Postencephalitic Parkinsonism in a Patient with Mumps Infection: A Case Report
Received: 08-May-2014 / Accepted Date: 25-Jun-2014 / Published Date: 03-Jul-2014
Abstract
In 1917, Von Economo described a disease named "Encephalitis Lethargica" which affected a large number of patients across the world in an epidemic between 1916 and 1927. It's spread paralleled with the spread of the pandemic influenza, but Von Economo and the contemporary authors did not believe it to be a post-influenzal sequela. Now parkinsonism is a well-known complication of acute infectious encephalitis. There are many potential infectious causes of parkinsonism reported in literature. In India, postencephalitic parkinsonism is commonly due to Japanese Encephalitis. Neurologic manifestations reported in Mumps include meningitis, encephalitis, deafness, facial neuritis, cerebellar ataxia, hydrocephalus, transverse myelitis and polyradiculitis. We describe a case of young girl who had Mumps parotitis and encephalitis and then subsequently developed parkinsonism as a sequela. Mumps was confirmed by positive serology and basal ganglia lesions were demonstrated by magnetic resonance imaging of the brain. To the best of our knowledge, this is the first case of postencephalitic parkinsonism following Mumps infection from India.
Keywords: Mumps, Parkinsonism
411892Introduction
In 1917, Von Economo described a disease named “Encephalitis Lethargica”. The acute stage of this disease was characterized by somnolence, oculomotor palsy, myoclonus and masked face. During recovery, the most common manifestations were parkinsonism, oculogyric crisis and central respiratory irregularities [1]. Histopathology showed varying degrees of vascular congestion and perivascular lymphocytic cuffing and degeneration in the basal ganglia and the midbrain, especially of the oculomotor nuclei [2]. Encephalitis Lethargica affected a large number of patients across the world in an epidemic between 1916 and 1927. Although it coincided with the spread of the pandemic influenza, current opinion is divided in it being a post-influenzal disease [3]. Currently, parkinsonism is a known sequela to acute infectious encephalitis and has been reported with viral infections such as Human Immunodeficiency virus (HIV), Coxsackie B virus types 2 and 4, Epstein-Barr virus (EBV), Herpes Simplex virus, Japanese Encephalitis (JE) virus, Measles virus, West Nile virus and Western equine encephalitis virus [4]. In India, postencephalitic parkinsonism occurs commonly in Japanese encephalitis patients who survive and magnetic resonance imaging (MRI) of the brain shows characteristic involvement of the thalamus, the basal ganglia and the midbrain [5].
Case Report
A 16-year-old girl had fever with parotid swelling, which resolved over seven days. On the fifth day of her illness, she had headache and giddiness. She vomited immediately after drinking milk and became unresponsive. Her body had gone pale and cold and she had stridor. She was rushed to a nearby hospital where she was immediately intubated and put on ventilator. She regained consciousness in a day and was weaned from ventilator by three days. She was discharged from the hospital with symptomatic treatment. She was apparently recovering from her illness. After two weeks, parents noticed that the girl was becoming weak, performed motor tasks slowly, had difficulty in getting up from bed and had stiffness of limbs. She developed postural instability and had a tendency to fall while walking. Subsequently she became mute. She could comprehend her parent’s commands and could communicate with some gestures. She smiled and cried inappropriately. The girl’s general examination was normal. On nervous system examination, she was conscious but mute, had normal comprehension; extraocular movements were full but she had hypometric saccades and apraxia of eyelid opening; there was no nystagmus and funduscopic examination was normal; her mouth remained open at times with drooling of saliva. She had bradykinesia, lead pipe rigidity of limbs and postural instability. There was no tremor or any other involuntary movement. Her deep tendon reflexes were exaggerated and plantar response was flexor. Examination of other systems was normal.
The parents said that the girl was regularly vaccinated but they did not have the records and they did not remember the names. There was no history of neuropsychiatric illness in the family. There was no history of toxin exposure or use of drugs that could cause parkinsonism. After few days of the girl’s illness, her younger sister also developed parotid swelling but there were no further complications.
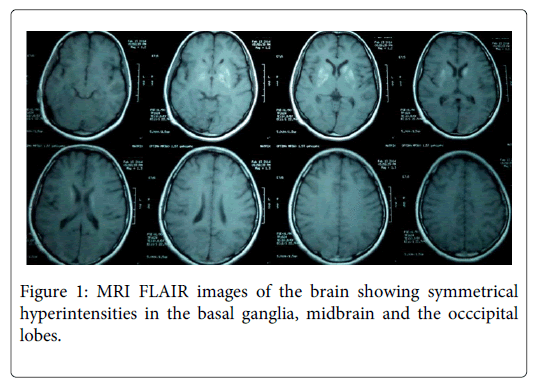
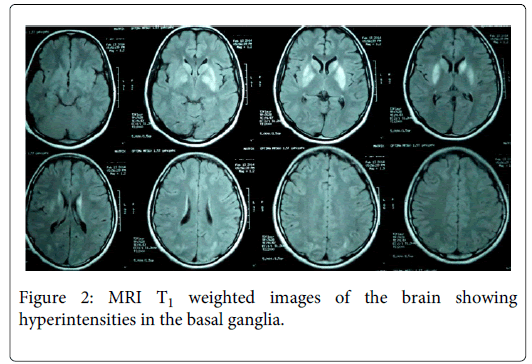
Laboratory investigations including complete hemogram, ESR, blood sugar, serum electrolytes, renal function tests, liver function tests, TSH, HIV, VDRL, creatine phosphokinase and serum ceruloplasmin were normal. Slit-lamp examination of the eyes did not show Kayser–Fleischer ring. MRI of the brain showed bilaterally symmetrical hyperintensities in the caudate, putamen, midbrain and the occcipital lobes, with sparing of globus pallidum and thalamus on T2 weighted and fluid attenuated inversion recovery (FLAIR) images (Figure 1). The basal ganglia lesions appeared Hyperintense even in T1 weighted images (Figure 2). Cerebrospinal fluid (CSF) analysis showed cells 3/mm3, protein 13 mg/dL and sugar 67 mg/dL and ELISA for Japanese encephalitis (JE) virus was negative. Mumps specific antibodies in serum were demonstrated by enzyme immunoassay - IgM index 2.13 (normal < 0.79) and IgG index 2.96 (normal < 0.90).
The girl was treated with syndopa (levodopa 110 mg, carbidopa 10 mg) thrice daily and trihexyphenidyl hydrochloride (2 mg) twice daily. She started showing improvement in rigidity. At two months follow up, her speech had returned, rigidity had decreased and there was no apraxia of eyelid opening. However, she still had postural instability and developed dyskinesia in one upper limb. Unfortunately we could not get follow up MRI brain due to financial constraints.
Discussion
Mumps virus belongs to the family Paramyxoviridae. It has an incubation period ranging from 2-4 weeks and typically causes a prodrome of low-grade fever, malaise and parotitis which resolves over seven days. Neurologic complications are the most common extrasalivary gland manifestations and usually start developing by one week. They include meningitis commonly and encephalitis, deafness, facial neuritis, cerebellar ataxia, hydrocephalus, transverse myelitis and polyradiculitis rarely. A recent case-control study showed significant association between mumps infection and later development of Parkinson’s disease [6]. But there has been no case report of acute to subacute parkinsonism following a bout of mumps encephalitis.
Recently, a study on the spectrum of movement disorders in encephalitis from India was published. Patients with encephalitis were categorized into JE, dengue, herpes simplex, miscellaneous (mumps and EBV) and nonspecific encephalitis. Movement disorders (which included parkinsonism and dystonia) were reported in JE, nonspecific encephalitis and dengue [5]. Although the authors concluded that movement disorder can occur in JE, dengue, EBV and mumps, there was no mention of parkinsonism following mumps in their study. In our region (North-East India), parkinsonism is a common sequela of encephalitis due to JE virus [7].
Our case had clinical features suggestive of encephalitis complicating mumps parotitis. Apraxia of eyelid opening, as seen in our case, has been reported as a postencephalitic sequela [8]. Owing to the development of stridor probably due to aspiration and documentation of decreased oxygen saturation by pulse oxymetry, she was intubated and put on mechanical ventilator. However she regained consciousness within a day and was weaned from ventilator within three days. Hypoxia induced brain injury (HI-BI) is a known cause of akinetic rigid syndrome, but the development of clinical features in young persons are usually delayed by months. Younger patients are more likely to develop dystonia. Moreover, parkinsonism following HI-BI is usually associated with necrosis of the globus pallidum [9]. Our patient had relative sparing of the globus pallidum on MRI. Early recovery from hypoxia followed by early development of parkinsonism and sparing of the globus pallidus makes HI-BI an unlikely cause of parkinsonism in our case. There was no history of exposure to any toxins or drugs (like antiemetic metoclopramide) that could cause parkinsonism.
In patients with postencephalitic parkinsonism, MRI abnormalities have been seen in the thalamus, globus pallidus, putamen, caudate, substantia nigra, and brainstem. The MRI signal changes are hyperintense in T2 and FLAIR and hypo- to isointense in T1 [5]. Our patient had bilateral symmetric hyperintensities in the caudate, putamen and midbrain on T2 weighted images. The basal ganglia appeared hyperintense in T1 weighted images, which is unusual following viral encephalitis. However, recently a case of postencephalitic parkinsonism with similar MRI features was reported to be caused by EBV [10].
Confirmation of mumps in our case was obtained by demonstration of positive mumps-IgM and IgG antibodies by enzyme immunoassays. As the patient presented to us late after the development of parkinsonism, we could not document the rise of mumps-IgG antibodies in acute and convalescent sera. Due to financial constraints and due to late presentation, we could not obtain PCR for mumps virus in the cerebrospinal fluid. But mumps encephalitis has been diagnosed based on clinical profile and positive serology [11]. Evidence of encephalitis was from history. CSF analysis was normal but it was done quite late in our case (the girl had presented to us after about 3 weeks of her illness). Parotitis can be caused by viruses other than mumps such as Influenza, EBV, Coxsackievirus A, Cytomegalovirus and HIV. We could not get the necessary tests to rule out EBV infection in our case due to financial constraints. However, with the typical presentation of unilateral parotid swelling resolving over a period of seven days in our country, transmission of infection to the younger sister in the same household, doubtful vaccination history and seropositivity, it will be hard to refute the diagnosis of mumps.
Neurotropism of mumps virus is well known and it has been suggested that early onset mumps encephalitis represents direct damage to neurons as a result of viral invasion, whereas late-onset disease is postinfectious demyelinating process related to the host response to infection [12-14]. Autoimmune pathogenesis has also been proposed in postencephalitic parkinsonism based on negative virological tests, oligoclonal banding in cerebrospinal fluid, perivenular lymphocyte aggregations on histological examination and detection of anti-basal ganglia neuronal antibodies [15]. Our case had relatively early onset of neurologic manifestations following onset of mumps parotitis and the development of parkinsonism as a sequela could be a direct effect of the virus, but this requires further studies for confirmation.
Conclusion
Our case report adds Mumps virus to the growing list of infectious causes of parkinsonism. The role of its pathogenicity in this context is yet to be explored.
References
- Von Economo C (1917) Encephalitis lethargica. Wiener KlinischeWochenschrift 30: 581–583.
- Buzzard EF, Greenfield JG (1919) Lethargic encephalitis: its sequelae and morbid anatomy. Brain. 42: 305–338
- Mortimer PP (2009) Was encephalitis lethargica a post-influenzal or some other phenomenon? Time to re-examine the problem. Epidemiology and Infection, 137: 449-455.
- Nisipeanu P, Paleacu D, Korczyn AD. Infectious and postinfectious parkinsonism. In Watts RL, Koller WC (2004) Movement Disorders: Neurologic Principles and Practice, 2nd ed. New York: McGraw Hill, pp. 373-382.
- Misra UK, Kalita J (2010) Spectrum of movement disorders in encephalitis. J Neurol 257:2052-2058.
- Vlajinac H, Dzoljic E, Maksimovic J, Marinkovic J, Sipetic S, et al. (2013) Infections as a risk factor for Parkinson's disease: a case-control study. Int. J. Neurosci. 123: 329-332.
- Basumatary LJ, Raja D, Bhuyan D, Das M, Goswami M, Kayal AK (2013) Clinical and radiological spectrum of Japanese encephalitis. J Neurol Sci. 15;325(1-2):15-21.
- Tan MH, Lim E (2004) Post-encephalitic segmental dystonia with apraxia of eyelid opening. Parkinsonism RelatDisord 10: 173-175.
- Lu-Emerson C,Khot S (2010) Neurological sequelae of hypoxic-ischemic brain injury. NeuroRehabilitation. 26:35-45.
- Kumar R S, Kuruvilla A (2009) Teaching NeuroImages: Acute hemorrhagic leukoencephalitis after mumps. Neurology 17: e98.
- Espay AJ, Henderson KK (2011) Postencephalitic parkinsonism and basal ganglia necrosis due to Epstein-Barr virus infection. Neurology 76:1529-1530.
- Bang HO, Bang J (1943) Involvement of the central nervous system in mumps. Acta Med Scand. 113:487.
- Donohue WL, Playfair FD, Whitaker L (1955) Mumps encephalitis. J Pediatr 47:395.
- Taylor FB, Toreson WE (1963) Primary mumps meningo-encephalitis. Arch Intern Med 112:216.
- Dale RC, Church AJ, Surtees RAH, Lees AJ, Adcock JE, et al. (2004) Encephalitis lethargica syndrome: 20 new cases and evidence of basal ganglia autoimmunity. Brain. 127:21–33.
Citation: Suvorit SB, Pawan S, Marami D, Ashok KK (2014) Postencephalitic Parkinsonism in a Patient with Mumps Infection: A Case Report. J Neuroinfect Dis 5:162
Copyright: © 2014 Subhas SB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 14291
- [From(publication date): 6-2014 - Dec 21, 2024]
- Breakdown by view type
- HTML page views: 9931
- PDF downloads: 4360