Post-mastectomy seroma: Does Dead Space Obliteration Have a Protective Effect?
Received: 26-Mar-2017 / Accepted Date: 04-May-2017 / Published Date: 10-May-2017
Abstract
Background: Seroma formation after breast surgery could result in significant morbidity and subsequent delay to commence the adjuvant therapy. A prospective randomized study was done to assess the effect of obliterating the axillary dead space by sutures with Flap fixation after Breast cancer surgery either by Modified Radical Mastectomy (MRM) or Conservative Breast Surgery (CBS) prospectively. In addition, Factors predicting the formation of seroma were analyzed and reported.
Methods: A total of 164 patients diagnosed as Breast cancer, they were randomized to have the post mastectomy dead space obliterated (intervention group) or standard wound closure (control group) following either MRM or CBS. Those had immediate reconstruction were excluded from the study. Drains were routinely left in place until the preceding 48-hour output was < 30 milliliters/day. The duration of the drains left in place and the incidence of seroma formation were reported. A multivariate analysis for the potential factors associated with seroma formation was done.
Results: Fifty-eight (n=58) patients were assigned to the treatment group and 106 (n=106) to the control group. MRM was performed on 105 patients (64%) and CBS on 59 (36%). Ten of the 58 patients (17.2%) in the intervention group developed a seroma in comparison to 33 of the 106 control patients (31.1%) (P=0.03). There was a significant reduction in the duration of suction drain in situ with obliteration of the dead space (P=0.001). No statistically significant differences were observed between intervention and control groups with respect to patient and pathological parameters or the incidence of other wound complications. Multivariate analysis revealed that Significant risk factors for seroma formation were Diabetes Mellitus (DM) (P=0.01), neoadjuvant CTH (P=0.019), number of retrieved L node (P=0.019), and dead space obliteration (P=0.04).
Conclusion: On multivariate analysis, the most significant factors affecting seroma formation were DM, neoadjuvant CTH, number of retrieved L node and Dead Space Obliteration. Dead Space Obliteration following breast cancer surgery is a simple technique that reduces the time of suction tubal drainage, and incidence of seroma formation.
Keywords: Breast cancer; Mastectomy; Dead space; Seroma
35975Introduction
Breast cancer is the most common non-cutaneous cancer diagnosed in women, accounting for more than 10% of the new cancer diagnosis each year. Breast cancer is the second most common cause of death from cancer among women [1].
The surgeon role in management of operable breast cancer, have many options including, breast conserving surgery and different types of mastectomies with or without reconstruction [2]. Several postoperative complications were reported such as necrosis of skin flaps, wound dehiscence, hematoma, seroma formation, and surgical site infection [3]. Among them, seroma, a clinically evident subcutaneous collection of serous fluid within a surgical cavity, is the most frequent post-operative complication after breast cancer surgery, developing in approximately 30% of cases [4].
Seroma formation could be superimposed by delayed wound healing, infection, skin flap necrosis and patient discomfort due to frequent seroma aspirations and repeated visits to the outpatient clinic to deal with seroma and its sequelae [5].
Pathogenesis of seroma formation is poorly understood. Numerous factors take part in the formation of seroma. Lee K-T et al. suggested that dead space formation could be one of the leading mechanisms of postoperative seroma formation. Prolonged postoperative leakage of disrupted lymphatics and minute blood vessels into the dead space mostly is the main contributing factor in seroma formation [6].
Type of operation [7], use of electrocautery [8], elderly patient, obesity and longer and higher drain outputs [9], are associated with increased risk, however, Porter et al. reported that the age of the patient, neoadjuvant chemotherapy, total number of retrieved axillary lymph nodes , the number of positive axillary lymph nodes, and the tumor size, were not [7].
Srivastava et al. reported that the surgical devices (laser scalpel, argon diathermy, and ultrasonic scalpel) have not proven to be superior to electrocautery in seroma reduction [10].
Seroma formation after axillary dissection for breast cancer is difficult to be avoided, but it can be minimized by mechanical closure of the dead space [11]. The trial performed by Almond et al. on flap fixation compared to closed suction drainage showed no difference in seroma rates, but patients without drains were discharged earlier [12]. Sakkary et al. reported that the amount of fluid drained was significantly less in the flap fixation group [13], and Ten Wolde B, et al. reported that quilting of the skin flaps led to less seroma formation, and fewer Surgical Site Infections (SSIs) [14].
The purpose of our study was to evaluate the effect of postmastectomy dead space obliteration (DSO) on seroma formation by suturing skin flaps to underlying muscles.
Patients and Methods
Patients
Our study included 164 women with operable breast cancer, who were admitted to three different tertiary hospitals (South Egypt Cancer Institute , Assuit University, General Surgery Department, Assuit University and General Surgery Department, South Valley University), from Jan 2015 and Dec 2016.
Exclusion criteria
Patients having additional procedures like pectoralis major muscle excision due to locally advanced tumor or those having immediate breast reconstruction were not involved in the study.
Type of the study
Prospective randomized study. Randomization was done by coin flip in every case by a nurse not included in the study.
Preoperative work-up
Preoperative comprehensive triple assessment was performed for all cases. History taking including comorbidities (DM, HTN, BMI, receiving neo-adjuvant chemotherapy), physical examination and routine laboratory investigations in the form of complete blood count, liver and kidney function tests, and evaluation of PT and INR were done to all patients. Radiological investigations in the form of mammography and breast ultrasound and metastatic work up were done as required. Histopathological diagnosis after Tru-Cut or open biopsy was done for all cases.
All patients signed an informed consent after explanations of the technique to be used. All the patients were fasting for six hours before the procedure. Prophylactic antibiotic (ceftriaxone 1 gm.) was given to all patients at the time of induction of general anesthesia. The antibiotics were continued for 48 hours and then switched to oral antibiotics if needed.
Intervention
Patients were randomly divided into two groups. Intervention group, Fifty eight (n=58) patients received dead space obliteration at the end of the procedure, and Standard group in which 106 patients had standard wound closure.
Patients had two types of surgical procedure; namely, modified radical mastectomy and breast conservative surgery. Skin flap dissection and excision of the breast tissue en bloc with pectoral fascia, and the proper dissection of axillary lymph nodes were performed in all cases.
At the end of surgery, 14F suction drain was inserted in the axilla. A second suction drain was inserted when mastectomy was performed. The surgical wounds were sutured and covered in a standard fashion.
The study got approval by south Egypt Cancer institute review board, and detailed informed consent was taken from all patients.
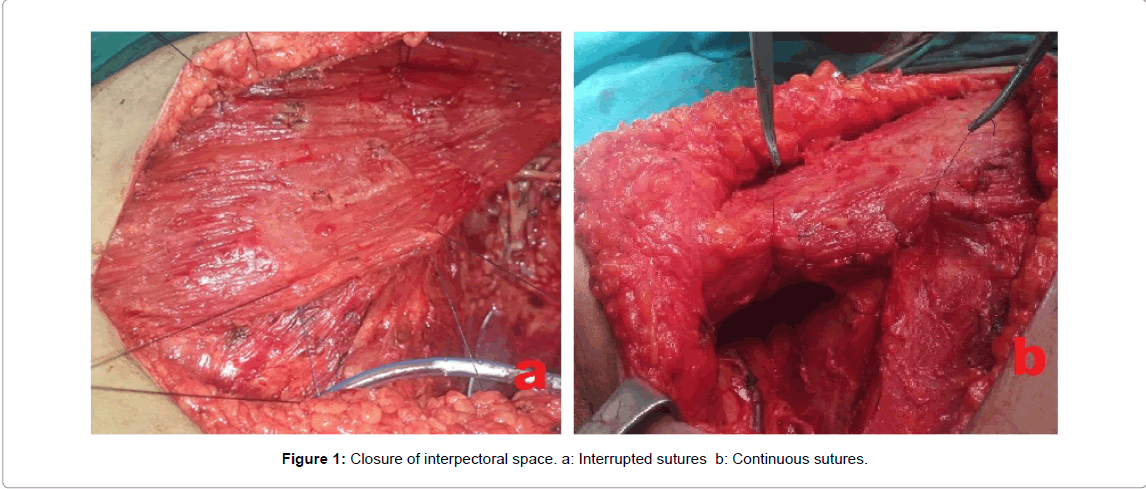
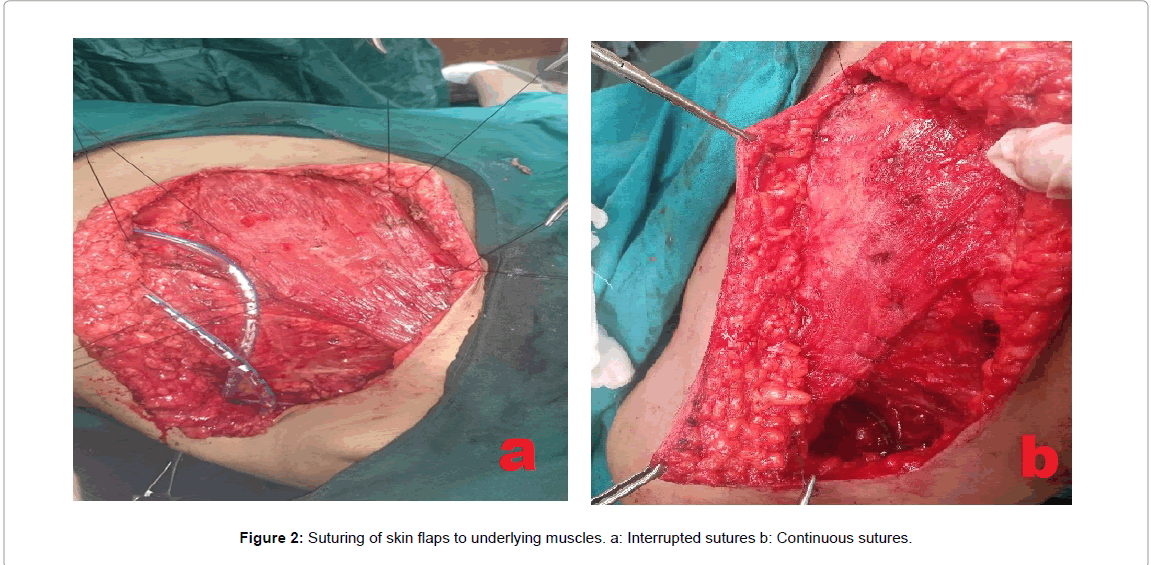
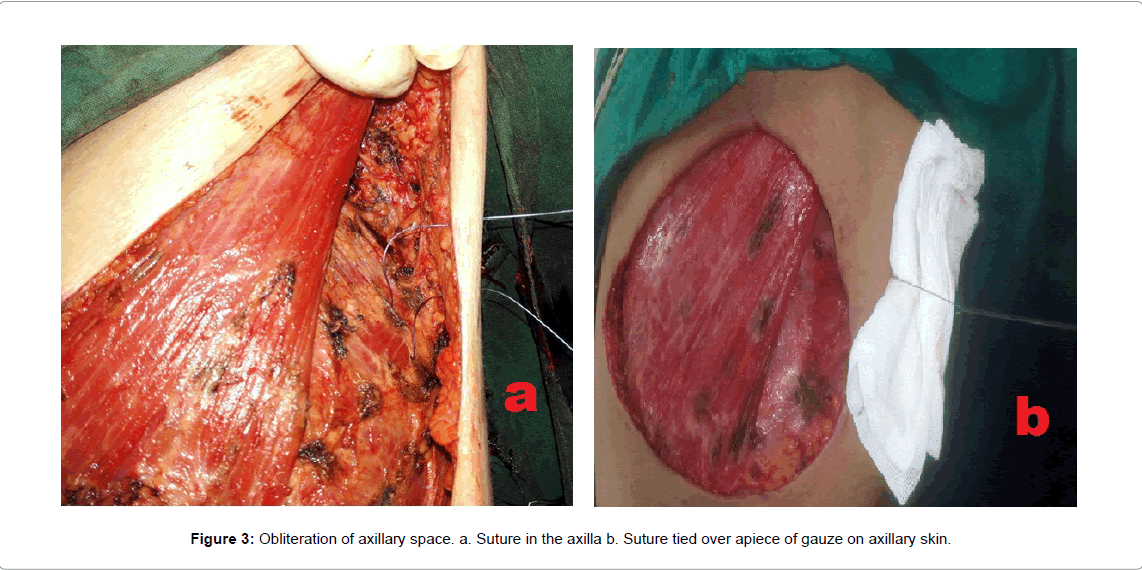
Operative technique for intervention group
In 58 patients, Obliteration of post-mastectomy dead space was done by a buttress sutures obliterating the axillary dead space (Figures 1-3) and by continuous or simple interrupted 2/0 Vicryl sutures (Figures 1 and 2) anchoring the mastectomy skin flaps to pectoralis major and the lateral chest wall muscles, also interrupted sutures were taken between pectoralis major and minor muscles.
Operative technique for standard group
In 106 patients, skin was closed by standard technique.
Post-operative follow-up
Drains were routinely left in place until the preceding 48 hours output was
Patient characteristics, operative time, type of intervention, pathological parameters including the pathological subtype, tumor grade, tumor size, hormonal receptors status, the total number of retrieved axillary lymph nodes, the number of positive axillary nodes as well as duration of the drains left in place and the incidence of seroma formation were recorded.
Statistics: statistical package SPSS version 19 (SPSS, Chicago, IL, USA) was used to analyze data. Chi square test used for categorical data (without Yates’ correction) and the Student’s t-test used for continuous data. Data presented as numbers, percentage, and arithmetic mean (M) and standard deviation (SD). All P values refer to two-sided tests, and were considered statistically significant if P value ≤ 0.05.
Results
One hundred and sixty four patients with operable breast cancer were operated in three different tertiary hospitals during the study period. There were 105 patients (64%) who underwent MRM and 59 patients (36%) who underwent CBS. 58 patients (35.4%) had fixing sutures to obliterate the dead space (Intervention group), and 106 patients (64.6%) had a standard wound closure (Standard group).
The 2 groups has no statistically significant difference regarding age (P=0.22), body mass index (P=0.14), nor comorbidity (P>0.1). The type of surgery performed, and tumor related variables (tumor size, histopathlogical type, grade, hormonal receptor status and lympho- Vascular Invasion (LVI) were statistically similar as summarized in Table 1.
The operative outcomes between the study groups are summarized in Table 1. The mean operative time was not statistically different in the 2 groups (80 minutes’ vs.70 minutes, (P value 0 .43). The mean duration of the suction left in place was much less in intervention group (12.79 ± 6.4 vs. 20.1 ± 6.6 days) (P value 0.001). The mean hospital stay was comparable in both groups (4.5 days vs. 5.6 days) (P value 0.78).
| Characteristics: | Standard Group | Obliteration Group | P value | |
| Age | 51.32 ± 11.3 | 49.91 ± 10.2 | 0.22 | |
| BMI | 29.95 ± 6.3 | 28.77 ± 4.8 | 0.14 | |
| DM | 30 (28.3%) | 12 (20.7%) | 0.19 | |
| HTN | 10 (9.4%) | 4 (6.9) | 0.4 | |
| Neoadjuvant CTH | 54 (50.9%) | 33 (56.9%) | 0.28 | |
| Operative time in minutes | 70 ± 6.5 | 80 ± 5.3 | 0.43 | |
|
12 (11.3%) 5 (8.6%) 0.86 | |||
| 2-5 | 65 (61.3%) | 37 (63.8%) | ||
| 5 | 29 (27.4%) | 16 (27.6%) | ||
|
|
92 (86.8%) | 49 (84.5%) | 0.87 |
|
6 (5.7%) | 5 (6.8%) | ||
|
5 (4.7%) | 2 (3.4%) | ||
|
3 (2.8%) | 2 (3.4%) | ||
| Tumor Grade G2 100 (90.7%) 46 (79.4%) |
G1 | 5 (4.7%) | 5 (6.6%) | 0.39 |
| G3 | 5 (4.7%) | 3 (5.1%) | ||
| ER + | 65 (61.3%) | 31 (53.4%) | 0.37 | |
| PR+ | 67 (63.2%) | 36 (62.1%) | 0.43 | |
| Operation Type | CBS | 66 (62.3%) | 39 (67.2%) | 0.32 |
| MRM | 40 (37.7%) | 19 (32.8) | ||
| No. of Retrieved LN | 18.52 ± 5.9 | 17.89 ± 5.3 | 0.96 | |
| Positive LN | 5.34 ± 2.5 | 5.88 ± 3.2 | 0.17 | |
| LVI | 22 (20.8%) | 13 (22.4%) | 0.47 | |
| Duration of drain in situ | 20.1 ± 6.6 | 12.79 ± 6.4 | 0.001 | |
| Complications | 28 (26.4%) | 12 (20.7%) | 0.12 | |
| Seroma | 33 (31.1%) | 10 (17.2%) | 0.03 | |
| Hospital stay in days (Mean) | 5.6 ± 2.1 | 4.5 ± 1.3 | 0.78 | |
Table 1: Demographic, clinical, and operative outcomes of the study groups.
Ten of the 58 patients (17.2%) in the Intervention Group developed a seroma in comparison to 33 of the 106 patients (31.1%) in the standard group. (P=0.03). In multivariate analysis (Table 2), the main factors that significantly predicts seroma formation were number of retrieved LN, dead space obliteration, DM and neoadjuvant radiotherapy, among them, the dead space obliteration is modifiable procedure.
| Characteristics | Coefficient | S.E. | Mean ± SD | 95.0% C.I. | P Value | |
| Age | -0.574 | 0.510 | 50.8 ± 11.4 | (0.207-1.528) | 0.26 | |
| Tumor Size (>5 cm) | -0.507 | 0.333 | - | (0.313-1.158) | 0.13 | |
| No of Retrieved LN | -0.516 | 0.583 | 18.4 ± 5.2 | (0.190 -1.870) | 0.37 | |
| Positive nodes(yes/No) | .037 | 0.072 | - | (0.901-1.194) | 0.08 | |
| BMI | -0.447 | 0.429 | 29.4 ± 5.9 | (0.276-1.483) | 0.093 | |
| HTN (yes/no) | 0.055 | 0.435 | - | (0.450-2.480) | 0.89 | |
| DS Obliteration (yes/no) | -0.881 | 0.462 | - | (0.168-1.024) | 0.04 | |
| DM(Yes/No) | 1.131 | 0.437 | - | (1.317-7.292) | 0.08 | |
| Neoadjuvant CTH (yes/No) |
0.537 | 0.416 | - | (0.757-3.867) | 0.019 | |
Table 2: Multivariate analysis (logistic regression) of Variables predicting postoperative seroma formation.
Discussion
Seroma is the most frequent feared post-operative complication after breast cancer surgery, developing approximately in around 30% of cases [4].
Seroma formation has a negative impact on patient recovery, initiation of further therapy and might be associated with ipsilateral arm lymphedema [15,7]. Variable incidence of post mastectomy seroma has been reported as 2.5-51% in different series [16,17].
Various studies have attempted to reduce seroma formation in order to improve surgical outcome and finally reduce postoperative morbidity.
In a trial to meaningfully decrease the incidence of this uncomfortable complication, many operative modification designed, the use of closed suction drains has been the initial step and then got widely adopted form [18].
Surgical techniques that had been designed include ipsilateral shoulder immobilization, suction drainage, certain surgical instruments, and post mastectomy dead space obliteration [19].
Different methods were used obliterate the dead space, Pressure wound dressing has no effect on the reducing the amount of the seroma, also different chemical methods such as fibrin glue, tissue adhesive and sclerotherapy agents failed to prove considerable impact [11]. Tetracycline is also tried for dead space obliteration but it has been abandoned, because of inducing pain [20].
There have been many prospective controlled trials that demonstrate the safety and efficacy of post mastectomy flap fixation for patients with breast cancer in decreasing the seroma formation [21-23]. In our study, the reported incidence of seroma was 26.2% (n=43) which is consistent in range with most of the previous studies [4].
In our study, physical examination was the main tool to diagnose seroma. Ultrasonography not used because of its cost inefficiency as well as seroma detected only by radiology doesn’t need surgical intervention. It is the same concept of Ozaslan et al. in his similar study [24].
In the present study we found the incidence of seroma formation in the interventional group was significantly lower 17.2% versus 31.1% in the control group emphasizing our hypothesis. This was consistent with prior studies [21-23].
The DSO did not increase neither the operative time nor the postoperative complications, emphasizing its safety after breast surgery and adding more advantages besides decreasing the incidence of seroma [21,22].
In our study, obliteration of dead space has a protective effect against seroma formation that confirmed by multivariate analysis , this agrees with Anjani et al. study that identified suture fixation of skin flap to underlying pectoral muscles significantly and dramatically resulted in seroma prevention, probably by reducing the dead space under the flaps [9].
Also, the efficacy of dead space reduction as a preventive technique was also confirmed previously by met–analysis of van Bemmel et al. [11]. Faisal et al. also, reported significantly reduction of the total amount of seroma formation after axillary exclusion [25].
Factors affecting seroma formation following breast cancer surgery are widely discussed in the literature. Patient related factors included patient age, obesity, comorbidity, and breast volume. Tumor related factors such as tumor size, number and presence of malignant nodes in the axillary region were documented. Also surgical factors like surgical instrument, number of dissected nodes, post mastectomy dead space, closed suction drain, early shoulder exercise, and the use of certain drugs, i.e. tamoxifen and heparin have been reported [26-29].
On multivariate analysis, the factors significantly affecting seroma formation were DM, neoadjuvant CTH, number of retrieved lymph nodes, and Dead Space Obliteration.
Similar to our results, previous studies reported that DM [30], and neoadjuvant chemotherapy [31] were associated with development of post mastectomy seroma and DSO [28-30] has a protective effect. In contrast to our results, previous studies failed to prove a constant association between seroma and number of retrieved axillary lymph nodes.
Conclusion
Dead Space Obliteration following breast cancer surgery is a simple technique that reduces the time of suction tubal drainage, and has a protective effect against seroma formation.
References
- Michele A, Gadd MD (2014) Screening For Breast Cancer.In: Current surgical therapy.(11th edn), Cameron JL, Cameron AM (editors), Elsevier Saunders: USA, pp: 568-571.
- Baker MK (2014) Breast Cancer: Surgical Management. In: Current surgical therapy. (11th edn), by Cameron JL, Cameron AM(editors), Elsevier Saunders: USA, pp: 568-571.
- Aitken DR, Minton JP (1983) Complications associated with mastectomy. SurgClin North Am 63: 1331-1352.
- Douay N, Akerman G, Clément D, Malartic C, Morel O, et al. (2008)Seroma after axillary lymph node dissection in breast cancer. GynecolObstetFertil36:130-135.
- Tadych K, Donegan WL (1987) Postmastectomyseromas and wound drainage. Surg Gynecol Obstet 165: 483-487.
- Lee K-T, Mun GH(2015) Fibrin Sealants and quilting suture for prevention of seroma formation following latissimusdorsi muscle harvest: a systematic review and meta-analysis. Aesth Plast Surg 39: 399-409.
- Gonzalez EA, Saltzstein EC, Riedner CS, Nelson BK (2009)Seroma formation following breast cancer surgery. Breast J 9: 385-388.
- Porter KA, O'Connor S, Rimm E, Lopez M (1998) Electrocautery as a factor in seroma formation following mastectomy. Am J Surg 176: 8-11.
- Anjani J, Amit O, Kuber S, Achal G (2016) Factors affecting seroma formation after modified radical mastectomy in patients of carcinoma breast: A prospective study. IJSS Journal of Surgery 21-5.
- Srivastava V, Basu S, Shukla VK (2012) Seroma formation after breast cancer surgery: what we have learned in the last two decades. J Breast Cancer 15: 373-380.
- vanBemmel AJ, van de Velde CJ, Schmitz RF, Liefers GJ (2011) Prevention of seroma formation after axillary dissection in breast cancer: a systematic review. Eur J Surg Oncol 37: 829-835.
- Almond LM, Khodaverdi L, Kumar B, Coveney EC (2010) Flap anchoring following primary breast cancer surgery facilitates early hospital discharge and reduces costs. Breast Care 5: 97-101.
- Sakkary MA (2012) The value of mastectomy flap fixation in reducing fluid drainage and seroma formation in breast cancer patients. World J Surg Oncol 10: 8.
- Ten Wolde B, van den Wildenberg FJ, Keemers-Gels ME, Polat F, Strobbe LJ (2014) Quilting prevents seroma formation following breast cancer surgery: closing the dead space by quilting prevents seroma following axillary lymph node dissection and mastectomy. Ann Surg Oncol 21: 802-807.
- Shamley DR, Barker K, Simonite V, Beardshaw A (2005) Delayed versus immediate exercises following surgery for breast cancer: a systematic review. Breast Cancer Research and Treatment 90: 263-271.
- Barwell J, Campbell L, Watkins RM, Teasdale C (1997) How long should suction drains stay in after breast surgery with axillary dissection? Ann R Coll Surg Engl 79: 435-437.
- Bryant M, Baum M (1987) Postoperative seroma following mastectomy and axillary dissection. Br J Surg 74: 1187.
- Aitken DR, Hunsaker R, James AG (1984) Prevention of seromas following mastectomy and axillary dissection. Surg Gynecol Obstet 158: 327-330.
- Purkayastha J, Hazarika S, Deo SV, Kar M, Shukla NK (2004) Post-mastectomy chylous fistula: anatomical and clinical implications. Clin Anat 17: 413-415.
- Ozaslan C, Yilmaz KB, Dogan L, Atalay C, Altinok M (2010) Effect of mechanical closure of dead space on seroma formation in modified radical mastectomy. Turk J Med Sci 40: 7515.
- Kontos M, Kothari A, Hamed H (2008) Effect of harmonic scalpel on seroma formation following surgery for breast cancer: a prospective randomized study. J BUON 13: 223-230.
- Schuijtvlot M, Sahu AN Cawthorn SJ (2002)Aprospective audit of the use of a buttress suture to reduce seroma formation following axillary node dissection without drains. Breast 11: 94-96.
- Khater A, Elnahas W, Roshdy S, Farouk O, Senbel A, et al. (2015) Evaluation of the Quilting Technique for Reduction of PostmastectomySeroma: A Randomized Controlled Study. Int J Breast Cancer 2015: 287398.
- Hashemi E, Kaviani A, Najafi M, Ebrahimi M, Hooshmand H, et al. (2004) Seroma formation after surgery for breast cancer. World J Surg Oncol 2: 44.
- Faisal M, Abu-Elela S, Mostafa W, and Antar O (2016) Efficacy of axillary exclusion on seroma formation after modified radical mastectomy. World J Surg Oncol 14: 39.
- Pogson CJ, Adwani A, Ebbs SR (2003) Seroma following breast cancer surgery. Eur J Surg Oncol 29: 711-717.
- Kuroi K, Shimozuma K, Taguchi T, Imai H, Yamashiro H, et al. (2006) Evidence-based risk factors for seroma formation in breast surgery. Jpn J Clin Oncol 36: 197-206.
- Kumar S, Lal B, Misra MC (1995) Post-mastectomy seroma: a new look into the aetiology of an old problem. J R Coll Surg Edinb 40: 292-294.
- Petrek JA, Peters MM, Nori S, Knauer C, Kinne DW, et al. (1990) Axillary lymphadenectomy. A prospective randomised trial of 13 factors influencing early or delayed arm mobilisation. Arch Surg 125: 378-383.
- Say CC, Donegan W (1974) Abiostatistical evaluation of complications from mastectomy. Surg Gynecol Obstet 138: 370-376.
- Browse DJ, Goble D, Jones PA (1996) Axillary node clearance: who wants to immobilize the shoulder? Eur J Surg Oncol 22: 569-570.
Citation: Jabir MA, Taha A, Shehata MR, Sayed MM, Yehia A (2017) Postmastectomy seroma: Does Dead Space Obliteration Have a Protective Effect?. Breast Can Curr Res 3: 119
Copyright: ©2017 Jabir MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 6601
- [From(publication date): 0-2017 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 5595
- PDF downloads: 1006