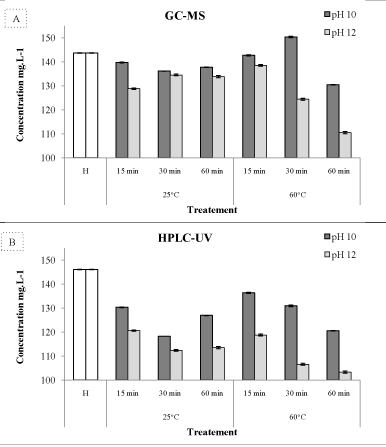
| figures 3A and 3B show the sum of the six major phenolic compounds as identified by HPLC-UV and GC-MS for each overliming treatment. Total amount of PCs identified by GC-MS was 143.7mgL-1 and a close value of 146.1mgL-1 was found by HPLC-UV. The highest PC amount was found in sample B2 (pH 12, 60°C and 60 min), with a value of 154.32 mgL-1 for the GC-MS analysis and in sample B1 (pH 12, 60°C and 60 min) with a value of 136.33 mgL-1 for HPLC-UV analysis. However, the harshest treatment D3 (pH12, 60°C and 60 min) showed the minimum amount of phenolic content for both GC-MS and HPLCUV with 110 and 103.2 mgL-1 respectively, showing consequently a decrease of 23.1 and 29.3%. These differences between the two analytical procedures maybe due to the matrix complexity, preconcentration, extraction with ethyl acetate and derivatisation with BSTFA. For all treatments combinations, overliming at pH 12 showed more efficiency in reducing the amount of total phenolic content. Furthermore, the difference between the two pH levels was more noticed at 60°C. Treatments at pH 12 and 60°C were function of time, the PC amount decreased with the overliming duration to reach a minimum after 60 min. | |
| Our results are in line with many others, showing that generally PCs were found to decrease after wood ash or alkaline treatment [11,13,14,30]. The amount of these compounds was lower in the treated hydrolysate enhancing therefore a better fermentability. The results suggested that the mechanisms behind the concentration changes of the investigated PC compounds after the different treatments are complex and deserve further attention in the future. However, there are some well-known reactions that might take place under the conditions used during the treatments and which could possibly account for some of the changes in concentration. For example, aldehydes could undergo nucleophilic addition to the carbonyl group in the presence of ammonia, or aldol-like reactions could occur when the aldehyde is transformed into a reactive nucleophile by formation of its enolate ion under alkaline conditions [31]. Furthermore, under alkaline conditions, phenolic compounds can be transformed into their corresponding phenolate ions, which are known for their high reactivity and can undergo further reactions [31]. | |
| Conclusion | |
| Phenolic compounds have been suggested to exert a considerable inhibitory effect in the fermentation of lignocellulosic hydrolysate, the low molecular weight being the most toxic. However, the mechanism of the inhibition has not been elucidated, largely due to lack of qualitative and quantitative analysis [7]. A simple and low cost method for the determination of polyphenolic compounds in DAH is proposed. A liquid-liquid microextraction procedure is used in conjunction with silylation prior to the analysis of the compounds by GC-MS. Ethyl acetate microextraction leads to the identification of 10 phenolic compounds. These compounds are derivatives of benzoic acid, cinnamic acid, simple phenols and aldehydes. Analysis by HPLC-UV contributes to a better understanding of the behavior of the major PCs. Tyrosol (T) was found to be the main phenolic compound of the DAH. | |
| Detoxification with lime at pH 12 was effective in reducing the total phenolic amount by almost 23.1 and 29.3% (as analyzed by GC-MS and HPLC-UV respectively). At the end of the treatment (pH 12, 60°C and 60 min), tyrosol concentration declined by 73%. Phenolic compounds seemed to present a well-defined trend when overlimed: acid phenolic compounds were more or less stable regardless of the treatment; aldehydes were stable at pH 10 and increased slightly at pH 12 while simple phenols were unstable, and their degradation increased with treatment severity. The increase in some PC concentrations could, apart from smaller deviations in the analysis results, be related to conversions taking place during the treatments. Conclusions were concordant with previous findings concerning the fluctuation and the elevation of the concentration of some PC during overliming. The mechanism of alkali detoxification is difficult to explain. The positive effects of added compounds or of compounds formed at high pH deserve future attention. | |
| Acknowledgements | |
| The authors would like to thank the Lebanese CNRS (Centre Nationale de Recherche Scientifique Libanais) for their financial support and for the opportunity to conduct research at the Université Saint-Esprit de Kaslik laboratory. | |
| References | |
References
|
|
Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
||
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 14991
- [From(publication date):
June-2013 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10351
- PDF downloads : 4640
