Research Article Open Access
Postural Instability in the ML Direction in Individuals with Parkinson's Disease Before, During and when Recovering from a Forward Reach
Shaun Porter, Christopher Dalton and Julie Nantel*School of Human Kinetics, University of Ottawa, 125 rue Université, Pavillon Montpetit, MNT 353, Ottawa, Canada
- *Corresponding Author:
- Julie Nantel
Assistant Professor, School of Human Kinetics
Faculty of Health Sciences, University of Ottawa
125 University, MNT 353 Ottawa, Canada, K1N 6N5
Tel: 613-562-5800
E-mail: jnantel@uottawa.ca
Received Date: September 20, 2016; Accepted Date: September 28, 2016; Published Date: October 05, 2016
Citation: Porter S, Dalton C, Nantel J (2016) Postural Instability in the ML Direction in Individuals with Parkinson’s Disease Before, During and when Recovering from a Forward Reach. J Alzheimers Dis Parkinsonism 6:267. doi: 10.4172/2161-0460.1000267
Copyright: © 2016 Porter S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Reaching forward requires preparing and regulating the trunk progression to avoid falling, but also controlling balance when recovering from it. Objective: To measure postural stability in individuals with Parkinson’s disease (PD) and healthy older adults before, during and when recovering from a Forward Reach Task (FRT). Methods: Nineteen PD (Dx: 5.3 ± 3.8 years; Hoehn and Yahr, 1-3) and sixteen older adults performed the FRT on a force platform and completed the Montreal Cognitive Assessment (MOCA) and self-reported falls (12 months). Center of pressure displacement variability (CoP) and CoP velocity (VCoP) were calculated before (T1), during (T2), and after (T3) reaching. Correlations assessed the relationship between postural stability during these periods, FRT, falls and MOCA scores. Results: Variability in CoP T2 medial-lateral (ML), velocity T1 and T3, p<0.05 were larger in PD compared to Controls. In anterior-posterior (AP) velocity in T1, p<0.05 and T2, p<0.001 and variability in CoP T3 were larger in controls than in PD, p<0.05. In PD, FRT distance was correlated with ML CoP T1 (r=-0.73, p<0.001), CoP T2, T3 (r=-0.59, p<0.01). Conclusion: Older adults showed postural instability in the main direction of the task (AP) whereas those with PD were mostly unstable in the ML direction. In PD, the large contribution of postural control in ML was correlated with decreased reach distance, increased number of falls and disease duration. Altogether this highlights that individuals with PD and more so fallers, rely heavily on postural control in the ML direction before, during and when recovering from a forward reach.
Keywords
Parkinson’s disease; Older adults; Forward reach; Postural control; Postural stability; Medial lateral; Falls
Introduction
Deficits in postural balance affect the independence and quality of life in individuals with Parkinson’s disease (PD). Activities that involve reaching forward, i.e., reaching for an object on a shelf are very common daily tasks and have been correlated to increased risks of falls in PD [1,2]. As the trunk is bent and moved forward, different postural control mechanisms are required to regulate its progression, avoid loss of balance and forward fall.
In a recent study, Huang and Brown [3] assessed the center of pressure (CoP) trajectories during a forward reach task (FRT) between young and older adults. As most reaching tasks involve not only reaching forward but also recovering balance after returning to the upright position, the authors also assessed postural control before and after the forward reach. They suggested that older adults lack the ability to control postural balance in preparation of a forward reach task as well as during the execution of the task and when regaining balance after the movement.
Deficits in postural stability, i.e., the inability to control the position of the center of mass in relation to the base of support, in individuals with PD increase with disease severity and with presence of mild cognitive impairments (MCI), impairments considered as independent factors for fall risk [4]. These deficits in postural stability have been reported both during static [5] and dynamic postural activities [6,7]. Furthermore, it has been shown that postural instability in the mediallateral (ML) direction was particularly affected in PD and was associated with increased risk of falls [8,9]. Van Wegen et al. investigated postural stability in individuals with PD during static forward and backward lean on force a platform [10]. The authors reported larger postural variability in the ML direction in PD compared to older adults and suggested that the large variability could be indicative of postural instability and changes in postural strategies in individuals with PD compared to older adults. This was similar to Mitchell et al. who suggested that postural variability in the ML direction could be used as a compensatory mechanism due to inflexibility of the postural control in the anterior-posterior (AP) direction in PD [11].
The purpose of our study was to assess postural stability in individuals with PD before, during and when recovering from a FRT, i.e., as they transition back to the upright position. We hypothesized that individuals with PD would show larger postural instability when preparing for the reach as well as during and moving back to the upright position. We also expected individuals with PD to display more postural compensation in the medial-lateral direction compared to healthy older adults. Finally, we expected that these results would be exacerbated in individuals with lower cognitive functions.
Methods
Participants
Nineteen individuals (16 men, 3 women; 60.0 ± 10.2 years old) diagnosed with PD, (Dx: 5.3 ± 3.8 years, Hoehn and Yahr stages 1-3, Motor Unified Parkinson’s Disease Rating Scale: 8.3 ± 3.2) and sixteen older adults (4 men, 12 women; 65.9 ± 9.7 years old) were recruited from the Parkinson’s disease and Movement Disorders Clinic of the Ottawa Hospital Research Institute and from the community. The inclusion criteria comprised of: no history of orthopedic impairments, musculoskeletal impairments or neurological conditions other than Parkinson’s disease that could impact balance and gait. Participants were also excluded if they were unable to walk unaided. Participants were tested on dopaminergic medications. The study was approved by the University Review Board.
Procedure
Before completing the FRT, participants were administered the Montreal Cognitive Assessment questionnaire (MOCA) and were asked if they had fallen in the previous 3 and 12 months. Subjects’ disease severity was assessed using the Unified Parkinson’s Disease Rating Scale (UPDRS) III (motor disability). For the FRT, participants were asked to stand on two force platforms for 10 s (T1) with their least affected arm at 90 degrees flexion. They were then asked to reach forward as far as possible, without bending the knees, while maintaining heel contact with the ground. This position was held with control for 10 s (T2) before returning to their starting position and standing for 10 s (T3). After the examiner explained and demonstrated the task, each subject completed 3 trials. A retractable measuring tape attached to a rigid stand placed at shoulder height was also used to measure the reach distance. The functional reach task is a simple measurement to assess dynamic balance during a forward reach, which has been reported as a valid and reliable test in assessing balance [12-15] However, while the 25.4 cm reaching distance cut-off used to assess risk for falls in older adults has good specificity (92%), it has poor sensitivity (30%). Therefore, FRT was considered not sensitive enough to be used as a screening tool for fall risks in PD [15]. In an attempt to maximize the sensitivity of the FRT in PD, Dibble et al. suggested to increase the from 24.4 cm to 31.75 cm, which resulted in 86% sensitivity and 52% specificity [16] rather than 90% specificity and 30% sensitivity. While this task has been reported to be a valid and reliable measure of postural control [12,17], Dibble et al. [16] concluded that the functional reach task should be used concurrently with other tests to accurately assess risks for falls in PD.
Data and Statistical Analysis
The FRT was performed on two-force platforms (Kistler, Winterthur, Switzerland) with kinetics captured at 200 Hz and filtered with a zero-lag fourth-order Butterworth filter, 10 Hz cut-off frequency. Center of pressure (CoP) displacement root mean square and mean velocity (VCoP) in the ML and AP directions were derived from the ground reaction forces to assess postural control. Mixed model ANOVA was used to analyze the between and within subject factors. Repeated measures ANOVAs were used when permitted, to further determine the CoPs and VCoPs between the three periods (T1-T3). According to the normality of the distribution, Pearson or Spearman Rank Order Correlations were used to assess the relationship between postural stability (CoPs and VCoPs) and both the FRT, falls and MOCA scores. FRT and the posturography data were averaged over the three trials.
Results
The average UPDRS III was 8.5 ± 3.2 and the MOCA scores ranged from 19 to 29 with an average of 25.9 ± 2.8. For falls, 11 reported 0 falls, four reported 1 fall and two reported 2 or more falls in the previous year. None reported falling in the previous 3 months. The average distance reached was 29.0 cm ± 8.2, with six participants reaching below the 25.4 cm cut-off [12], but nine participants above the 31.75 cm cut-off. In the control group, the MOCA scores ranged from 26 to 30 with an average of 27.4 ± 1.7. Fourteen control subjects reported 0 falls, one reported 1 fall and one reported 2 or more falls in the past three months while four reported 1 fall and two, 2 or more falls in the past year. The average distance reached was 31.5 cm ± 4.5, with two participants reaching below the 25.4 cm cut-off [12] and nine reaching above the 31.75 cm cut-off.
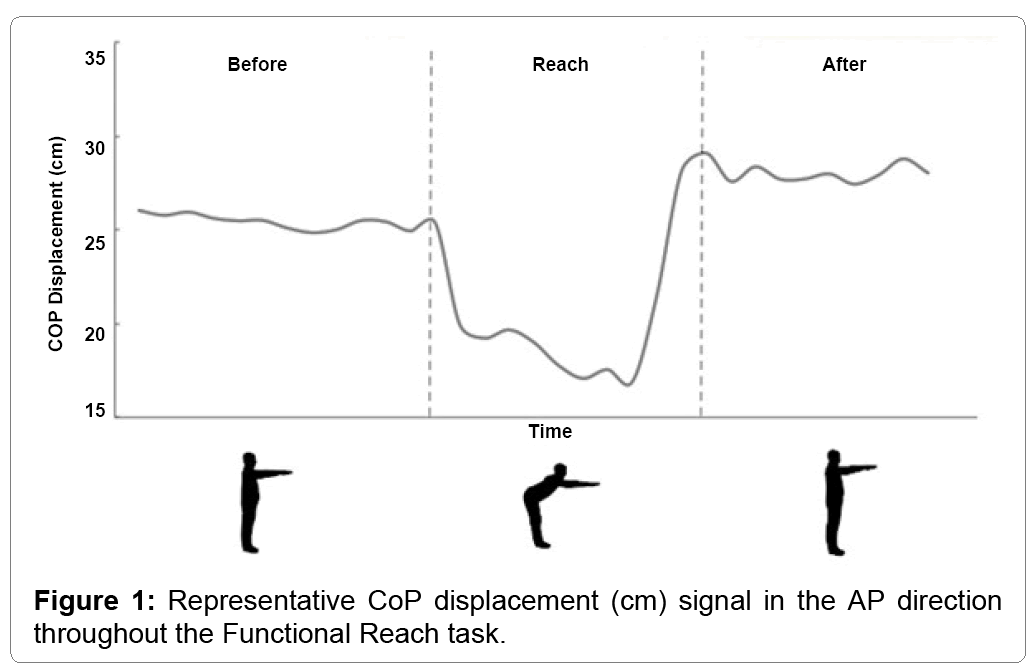
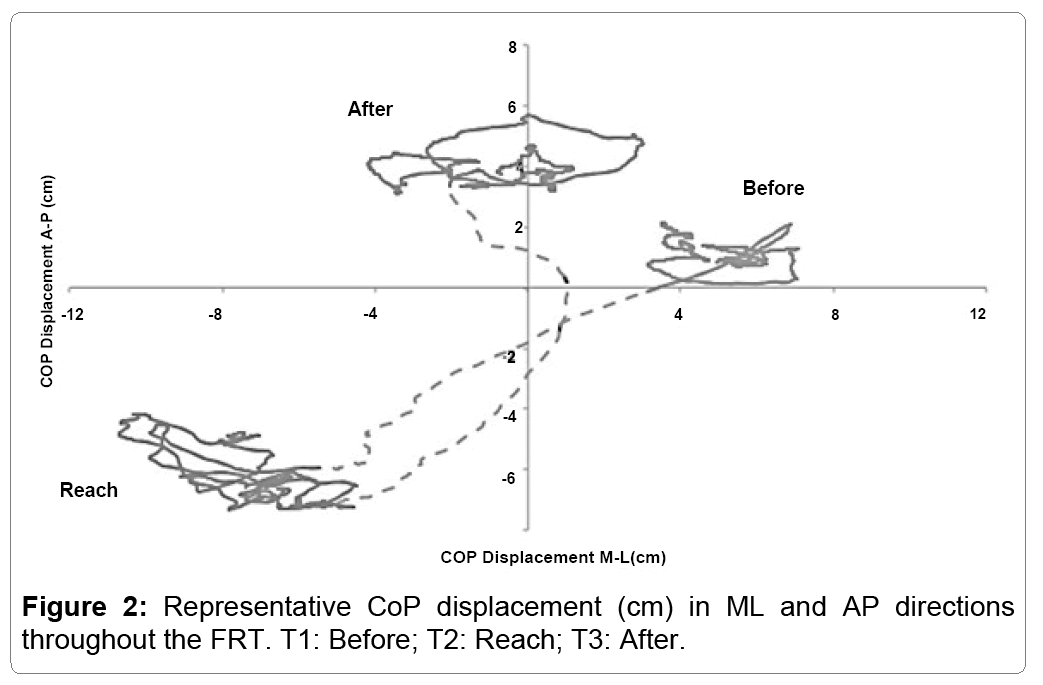
The trajectory of the CoP throughout the FRT is represented in both (Figures 1 and 2). The figures present the reach in AP (Figure 1) and in both AP and ML (Figure 2).
Both figures display the relative stability of the participant prior to the reach (T1), with minimal displacement seen in either the AP or ML direction. During T2, the participants reach forward, resulting in a forward displacement of the CoP (Figure 1). However, Figure 2 reveals that while the CoP is displaced in AP, there is also an increase in ML displacement. During the final phase of the FRT (T3), the CoP displaces further posteriorly compared to the beginning (Figure 1) while the displacement remains larger for the entirety of the 10 seconds recorded post-reach (Figure 2).
The CoPs and VCoPs in AP and ML directions are summarized in Table 1. Significant differences were found between groups in CoP displacement variability in both ML and AP directions (p<0.05). Variability in CoP T2 ML was larger in PD compared to controls F(1, 33)=5.19, p<0.05, while CoP T3 in AP was larger in controls than in PD F (1, 33)=5.85, p<0.05. For the CoP ML velocity T1 F (1, 33)=5.12, p<0.05 and T3 F (1, 33)=5.14 p<0.05 were larger in the PD group compared to Controls, while in AP direction T1 F(1, 33)=5.20, p<0.05 and T2 F(1, 33)=20.50, p<0.001 were larger in Controls than PD.
| Controls | PD | |||||
|---|---|---|---|---|---|---|
| AP | ML | AP | ML | |||
| Mean ± SD | Mean ± SD | |||||
| CoP cm |
T1 | 0.69 ± 0.28 | 0.48 ±0.30 | 0.57 ± 0.27 | 0.70 ±0.59 | |
| T2 | 1.53 ± 0.31 | 0.91 ± 0.24 | 1.64 ± 0.70 | 1.51 ± 1.01Ç? | ||
| T3 | 1.05 ± 0.27 | 0.83 ± 0.33 | 0.79 ± 0.35Ç? | 1.00 ± 0.72 | ||
| VCoP cm/s |
T1 | 1.23 ± 0.35 | 0.72 ± 0.24 | 0.96 ± 0.35Ç? | 1.13 ± 0.69Ç? | |
| T2 | 1.83 ± 0.58 | 0.93 ± 0.32 | 1.05 ± 0.43Ç? | 1.13 ± 0.65 | ||
| T3 | 1.18 ± 0.34 | 0.72 ± 0.28 | 1.08 ± 0.48 | 1.25 ± 0.89Ç? | ||
T1: Before the reach
T2: During the reach
T3: recovering from the reach
Adjustment for multiple comparisons: Bonferroni
Table 1: Centre of pressure displacement root mean square (CoPs) in cm and velocity of the centre of pressure (VCoPs) in cm/s, in both the anterior-posterior and mediallateral directions, divided into the FRT intervals (T1-T3).
In PD, CoP T2 was larger compared to CoP T1 (p<0.001) in both AP and ML directions and larger compared to T3 (p=0.001), but in the ML direction only. VCoP in AP and ML were not different between periods. In control group, CoP in T2 and T3 in both directions were larger than T1 and T2 larger than T3 in the AP direction only (p<0.001). In VCoP T2 was larger than both T1 and T3 in ML and AP directions (p<0.001).
In PD, the FRT distance was negatively associated with the CoP T1 (r=-0.73, p < 0.001), CoP T2 and CoP T3 (both r=-0.59, p<0.01) displacement in the ML direction. The FRT was also correlated with VCoP T1 (r=-0.58, p<0.01), T2 (r=-0.59, p<0.01) and T3 (r=-0.56, p=0.01) in the ML direction. No correlations were shown in the AP direction. In controls, the reach distance was positively associated with the VCoP T2 in the AP direction (r=0.62, p=0.02) and negatively associated with CoP T3 (r=-0.51, p<0.05) in the ML direction. In PD, self-reported falls were correlated with the CoP displacement in the ML direction at T1 (r=0.48) and at T2 in the AP direction (r=0.52), p<0.05). Disease duration was moderately associated with VCoP in the ML direction (T1: r=0.47, T2: 0.40 and T3: 0.51, p<0.05) as well as with VCoP in the AP direction (T1: r=0.48 and T3: 0.53, p<0.05). The MOCA scores in PD did not show correlation with the CoPs or VCoPs in neither AP nor ML directions. In controls, the MOCA scores were negatively correlated with CoP T3 in both AP (r=-0.68) and ML (r=- 0.62) p<0.01, as well as with VCoP T1 in the ML direction (r=-0.50, p<0.05). Self-reported falls did not correlate with CoPs in controls.
Discussion
The main objectives of the present study were to assess postural stability before, during and recovering from a self-initiated forward reaching task in individuals with PD and compare postural control and strategies with healthy, age-matched adults. We also sought to determine the relationship between postural stability and the distance reached during the FRT, self-reported falls, disease duration and mild cognitive impairments. As expected, postural instability increased in both groups as participants reached forward. However, while older adults had larger velocity (T1 and T2) and variability (T3) in the principal direction of the task (AP direction), individuals with PD displayed larger CoP displacement variability (T2) and velocity (T1 and T3) in the ML direction. In addition, in this latter group, the larger CoP displacement variability and velocity in the ML direction were moderately associated with smaller reaching distance, greater number of falls as well as with longer disease duration. Our results suggest that individuals with PD rely on a greater contribution of postural control in the ML direction compared to older adults when performing and recovering from a reaching task.
Postural instability and difficulty recovering balance following external perturbations has been widely reported in individuals with PD [14,18].This was attributed to smaller margin of stability as well as an inability to efficiently adapt to a changing environment due to the defective basal ganglia circuitry [18]. Bloem et al. [19] reported that most falls in PD occur due to internal disturbances of balance rather than being the consequence of external triggers. In our PD group, based on the large ML velocity during the phase preceding the reach, it is most likely that the postural instability in the ML direction could have interfered with the ability to regulate postural balance during the more challenging forward reach. It is also interesting to emphasize that while older adults displayed larger velocity in the main direction of the task (AP), individuals in the PD group displayed significantly larger variability and velocity in the ML direction. As large CoP velocity in the ML direction has been associated with higher risks for falls, these results suggest that individuals with PD might have needed to increase postural control in the ML direction to preserve balance when preparing for, during and when recovering from the reach. Furthermore, similarly to the reduced COP excursions and velocity during gait initiation in PD [20,21], the smaller postural adjustment in the AP direction in PD could reflect the inefficiency of the basal ganglia in planning and initiating the reaching movement.
In the PD group, the large postural variability during the reach and velocity before and when recovering from the task in the ML direction were associated with lower functional reaching distance. This illustrates a greater need for controlling postural stability in ML direction in those with lower FRT performance. Also, larger ML variability before the reach and AP variability during the reach was associated with falls in PD. This is consistent with studies, reporting an association between disease duration, postural instability in the ML direction and higher risk of falls in PD [22] and between ML stability and prediction of falls in older adults [23]. Furthermore, in a cohort of 27 participants classified as “early” stage PD, and of whom 16 had never taken dopaminergic medications, Nantel et al. [24] reported larger CoP displacement variability in the ML direction compared to healthy older adults. Interestingly, in older adults CoP variability in ML during the recovery period was negatively associated with reaching distance. This is in line with Huang and Brown [3] who reported a decrease in CoP trajectory smoothness in older adults and suggested that this could reflect difficulty in controlling balance when recovering from a dynamic task. This deficit in controlling balance in the ML direction was exacerbated in PD as both ML variability and velocity throughout the reach were negatively correlated with the reaching distance and that velocity in ML before and after the reach was greater in PD compared to older adults.
Within the older adults, MOCA was associated with both velocity in preparation of the reach (T1) and variability when recovering from the reach (T3) in the ML direction, whereas no such correlation was seen in the PD group. This is surprising, as MCI has been associated with an increased risk of falls and reported as a predictor of future falls in PD [4,25]. This illustrates the complexity of evaluating the risks of fall in individuals with PD, as many factors such as freezing of gait, past history of falls and MCI may contribute to the occurrence of falls [4]. Furthermore, these factors also have an impact on fear of falling [26]. This could be due to two distinctive behaviors in individuals with PD, as fear of falling leads some individuals to restrain their participation in activities of daily living, whereas lack of fear of falling increases the risks of falls and injuries in others [19,26-28].This is in line with the moderate positive correlation between self-reported falls in PD and CoP variability in the AP direction during the reach. A larger sample size could have allowed us to statistically assess the association between falls and the FRT scores in those above the 25.4 cm and 31.75 cm cut-offs, thus documenting the impact of risk-taking behaviour on the occurrence of falls in these individuals. Based on the occurrence of falls in these individuals and the impact of falls on participation and functional mobility, this seems to be an important area of exploration in future studies.
Conclusion
Our posturographic analysis of a self-initiated forward reach showed large postural instability throughout the task in individuals with PD compared to older adults. It also identified different strategies in PD compared to healthy older adults to control postural balance before, during and when recovering from the reach. In healthy older adults, postural instability was seen mainly in the main direction of the task, whereas those with PD showed larger instability in the ML direction. This ML focused strategy in the PD group was correlated with smaller reaching distance, increased number of falls and longer disease duration. These results suggest that as disease progresses individuals with PD, and particularly fallers, rely highly on postural control in the ML direction. As this strategy was exacerbated when performing and recovering from the reach task executed in the AP direction, it seems critical to assess postural balance in the ML direction when assessing postural stability and risks of falling. It is also relevant to bring attention to the occurrence of falls in some individuals reaching well above the FRT cut-off as this could indicate the presence of risk-taking behavior and potential risks for injuries related to falls. Finally, since the distance reached during the FRT showed no differences between groups, it appears preferable to use an instrumented version of the FRT when assessing postural stability and risks for falls in individual with PD.
Acknowledgement
We would like to thank Dr Grimes and his team at the Parkinson’s disease and Movement Disorders Clinic of the Ottawa Hospital Research Institute, for the help recruiting participants.
Financial support: This study was supported by Parkinson Society Canada (JN), by the University of Ottawa, Faculty of Health Sciences Research Development Program Grant (JN) and by Racquetball Canada (JN).
References
- Ryckewaert G, Luyat M, Rambour M, Tard C, Noël M, et al. (2015) Self-perceived and actual ability in the functional reach test in patients with Parkinson's disease. NeurosciLett 589: 181-184.
- Morris ME (2000) Movement disorders in people with Parkinson disease: A model for physical therapy. PhysTher 80: 578-597.
- Huang MH, Brown SH (2013) Age differences in the control of postural stability during reaching tasks. Gait Posture 38: 837-842.
- Camicioli R, Majumdar SR (2010) Relationship between mild cognitive impairment and falls in older people with and without Parkinson's disease: 1year prospective cohort study. Gait Posture 32: 87-91.
- Blaszczyk JW (2016)The use of force-plate posturography in the assessment of postural instability. Gait Posture 44: 1-6.
- Mancini M, Rocchi L, Horak FB, Chiari L (2008) Effects of Parkinson's disease and levodopa on functional limits of stability. ClinBiomech (Bristol, Avon) 23: 450-458.
- Hasmann SE, Berg D, Hobert MA, Weiss D, Lindemann U, et al. (2014) Instrumented functional reach test differentiates individuals at high risk for Parkinson's disease from controls. Front Aging Neurosci 6: 286.
- Nantel J, Bronte-Stewart H (2014)The effect of medication and the role of postural instability in different components of freezing of gait (FOG). Parkinsonism RelatDisord 20: 447-451.
- Dimitrova D, Horak FB, Nutt JG (2004) Postural muscle responses to multidirectional translations in patients with Parkinson's disease. J Neurophysiol 91: 489-501.
- van Wegen EE, van Emmerik RE, Wagenaar RC, Ellis T (2000) Stability boundaries and lateral postural control in Parkinson’s disease. Motor Control 5: 254-269.
- Mitchell SL, Collins JJ, De Luca CJ, Burrows A, Lipsitz LA (1995) Open-loop and closed-loop postural control mechanisms in Parkinson's disease: increased mediolateral activity during quiet standing. NeurosciLett 197: 133-136.
- Duncan PW, Weiner DK, Chandler J, Studenski S (1990) Functional reach: A new clinical measure of balance. J Gerontol 45: M192-197.
- Jenkins ME, Johnson AM, Holmes JD, Stephenson FF, Spaulding SJ (2010) Predictive validity of the UPDRS postural stability score and the functional reach test, when compared with ecologically valid reaching tasks. Parkinsonism RelatDisord 16: 409-411.
- Smithson F, Morris ME, Iansek R (1998) Performance on clinical tests of balance in Parkinson's disease. Physical Therapy 78: 577-592.
- Behrman AL, Light KE, Flynn SM, Thigpen MT (2002) Is the functional reach test useful for identifying falls risk among individuals with Parkinson's disease? Archives of Physical Medicine and Rehabilitation 83: 538-542.
- Dibble LE, Lange M (2006) Predicting falls in individuals with Parkinson disease: A reconsideration of clinical balance measures. J NeurolPhysTher 30: 60-67.
- Duncan PW, Weiner DK, Chandler J, Studenski SF (1990) Functional reach: A new clinical measure of balance. J Gerontol 45: M192-197.
- Horak FB, Dimitrova D, Nutt JG (2005) Direction-specific postural instability in subjects with Parkinson's disease. ExpNeurol 193: 504-521.
- Bloem BR, Grimbergen YA, Cramer M, Willemsen M, Zwinderman AH (2001) Prospective assessment of falls in Parkinson's disease. J Neurol 248: 950-958.
- Fernandez KM, Roemmich RT, Stegemöller EL, Amano S, Thompson A, et al. (2013) Gait initiation impairments in both essential tremor and Parkinson's disease. Gait & Posture 38: 956-961.
- Halliday SE, Winter DA, Frank JS, Patla AE, Prince F (1998) The initiation of gait in young, elderly and Parkinson's disease subjects. Gait Posture 8: 8-14.
- Mancini M, Carlson-Kuhta P, Zampieri C, Nutt JG, Chiari L, et al. (2012) Postural sway as a marker of progression in Parkinson's disease: A pilot longitudinal study. Gait Posture 36: 471-476.
- Hilliard MJ, Martinez KM, Janssen I, Edwards B, Mille ML, et al. (2008) Lateral balance factors predict future falls in community-living older adults. Arch Phys Med Rehabil 89: 1708-1713.
- Nantel J, McDonald JC, Bronte-Stewart H (2012) Effect of medication and STN-DBS on postural control in subjects with Parkinson's disease. Parkinsonism RelatDisord 18: 285-299.
- Latt MD, Lord SR, Morris JG, Fung VS (2009) Clinical and physiological assessments for elucidating falls risk in Parkinson's disease. MovDisord 24: 1280-1289.
- Thomas AA, Rogers JM, Amick MM, Friedman JH (2010) Falls and the falls efficacy scale in Parkinson’s disease. Journal of neurology 257: 1124-1128.
- Bloem BR, Grimbergen YA, van Dijk JG, Munneke M (2006) The "posture second" strategy: A review of wrong priorities in Parkinson's disease. J NeurolSci 248: 196-204.
- Adkin AL, Frank JS, Jog MS (2003) Fear of falling and postural control in Parkinson's disease. MovDisord 18: 496-502.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 12011
- [From(publication date):
October-2016 - Jul 01, 2025] - Breakdown by view type
- HTML page views : 11056
- PDF downloads : 955