Potential Anti-angiogenic Effect of a Combination of Boswellic Acids and Cisplatin against Chemically-induced Colon Cancer in Mice
Received: 31-Jul-2016 / Accepted Date: 19-Sep-2016 / Published Date: 23-Sep-2016 DOI: 10.4172/2572-0406.1000111
Abstract
Boswellic acids (BA) have been found to possess potent anti-inflammatory and anti-cancer activity. We aimed to explore the possible effect of BA as an adjuvant therapy with Cisplatin on chemically-induced colon cancer in mice. Colon cancer was induced in seventy healthy male albino mice using 1,2-dimethylhydrazine (DMH, 20 mg/kg/week, s.c.) for fifteen weeks. Treatment with BA, Cisplatin or both for another 3 weeks was applied. The mice were divided into seven groups: (i) Saline, (ii) DMH, (iii) Cisplatin, (iv-v): BA standardized extract (250 or 500 mg/kg) and (vi-vii): Cisplatin+BA (250 or 500 mg/kg), respectively. Serum interleukin-6, vascular endothelial growth factor and Cyclooxygenase 2 levels have been measured by ELISA assays. Immunohistochemical staining for CD31, in addition, was done. On examination of the colon tissue at the end of the therapeutic period, there was a significant decrease in the CD31 immunostaining score as a marker of tumor proliferation index and a significant decrease in measured serum markers in combination therapy treated group than Cisplatin monotherapy treated group. These results suggested that BA could augment the antitumor effects of Cisplatin. Hence, BA could be used as an adjuvant therapy in the clinical management of CRC after its validation in humans.
Keywords: Boswellic acids; CD31; Cisplatin; Colon cancer; COX-2; IL-6; VEGF
9789Introduction
Although, screening modalities for early detection and therapeutic management of human Colorectal cancer (CRC) have improved considerably, the World Health Organization (WHO) estimates an increase of 80% in deaths from CRC by 2030 [1]. In Egyptian patients, CRC attends relatively higher rates than reported in the West [2]. A combination of increased consumption of fat and an elevated prevalence of obesity and smoking contributes to this increment in various regions [3].
Cyclooxygenase (COX)-2 is an inducible enzyme produced in inflammatory cells and colorectal carcinomas [4]. A large body of evidence suggested that COX-derived prostaglandins contribute to tumorigenesis in humans [5], including CRC that represents the first cancer where the role of COX-2 and prostaglandins were suspected [6].
Interleukin 6 (IL-6) is regarded as an important tumor promoting factor in various types of human cancer, including CRC [7]. Recently, it has been found that IL-6 enhanced vascular endothelial growth factor (VEGF) production by CRC fibroblasts, thereby inducing angiogenesis [8]. VEGF release, which is secreted by cancer cells, in addition, targets endothelial cells and is regarded as one of the most important angiogenic factors that play an important role in the progressive growth of malignant tumors [9].
Although surgical excision, chemotherapy and radiotherapy are available, survival is still very poor when the disease is diagnosed at an advanced stage, and better treatments are desperately needed. Thus, novel therapeutic agents, multitargeted to several mechanisms of carcinogenesis, are needed [10].
Cisplatin is one of the most commonly used antitumor drugs that used for advanced stages and persistent cancers. It directly targets DNA, inhibiting its synthesis by producing interstrand and intrastrand cross-links. However, one of its chemotherapy problems is its inherent nephrotoxicity and myelosuppression, which are limiting factors in its use and, especially, in its useful dosage levels [11].
One increasingly promising source of therapeutic agents is traditional medicine from natural compounds; from which Boswellic acids (BA), a pentacyclic triterpene derived from the fragrant gum resin of the bark of the Boswellia serrata tree, has been used for centuries in traditional Ayurvedic medicine as a treatment for a wide variety of proinflammatory disorders in human like osteoarthritis, inflammatory bowel disease, chronic colitis and collagenous colitis [10,12]. The acids have been shown to inhibit azoxymethane-induced formation of aberrant crypt foci in the colon of mice [13] and exhibited a range of cytotoxicity against various human cancer cell lines [14,15].
While a combination-chemotherapy with Cisplatin is a cornerstone for the treatment of multiple cancers, the challenge is that cancer cells could become Cisplatin-resistant. To minimize this resistance, combinatorial therapies were developed and have proven more effective to defeat cancers [16].
In this regard, the combination of Cisplatin with Boswellic acids may provide valuable insight into new approaches for the prevention of Cisplatin side effects without affecting its efficacy. So, the current study used a mouse model of CRC to evaluate the effect of combined therapy, Cisplatin and BA, on the pathobiology of CRC. We tested the hypothesis that BA may enhance the efficacy and the anti-angiogenic effect of Cisplatin chemotherapy against CRC in a mouse model and thus may be an effective way to reduce the angiogenesis and tumor growth in colon cancer.
Material and Methods
Animals
The current animal-based experimental study was conducted at the Faculty of Pharmacy, Suez Canal University in the period June- December 2014. Male Swiss albino mice weighing 24-32 g were purchased from the Modern Veterinary Office for Laboratory Animals (Cairo, Egypt). Mice were housed in groups of ten in polyethylene cages under hygienic laboratory conditions and normal dark/light cycle. Mice were acclimatized to the housing conditions for ten days before starting the experiment. Food and water were allowed ad libitum during the study period. The experiment was conducted according to the guidelines of the Laboratory Animal Care and Use of the Scientific Research Ethics Committee at Suez Canal University (Research approval no. 20146A17).
Pharmacological treatments
For induction of colonic cancer, mice were injected subcutaneously with 1,2-dimethylhydrazine (DMH, 20 mg/kg/week, body weight) for fifteen weeks [17]. DMH (Sigma-Aldrich®, MO, USA) was diluted using phosphate-buffered sterile saline. Treatment with Boswellic acids (B. Serrata-standardized extract containing 65% boswellic acids from Advance Physician Formulas Inc., California, USA) in the form of tablets suspended in distilled water began after finishing the course of DMH (i.e., at the beginning of week 16) and continued for the next three weeks until the mice were sacrificed at the end of the therapeutic period. Cisplatin (Hospira Inc., IL, Australia) was freshly prepared every week. For combination therapies, Cisplatin treatment (4 mg/kg, i.p.) was given on day 7, 14, and 21 from the beginning of therapy with BA and Cisplatin. This dose of Cisplatin (i.e., one injection on the last day of every week of therapy with total three injections) was recommended by Park et al. [18] for maximal tolerated dose for Cisplatin in mice.
Experimental groups
The current study was carried out on seventy healthy male albino mice allocated randomly into seven groups, ten mice per each. (Group i) normal mice that were injected with normal saline (0.1 ml/mouse, i.p.) at the first day of the experiment till the end of the first 15 weeks (i.e., as it is the route of administration of DMH) and then treated with saline (5 ml/kg/day, p.o.) starting from day 8 until the last day of week 18 of the experiment (i.e., as it is the route of BA administration in the current experiment). (Group ii) DMH, (Group iii) DMH+Cisplatin, (Group iv) DMH+BA (250 mg/kg), (Group v) DMH+BA (500 mg/kfg), (Group vi) DMH+Cisplatin+BA (250 mg/kg) and (Group vii) DMH +Cisliplatin+BA (500 mg/kg).
Blood and tissue collection
At the end of the therapeutic period, under light ether anaesthesia, blood samples were collected from the orbital sinus and centrifuged at 1500 × g for 15 min. Serum samples were then separated and collected in clean Eppendorf ’s tubes and stored at -20°C until used for the ELISA assays. After that, mice were sacrificed by decapitation and tissue specimens from the colon were dissected and fixed in phosphate-buffered formalin (4% paraformaldehyde in 0.1 M phosphate buffer, pH = 7.2) overnight and then embedded in paraffin wax. All paraffin-embedded sections were cut at 4 μm and left to dry overnight. Sections were then prepared for histopathological staining with hematoxylin and eosin (H&E) or for immunohistochemical staining for cluster of differentiation 31 (CD31; a marker for microvessel density).
Enzyme Linked Immune Sorbent Assay (ELISA) for IL-6, COX 2 and VEGF
Serum IL-6 and COX-2 levels were determined using mouse IL-6 and COX-2 ELISA kits (Glory Science Co., Ltd., Del Rio, TX, USA) whereas VEGF was determined using a mouse VEGF ELISA kit (Sun Red Biotechnology Company, Shanghai, China). Assays were determined according to the manufacturer’s instructions. It is based on the principle of double-antibody sandwich technique. The reaction was assessed by measuring the optical density using an automated microplate reader (Metertech, M960) at 450 nm. Standard curve for each parameter, from each kit, was generated by using the reference concentrations supplied by the manufacturers. By comparing the absorbance of the samples with the standard curve, each assessed parameter concentration in the unknown samples was determined. The sensitivity of the assay was 5.1 ng/L, 0.02 ng/mL and 10.127 ng/L for IL-6, COX-2 and VEGF, respectively (samples below this limit were considered negative).
Microscopic examination of colon tissue
Tissue sections stained with H and E were examined using a light microscope. Each tissue section was first examined at 10X magnification. Whereas, the scoring was performed at a higher power (40X magnification). Tumor cells in the colon were evaluated using a morphometric point-counting procedure. The histopathological changes were graded in a blind fashion as 1, 2, 3 and 4 for low, moderate, high and intensive pathological changes, respectively.
Immunohistochemistry and image analysis
In brief, immunostaining was performed using streptavidin-biotinimmunoperoxidase complex method with 4 μm thick sections which have been deparaffinized and heated in 0.01 M citrate buffer solution (pH = 6) for 15 min for antigen retrieval. Sections were then incubated overnight with rabbit monoclonal CD31 primary antibody, 1:50 in phosphate buffered saline (Thermo Scientific®, Fremont, USA) at 4°C. After conjugation with streptavidin-biotin-peroxidase complex (broad spectrum LAB-SA detection system, Invitrogen), 3,3- diaminobenzidine (DAB, Sigma-Aldrich®, MO, USA) was used as a chromogen and Mayer’s hematoxylin was used as a counter stain. Negative control was prepared by omitting the primary antibody. Then, tissue sections were examined using a light microscope and photomicrographs were captured. CD31 cells were counted by using the ImageJ software developed by the National Institute of Health (Bethesda, Maryland, USA). Any single cell or spot that stained with the immunohistochemical marker was counted as a vessel. The numbers of CD31 positive vessels were counted in eight sections representing the whole tissue section in each slide [19].
Statistical Analysis
Data analysis was performed employing the Statistical Package for Social Science, version 16 (SPSS Software, SPSS Inc., Chicago, USA). The survival rate of the studied mice was expressed as a frequency (percentage) and compared between groups using chi-square (χ2) test. Quantitative Data were expressed as mean ± S.E.M. The difference of mean values among groups was assessed by using two-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. A P value less than 0.05 were considered significant.
Results
The survival rate was decreased significantly in mice treated with Cisplatin alone (60%) and the group treated with combination therapy with Boswellic acids in a dose of 500 mg/kg (60%) versus the normal group (100%) (P = 0.029). Also, it was decreased in the DMH control group than that the normal group (70% versus 100%) but this was not statistically significant (P = 0.067). Moreover, the survival rate of the group treated with combination therapy (Cisplatin + BA 500 mg/kg) was decreased than that the group treated with combination therapy (Cisplatin + BA 250 mg/kg) (60 % versus 70%, respectively). However, this did not reach statistical significance (P = 0.648) (Figure 1).
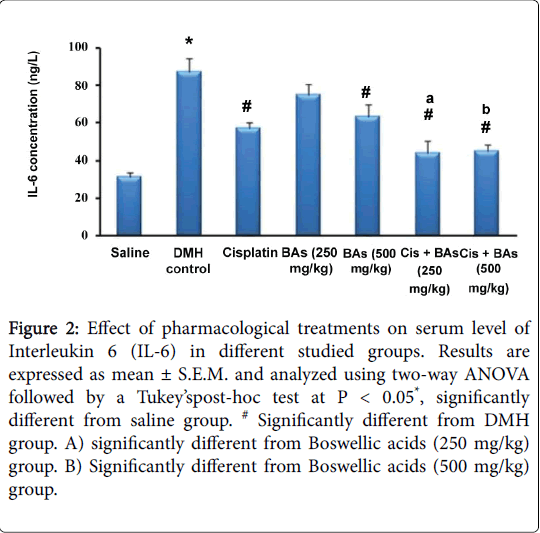
Regarding the effect of pharmacological treatments on serum level of Interleukin 6 (IL-6) in different studied groups, the concentration of IL-6 was increased significantly in the DMH control group versus the normal group (87 ± 7.1 versus 31 ± 2.8 ng/L) and was decreased significantly in treated groups versus the DMH control group (P < 0.05), (Figure 2). The treated groups with combined therapy showed significant decrease of IL-6 levels (44 ± 6.5 and 45 ± 3.6 ng/L) than in groups treated with Boswellic acids alone (75 ± 5.3 and 63 ± 6.9 ng/L) or Cisplatin alone (57 ± 3.2 ng/L). Hence combination therapy was better than monotherapy (Figure 2).
Figure 2: Effect of pharmacological treatments on serum level of Interleukin 6 (IL-6) in different studied groups. Results are expressed as mean ± S.E.M. and analyzed using two-way ANOVA followed by a Tukey’spost-hoc test at P < 0.05*, significantly different from saline group. # Significantly different from DMH group. A) significantly different from Boswellic acids (250 mg/kg) group. B) Significantly different from Boswellic acids (500 mg/kg) group.
Figure 3 shows the effect of pharmacological treatments on serum concentration of COX-2 in different studied groups. The concentration of COX-2 was increased significantly in the DMH control group versus the normal group (12 ± 0.5 versus 9 ± 0.4 ng/mL). In addition, it was increased in all treated groups (iii, iv, v, and vii); 14 ± 0.9, 13 ± 1.1, 12 ± 1.0, and 13 ± 0.78, respectively, except group vi that was treated with (Cisplatin + BA 250 mg/kg); 10 ± 0.76 ng/mL. There were no significant differences in these treated groups versus the DMH control group (12 ± 0.5 ng/mL); P > 0.05.
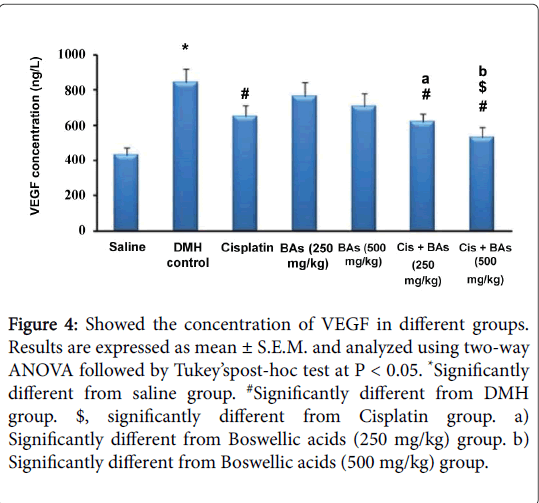
The effect of pharmacological treatments on the concentration of VEGF in different groups is illustrated in Figure 4. The serum VEGF level in the DMH control group was significantly higher than its level in the normal saline group (846 ±76 versus 432 ± 43 ng/L). The serum VEGF decreased significantly in all treated groups either with monotherapy or with combination therapy than that the DMH control group (650 ± 63, 765 ± 79, 710 ± 73, 618 ± 46 and 530 ± 58 versus 846 ± 76 ng/L, respectively). Combination therapy with BA leads to significant reduction in the level of serum VEGF versus the monotherapy with Cisplatin or BA in different concentrations (618 ± 46, 530 ± 58 versus 650 ± 63, 765 ± 79, 710 ± 73 ng/L, respectively).
Figure 4: Showed the concentration of VEGF in different groups. Results are expressed as mean ± S.E.M. and analyzed using two-way ANOVA followed by Tukey’spost-hoc test at P < 0.05. *Significantly different from saline group. #Significantly different from DMH group. $, significantly different from Cisplatin group. a) Significantly different from Boswellic acids (250 mg/kg) group. b) Significantly different from Boswellic acids (500 mg/kg) group.
Histopathological results
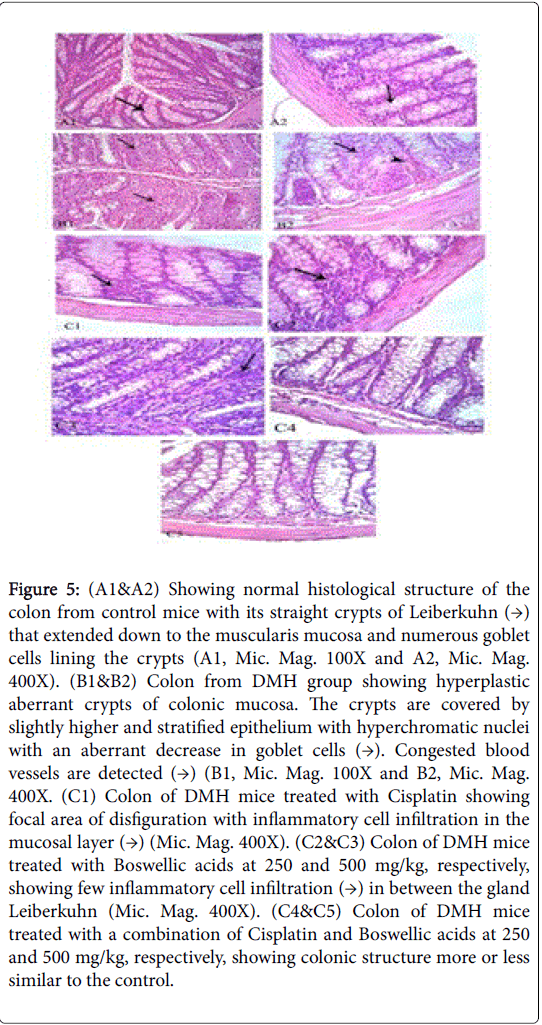
Examination of the H & E-stained sections of the colon of control mice (group i) showed its usual layers of mucosa, submucosa, muscularis externa, and serosa. The mucosa revealed straight tubular glands or crypts of Lieberkühn that extended down to the muscularis mucosa. The goblet cells were the most conspicuous cells lining the crypts. Columnar absorptive cells were tall columnar with vesicular oval nucleus and acidophilic cytoplasm (Figure 5 (A1&A2)). Histological examination of Group ii (DMH) revealed hyperplastic and dysplastic epithelial lesions and aberrant crypt foci. Hyperplastic crypt foci are composed of a mixture of goblet and absorptive cells with disorganized epithelial cells with varying degrees of nuclear stratification; the nuclei were large and hyperchromatic. The number of goblet cells was decreased and mucin depleted. Isolated islands of preneoplastic cells with characteristic leukocytic infiltration were noticed (Figure 5 (B1&B2)). However, histological examination of Group iii (DMH+Cisplatin) showed normalization of the histopathological changes recorded in the DMH administrated group. Similar results were obtained when Boswellic acids were administrated alone at 250 and 500 mg/kg (group iv and v) and also when combined with Cisplatin in group vi and vii (Figure 5 (C1toC5)).
Figure 5: (A1&A2) Showing normal histological structure of the colon from control mice with its straight crypts of Leiberkuhn (→) that extended down to the muscularis mucosa and numerous goblet cells lining the crypts (A1, Mic. Mag. 100X and A2, Mic. Mag. 400X). (B1&B2) Colon from DMH group showing hyperplastic aberrant crypts of colonic mucosa. The crypts are covered by slightly higher and stratified epithelium with hyperchromatic nuclei with an aberrant decrease in goblet cells (→). Congested blood vessels are detected (→) (B1, Mic. Mag. 100X and B2, Mic. Mag. 400X. (C1) Colon of DMH mice treated with Cisplatin showing focal area of disfiguration with inflammatory cell infiltration in the mucosal layer (→) (Mic. Mag. 400X). (C2&C3) Colon of DMH mice treated with Boswellic acids at 250 and 500 mg/kg, respectively, showing few inflammatory cell infiltration (→) in between the gland Leiberkuhn (Mic. Mag. 400X). (C4&C5) Colon of DMH mice treated with a combination of Cisplatin and Boswellic acids at 250 and 500 mg/kg, respectively, showing colonic structure more or less similar to the control.
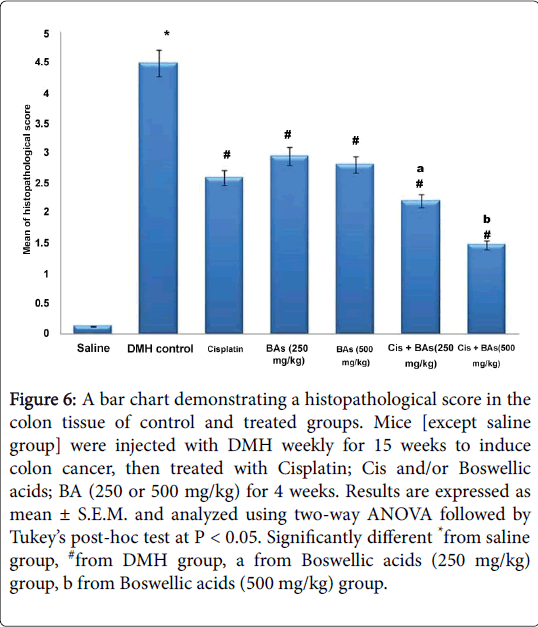
Statistical analysis showed a significant difference in histological score between DMH group and control group. However, all treated groups with Cisplatin as well as with Boswellic acids either alone or in combination showed successful amelioration in the histopathological score compared to the DMH control (Figure 6).
Figure 6: A bar chart demonstrating a histopathological score in the colon tissue of control and treated groups. Mice [except saline group] were injected with DMH weekly for 15 weeks to induce colon cancer, then treated with Cisplatin; Cis and/or Boswellic acids; BA (250 or 500 mg/kg) for 4 weeks. Results are expressed as mean ± S.E.M. and analyzed using two-way ANOVA followed by Tukey’s post-hoc test at P < 0.05. Significantly different *from saline group, #from DMH group, a from Boswellic acids (250 mg/kg) group, b from Boswellic acids (500 mg/kg) group.
Immunohistochemical results
Examination of control tissue specimens for immunohistochemical staining for CD31 showed CD31 positive endothelial cells of blood vessels of the mucosa and submucosa of the colon. On the other hand, sections from DMH administrated mice showed much increase in the number of CD31 positive endothelial cells indicating angiogenesis. However, treatment of DMH mice with Cisplatin showed lower CD31 immunostaining compared to DMH which appeared more or less similar to the control group. In addition, Boswellic acids groups showed a reduction in the expression of CD31 but to a lesser degree when compared with group III. Moreover, the combination therapies showed more decreases in immunostaining for CD31 in colon tissues compared to their corresponding monotherapies (Figure 7).
Figure 7: (A) Colon of control mice showing expression of CD31 (→) in endothelial cells of blood capillaries in between mucosal gland of colon (CD31-immunostaining & hematoxylin counterstain, Mic. Mag. 400X). (B) Colon from DMH group showing a high grade of immunoreactivity of CD31 in mucosal and submucosal blood vessels indicating angiogenesis (CD31- immunostaining & hematoxylin counterstain, Mic. Mag. 400X). (C) Colon of DMH mice treated with Cisplatin showing significant reduction in the immunoexpression of CD31 compared to DMH group (CD31-immunostaining & hematoxylin counterstain, Mic. Mag. 400X). (D&E) Colon of DMH mice treated with BA at 250 and 500 mg/kg, respectively, showing reduced immunoexpression of CD31 in mice treated with 500 mg/kg BA more than those treated with 250 mg/kg when compared to DMH group (CD31- immunostaining & hematoxylin counterstain, Mic. Mag. 400X). (F&G) Colon of DMH mice treated with a combination of Cisplatin and BAs at 250 and 500 mg/kg, respectively; showing a marked reduction in immunoexpression of CD31 (CD31-immunostaining & hematoxylin counterstain, Mic. Mag. 400X).
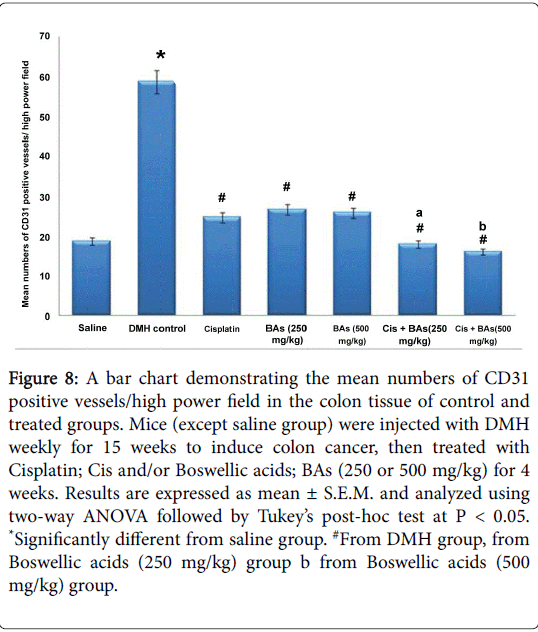
Statistically, there was a significant difference in immunoreactivity for CD31 between DMH group and control group, however, all treated groups with Cisplatin as well as with Boswellic acids either alone or in combination showed lower CD31 immunostaining compared to the DMH group. Importantly, the combination therapy showed lower CD31 immunostaining compared to the corresponding monotherapies (Figure 8).
Figure 8: A bar chart demonstrating the mean numbers of CD31 positive vessels/high power field in the colon tissue of control and treated groups. Mice (except saline group) were injected with DMH weekly for 15 weeks to induce colon cancer, then treated with Cisplatin; Cis and/or Boswellic acids; BAs (250 or 500 mg/kg) for 4 weeks. Results are expressed as mean ± S.E.M. and analyzed using two-way ANOVA followed by Tukey’s post-hoc test at P < 0.05. *Significantly different from saline group. #From DMH group, from Boswellic acids (250 mg/kg) group b from Boswellic acids (500 mg/kg) group.
Discussion
Colorectal cancer (CRC) incidence has been increasing to become a major cause of morbidity and mortality worldwide from cancers, with high rates in westernized societies and increasing rates in developing countries [20]. Although screening modalities for early detection and therapeutic management of human CRC has improved, this disease still remains the second leading cause of cancer related deaths in the USA and other developing countries [21].
The current study was designed to investigate the possible antiangiogenic and anti-proliferative effect of BA in chemicallyinduced colon cancer that had been induced by s.c. injection of DMH (20 mg/kg/week) for fifteen weeks. Induction of colon cancer by other chemical carcinogens, such as nitrosamines or heterocyclic amines is less frequently used because DMH is less expensive, more potent and more convenient to use. Its induced model shares many similarities to human sporadic colon cancer, including similarities in the response to some promotional and preventive agents [22]. DMH is a highly specific carcinogen that induces colorectal tumors in a dose-dependent manner. It metabolically activated in the liver by a series of reactions through intermediates azoxymethane and methylazoxymethanol to the ultimate carcinogenic metabolite, highly reactive methyldiazonium ion.
Some studies have also demonstrated that rat colon epithelial cells are capable of metabolizing DMH into the carcinogenic metabolite without previous metabolism by other tissues or colon bacteria [23]. The ultimate carcinogenic metabolite of DMH is responsible for methylation of the DNA bases of various organs, including epithelial cells in the proliferative compartment of the crypts, which result in a great loss of colonic cells by apoptosis, an increase in proliferation, and an apparent increase in mutations of colonic epithelial cells [22].
Interleukin-6 (IL-6), cyclooxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF) had been found previously to be triggered by inflammatory stimuli leading to angiogenesis [24]. The progressive growth of malignant tumors among which the CRC is dependent on this phenomenon [9]. For instance, increased expression of VEGF has been found in patients with CRC, localized and metastatic disease, and early preclinical studies on tumor specimens have revealed an association between higher levels of VEGF and CRC proliferative activity [25,26].
The release of VEGF by tumor cells may account for the increased microvascular permeability found in a wide variety of tumors, and is considered to play a role in tumor angiogenesis [27]. These were congruent with our results as we found that the use of BA as monotherapy (250 and 500 mg/kg) reduced the serum VEGF. In addition, the combination therapy of BA (250 and 500 mgd/kg) and Cisplatin significantly reduced the serum VEGF level compared to monotherapies. This pointed to the possible potentiating effect of BA on the anti-angiogenic effect of Cisplatin.
Other than VEGF, several pro-inflammatory cytokines released by innate and adaptive immune cells have been shown to regulate cancer cell growth and thereby contribute to tumor promotion and progression. Among these, IL-6 seems to take a center stage in human cancer development. An increased expression of IL-6 has been detected and associated with an unfavorable prognosis in patients with various types of cancer including both sporadic and colitis-associated CRC. Many experimental studies found an activation of important oncogenic pathways in cancer cells through IL-6 and various studies have found an increased its expression in patients with CRC, where its level is elevated in the serum of patients and in tumor tissue itself [7]. In the current study, we found that colon of DMH mice treated with a combination of Cisplatin and Boswellic acids at 250 and 500 mg/kg showed a marked reduction in IL-6 levels, which in partial could be related to the inhibitory effect of Acetyl-11-keto-beta-Boswellic acid (AKBA). This latter compound, one of the active principles present in BA, is known to be a non-redox and non-competitive inhibitor of 5- lipooxygenase that inhibits nuclear factor–kappa B and signal transducer and activator of transcription-3 (STAT-3) related pathways, leading to an induction of apoptosis and inhibition of angiogenesis in cancer cells [14]. This result supports the possible role of the BA as an anticancer agent through suppression of IL-6 induced angiogenesis.
In the present study, the level of COX-2 was increased insignificantly in all treated groups versus DMH group except the one that treated with (Cisplatin+BA 250 mg/kg) where the level of COX -2 was decreased than the DMH group. Research studies have demonstrated the importance of COX-2 in colorectal tumorigenesis and in the development of intestinal neoplasms [28], and its overexpression enhances neovascularization thus conferring a survival advantage of colon cancer cells [29].
COX-2 derived PGs can stimulate cell proliferation, promote angiogenesis, and inhibit apoptosis and immune surveillance [30]. In addition, these compounds may also promote metastasis by stimulating cell invasion [31]. We found that the expression of COX-2 was significantly higher in the Cisplatin treated mice than that of the control cells. However, the COX-2 expression was markedly decreased by a combination of Cisplatin and Boswellic acids at 250 mg/kg. It is proposed that the down-regulation of COX-2 by BA may have beneficial effects on reversing the Cisplatin-induced apoptosis inhibition, thus, offering protection against cancer development [32]. These results suggested that combination therapy of Cisplatin with BA in a dose of 250 mg/kg may decrease the production of proinflammatory prostaglandins to inhibit tumor growth. However, by increasing the dose of BA to 500 mg/kg in combination with Cisplatin, the level of COX-2 was increased. There are two possibilities that can explain this finding; firstly, this may be a sign of adverse effect of BA as its elimination half-life ranges from 10.5 to 69.3 h and it has a high volume of distribution. Therefore, accumulation and side effects can occur easily with high doses. The second possibility is that lipophilic tissues as fat cells accumulate BA and so its therapeutic effect on the COX-2 level was decreased than with small doses [33]. These findings still need to be validated by preclinical trials.
As angiogenesis is a prerequisite to tumor growth and its metastatic spread, the microvessel density that reflects the inter-capillary distance has been assessed in the current study by measuring the CD31 index. It is influenced by both angiogenic and non-angiogenic factors, and is considered to be a powerful candidate for prognosis [34]. We found that colon of DMH mice treated with a combination of Cisplatin and Boswellic acids at 250 and 500 mg/kg showing a marked reduction in immunoexpression of CD31. The combination therapy produced a greater reduction in microvessel density compared to monotherapies; this indicates that the greater tumor inhibition exerted by the combination therapy was, at least in part, attributed to the combined anti-angiogenic effect of both drugs.
Conclusion
BA augmented the antitumor effects of Cisplatin by downregulating significantly the marker of tumor proliferation index; the microvessel density CD31 and decreasing the levels of vascular endothelial growth factor, COX-2 and interleukin-6 biomarkers. Collectively, our findings showed that combination of Cisplatin with BA could be used to improve the treatment in CRC through decreasing angiogenesis and enhancing apoptosis. This combination is a less toxic option in the treatment of CRC than monotherapy with Cisplatin. Therefore, this study showed that BA can have a chemotherapeutic use. However, until appropriate safety data are available, further investigations, including one that could detect the histopathological changes in different tissues that accompanied the prolonged use of this combined therapy, are needed as it is known that chemotherapeutic agents must be used for more than one cycle. Such studies are prerequisite to ensure the efficacy of Cisplatin and Boswellic acids as an adjuvant therapy in the clinical management of patients with CRC.
References
- Binefa G, RodrÃguez-Moranta F, Teule A, Medina-Hayas M(2014) Colorectal cancer: From prevention to personalized medicine. World J Gastroenterol 20: 6786-6808.
- Gadoa A,Ebeidb B, Abdelmohsenc A, Axon A (2014) Colorectal cancer in Egypt is commoner in young people: Is this cause for alarm?AJM 50: 197-201.
- Ferrarotto R, Hoff PM (2013) Antiangiogenic Drugs for Colorectal Cancer: Exploring New Possibilities. Clin Colorectal Cancer 12: 1-7.
- Majerus PW (1998) Prostaglandins: Critical roles in pregnancy and colon cancer. Current Biology 8: 87-89.
- Greenhough A, Smartt HJM, Moore AE, Roberts HR, Williams AC, et al. (2009) The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 30: 377-386.
- Cuzick J, Otto F, Baron JA, Brown PH, Burn J, et al. (2009) Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet 10: 501-507.
- Chung YC, Chang YF (2003) Serum interleukin-6 levels reflect the disease status of colorectal cancer. J SurgOncol 83: 222-226.
- Nagasaki T, Hara M, Nakanishi H, Takahashi H, Sato M, et al. (2014) Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer 110: 469-478.
- Sakurai T, Kudo M (2011) Signaling pathways governing tumor angiogenesis. Oncology 81: 24-29.
- Yadav VR, Prasad S, Sung B, Gelovani JG, Guha S, et al. (2012) Boswellic Acid Inhibits Growth and Metastasis of Human Colorectal Cancer in Orthotopic Mouse Model By Downregulating Inflammatory, Proliferative, Invasive, and Angiogenic Biomarkers. Int J Cancer 130: 2176-2184.
- Mizutani Y, Nakao M, Ogawa O, Yoshida O, Bonavida B, et al. (2001) Enhanced sensitivity of bladder cancer cells to tumor necrosis factor related apoptosis inducing ligand mediated apoptosis by cisplatin and carboplatin. J Urol 165: 263-270.
- Liu HP, Gao ZH, Cui SX, Wang Y, Li BY, et al. (2013) Chemoprevention of intestinal adenomatous polyposis by acetyl-11-keto-beta-boswellic acid in APC (Min/+) mice. Int J Cancer 132:2667-2681.
- Liu JJ, Nilsson A, Oredsson S, Badmaev V, Zhao WZ, et al. (2002) Boswellic acids trigger apoptosis via a pathway dependent on caspase-8 activation but independent on Fas/Fas ligand interaction in colon cancer HT-29 cells. Carcinogenesis 23: 2087-2093.
- Kumar A, Shah BA, Singh S, Hamid A, Singh SK, et al. (2012) Acyl derivatives of boswellic acids as inhibitors of NF-κB and STATs. Bioorg Med ChemLett 22:431-435.
- Qurishi Y, Hamid A, Sharma PR, Wani ZA, Mondhe DM, et al. (2012) PARP cleavage and perturbance in mitochondrial membrane potential by 3-α-propionyloxy-β-boswellic acid results in cancer cell death and tumor regression in murine models. Future Oncol 8:867-881.
- Florea AM, Büsselberg D (2011) Cisplatin as an Anti-Tumor Drug: Cellular Mechanisms of Activity, Drug Resistance and Induced Side Effects. Cancers 3: 1351-1371.
- Colussi C1, Fiumicino S, Giuliani A, Rosini S, Musiani P, et al. (2001) 1,2-dimethylhydrazine-induced colon carcinoma and Lymphoma in msh2(-/-) mice. JNCI 93: 1534-1540.
- Park HR, Ju EJ, Jo SK, Jung U, Kim SH, et al. (2009) Enhanced antitumor efficacy of cisplatin in combination with HemoHIM in tumor-bearing mice. BMC Cancer 9:85.
- Vermeulen PB, Gasparini G, Fox SB, Toi M, Martin L, et al. (1996) Quantification of angiogenesis in solid human tumors: an international consensus on the methodology and criteria of evaluation. Eur J Cancer 32: 2474-2484.
- Schnekenburger M, Diederich M (2012) Epigenetics Offer New Horizons for Colorectal Cancer Prevention. Curr Colorectal Cancer Rep 8:66-81.
- Takahashi M, Sung B, Shen Y, Hur K, Link A, et al. (2012) Boswellic acid exerts antitumor effects in colorectl cancer by modulating expression of the let-7 and miR-200 microRNA family. Carcinogenesis 33: 2441-2449.
- Perše M, Cerar A (2011) Morphological and molecular alterations in 1,2 dimethylhydrazine and azoxymethane induced colon carcinogenesis in rats. J Biomed Biotechnol 2011:473964.
- Oravec CT, Jones CA, Huberman E (1986) Activation of the colon carcinogen 1,2-dimethylhydrazine in a rat colon cell-mediated mutagenesis assay. Cancer Research46: 5068-5071.
- Karimzadeh L, Nabiuni M,Kouchesfehani H, Adham H, Bagheri A, et al. (2013) Effect of bee venom on IL-6, COX-2 and VEGF levels in polycystic ovarian syndrome induced in Wistar rats by estradiolvalerate. J Venom Anim Toxins Incl Trop Dis 19:32.
- Fan F, Schimming A, Jaeger D, Podar K (2012) Targeting the tumor microenvironment: focus on angiogenesis. J Oncol 2012:281261.
- Mulder K, Koski S, Scarfe A, Chu Q, King K, et al. (2010) Antiangiogenic agents in advanced gastrointestinal malignancies: past, present and a novel future. Oncotarget 1:515-529.
- Dvorak HF, Nagy JA, Berse B, Brown LF, Yeo KT, et al. (1992) Vascular permeability factor, fibrin, and the pathogenesis of tumorstroma formation. Ann NY AcadSci 667: 101-111.
- Kawai N, Tsujii M, Tsuji S (2002) Cyclooxygenases and colon cancer. Prostaglandins and Other Lipid Mediators69:187-196.
- Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, et al. (1998) Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93:705-716.
- Kim HS, Kim T, Kim MK, Suh DH, Chung HH, et al. (2013) Cyclooxygenase-1 and -2: Molecular Targets for Cervical Neoplasia. Journal of Cancer Prevention 18:123-134.
- Brown JR, DuBois RN (2005) COX-2: A Molecular Target for Colorectal Cancer Prevention. J ClinOncol 23:2840-2855.
- Hong J, Smith TJ, Ho CT, August DA, Yang CS (2001) Effects of purified green and black tea polyphenols on cyclooxygenase- and lipoxygenase-dependent metabolism of arachidonic acid in human colon mucosa and colon tumor tissues. BiochemPharmacol 62: 1175-1183.
- Ammon P (2006) Boswellic acids in chronic inflammatory diseases. Planta Med 72:1100-1116.
- Lunt SJ, Chaudary N, Hill RP (2009) The tumor microenvironment and metastatic disease. ClinExp Metastasis 26:19-34.
Citation: Abdelaziz EZ, Hegazy AMS, Badawy A, Bayomy NA, Rabat AE, et al. (2016) Potential Anti-angiogenic Effect of a Combination of Boswellic Acids and Cisplatin against Chemically-induced Colon Cancer in Mice. J Chem Biol Ther 2: 111. DOI: 10.4172/2572-0406.1000111
Copyright: © 2016 Abdelaziz EZ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 12725
- [From(publication date): 11-2016 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 11722
- PDF downloads: 1003