Post-Transplant Lymphoproliferative Disorders after Allogeneic Hematopoietic Stem Cell Transplantation: Case Report and Literature Review
Received: 31-Aug-2021 / Accepted Date: 14-Sep-2021 / Published Date: 21-Sep-2021
Abstract
PTLDs are disorders with a spectrum ranging from “early” polyclonal proliferations resembling nonspecific plasma cell hyperplasia or infectious mononucleosis to clonal proliferations of B or T/NK cells. The incidence of PTLD after HSCT is approximately 0.8%-4.0%. The clinical manifestations of PTLD are variable related to the morphological types, sites, and patient’s general condition. Four basic histological types of PTLD have been identified: Nondestructive PTLD, polymorphic PTLD, monomorphic PTLD and classical Hodgkin Lymphoma. Treatments for PTLD comprise reduction of immunosuppression, rituximab, chemotherapy, and adoptive immunotherapy.
Keywords: Hematopoietic stem cell transplantation; Post-transplant lymphoproliferative disorders; Leukemia; Epstein-Barr virus
Introduction
Hematopoietic stem-cell transplantation (HSCT) is the transplantation of multipotent hematopoietic stem cells, usually derived from bone marrow, peripheral blood, or umbilical cord blood. It may be autologous, allogeneic, or syngeneic.
It is most often performed for patients with certain cancers of the blood or bone marrow, such as multiple myeloma or leukemia. In these cases, the recipient's immune system is usually destroyed with radiation or chemotherapy before the transplantation. Infection and graft-versus-host disease are major complications of allogeneic HSCT.
HSCT remains a dangerous procedure with many possible complications; it is reserved for patients with life-threatening diseases. As survival following the procedure has increased, its use has expanded beyond cancer to autoimmune diseases and hereditary skeletal dysplasia; notably malignant infantile osteopetrosis and mucopolysaccharidosis.
Lymphoproliferative disorders (LPDs) refer to a specific class of diagnoses, comprising a group of several conditions, in which lymphocytes are produced in excessive quantities. These disorders primarily present in patients who have a compromised immune system. Due to this factor, there are instances of these conditions being equated with "immunoproliferative disorders"; although, in terms of nomenclature, lymphoproliferative disorders are a subclass of immunoproliferative disorders-along with hypergammaglobulinemia and paraproteinemia’s.
Case Study
Clinical history of patient
Twenty-one years old male got fever for no obvious reason 1 year ago and presented with right chest pain later. The CT scan showed an antero-superior mediastinal mass 1 month later and was diagnosed as T lymphoblastic leukemia/lymphoma (T-LBL). The bone marrow aspiration also supported the diagnosis. The flow cytometry detected abnormal precursor T lymphocytes. He received chemotherapy of Hyper-CVAD-A protocol with Pegaspargase and Hyper-CVAD-B protocol later. Three months ago, he was treated by HSCT after modified TBI-CY-ATG pretreatment, whose donor was his mother (HLA3/6). Ten days later, he presented with fever, vomit, and sore throat, with elevated EBV DNA. A tonsil biopsy was made.
The pathological features of T-LBL and PTLD
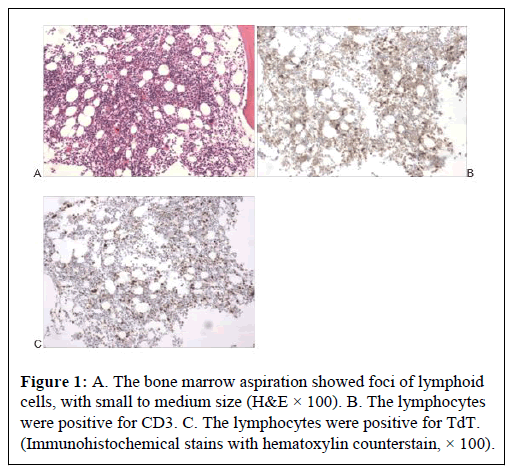
The mediastinal biopsy demonstrated medium sized cells with fine chromatin, expressing CD3, CD5, CD7, CD43 and TdT, but not CD20, CD2, MPO and index of Ki67 was 25%. The bone marrow aspiration showed foci of lymphoid cells, with small to medium size, unclear chromatin observed in Figure 1, expressing CD3, CD2, CD7 and TdT, but not CD20, and index of Ki67 was 60%. The findings were consistent with T-LBL.
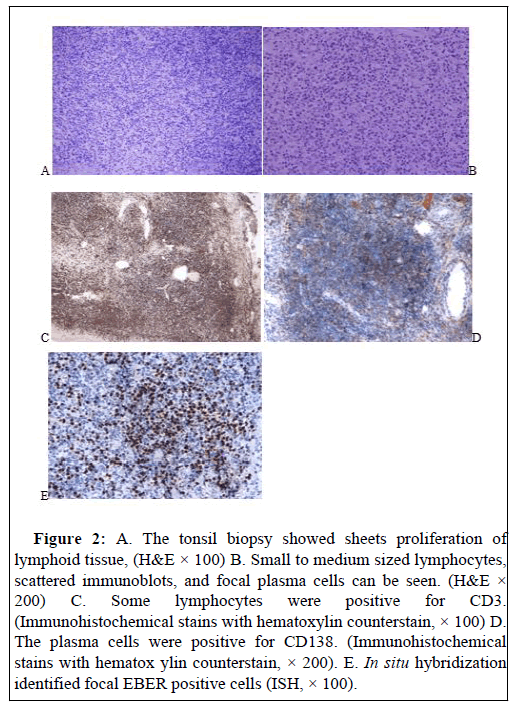
The tonsil biopsy showed sheets proliferation of lymphoid tissue, with small to medium sized lymphocytes, scattered immunoblots, and foci of plasma cells seen in Figure 2. Immunohistochemistry stains showed some cells were positive for CD3, CD5, CD2, CD7, CD20, CD138, negative for TdT, and index of Ki67 was 30%. In situ hybridization (ISH) showed EBER was positive. The findings were consistent with polymorphic PTLD.
Figure 2: A. The tonsil biopsy showed sheets proliferation of lymphoid tissue, (H&E × 100) B. Small to medium sized lymphocytes, scattered immunoblots, and focal plasma cells can be seen. (H&E × 200) C. Some lymphocytes were positive for CD3. (Immunohistochemical stains with hematoxylin counterstain, × 100) D. The plasma cells were positive for CD138. (Immunohistochemical stains with hematox ylin counterstain, × 200). E. In situ hybridization identified focal EBER positive cells (ISH, × 100).
Results and Discussion
Introduction of the PTLD
PTLD was first described by Starzl in 1984 [1]. The 2016 World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues defines PTLD as lymphoid or plasmocytic proliferations of immunosuppression in recipients of solid organ, bone marrow, or Hematopoietic Stem Cell Transplants (HSCT) [2]. PTLDs are disorders with a spectrum ranging from “early” polyclonal proliferations resembling nonspecific plasma cell hyperplasia or infectious mononucleosis to clonal proliferations of B or T/NK cells. The incidence of PTLD after HSCT is approximately 0.8%-4.0%, lower than that after Solid Organ Transplantation (SOT) [3].
The clinicopathologic features of the PTLD
The clinical manifestations of PTLD are variable related to the morphological types, sites, and patient’s general condition; different from those of lymphomas occurred in the general population. Symptoms may be mild, such as fever, mononucleosis-like syndrome, lymphadenopathy, recurrent infections, or severe organ dysfunction.
Several classifications of PTLD have been put forward. According to the 2016 WHO classification, four basic histological types of PTLD have been identified as following: (1) Non-destructive PTLD, including PH, IM-like and florid follicular hyperplasia PTLD which was recently proposed. (2) Polymorphic PTLD. Knoles et al. [4] proposed to separate it into polymorphic B-cell hyperplasia’s and polymorphic B-cell lymphoma, the former composed of a mixture of lymphoid cells with prominent plasmacytoid differentiation and abundant immunoblasts without cytologic atypia, whereas the latter lacking prominent plasmacytoid differentiation with significant cytologic atypia. (3) Monomorphic PTLD, including most B-cell lymphomas and all T/NK neoplasms. (4) Classical Hodgkin Lymphoma.
Tumorigenesis about the PTLD
There are several known risk factors for PTLD, including T-cell depletion strategy, relation between donor and recipient, conditioning regimen, acute GVHD development, mesenchymal stem cell, CMV reactivation, patient baseline (age, disease, past medical history, number of allogeneic HSCT (allo-HSCT), EBV serological mismatch) [3,5].
Most PTLDs are associated with Epstein-Barr virus (EBV) infection, which can cause a range of neoplasms attributable to dysregulated proliferation of EBV-infected B-cells due to dysfunction or suppression of the host immune system after transplantation. Three important factors associated with the pathogenesis of EBV-PTLD were suggested: EBV-encoded oncogenes, host immune suppression, and genetic or epigenetic alternations in the recipient [6].
The treatment of rejection episodes with OKT3 or ATG enhances the PTLD risk in patients who did not receive antibody induction, rejection therapy with OKT3 or ATG adds to the already increased lymphoma risk [7].
HLA matching is also a risk factor in the pathogenesis of PTLD, and HLA-B or HLA-DR mismatches especially seem to be critical. The number of HLA mismatches parallels with an increased risk of PTLD [8]. In some studies, the magnitude of increased risk of PTLD was graded based on conditioning, umbilical-cord transplantation, Transplant from unrelated donors, matched, related donors [9].
Treatment of PTLD
The ECIL-6 guideline of PTLD classifies management strategies for PTLD into three categories: prophylaxis, pre-emptive therapy, and targeted therapy. Treatments for PTLD comprise reduction of immunosuppression, rituximab, chemotherapy, and adoptive immunotherapy [10]. This patient was treated by reduction of immunosuppression, rituximab, and anti-virus therapy. He was well after following up for 11 months.
Conclusion
PTLDs are disorders with a spectrum ranging from “early” polyclonal proliferations to clonal proliferations of B or T/NK cells. Four basic histological types of PTLD have been identified. A case of polymorphous PTLD was introduced and the patient can benefit from rational treatment.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
Supported by grants from the Xinjiang Uygur Autonomous Region Natural Science Foundation (2014211C107).
References
- Starzl TE, Nalesnik MA, Porter KA (1984) Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporine-steroid therapy. Lancet 1: 583-587.
- Swerdlow S, Campo E, Pileri SA (2016) The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood 127: 2375-2390.
- Ayumi F, Ritsuro S (2020) Epstein-barr virus-associated post-transplant lymphoproliferative disorders after hematopoietic stem cell transplantation: Pathogenesis, risk factors and clinical outcomes. Cancers 12: 328.
- Knowles DM, Cesarman E, Chadburn A (1995) Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of post transplantation lymphoproliferative disorders. Blood 85: 552-565.
- Ru YH, Chen J, Wu DP (2018) Epsein-Barr virus post-transplant lymphoproliferative disease (PTLD) after hematopoetic stem cell transplantation. Eur J Haematol 101: 283-290.
- Murata T, Sato Y, Kimura H (2014) Modes of infection and oncogenesis by the Epstein-Barr virus. Rev Med Virol 24: 242-253.
- Opelz G, Dohler B (2003) Lympphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant 4: 222-230.
- Opelz G, Dohler B (2010) Impact of HLA mismatching on incidence of posttransplant at non-Hodgkin lymphoma after kidney transplantation. Transplantation 89: 567-572.
- Abbas F, El Kossi M, Shaheen IS, Sharma A, Halawa A (2020) Post-transplantation lymphoproliferative disorders: Current concepts and future therapeutic approaches. World J Transplant 10: 29-46.
- Styczynski J, Fox CP, Engelhard D, Cordonnier C, Ljungman, P (2016) Management of Epstein-Barr Virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica 101: 803-811.
Citation: Chen D, Jiang , Shen D (2021) Post-Transplant Lymphoproliferative Disorders After Allogeneic Hematopoietic Stem Case Report and Literature Review. J Clin Exp Pathol 11: 397.
Copyright: © 2021 Chen D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1538
- [From(publication date): 0-2021 - Dec 20, 2024]
- Breakdown by view type
- HTML page views: 1077
- PDF downloads: 461