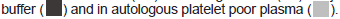Possible Role of P-Selectin Adhesion in Long-COVID: A Comparative Analysis of a Long-COVID Case vs. an Asymptomatic Post-COVID Case
Received: 03-Mar-2022 / Manuscript No. JIDT-22-58151 / Editor assigned: 07-Mar-2022 / PreQC No. JIDT-22-58151(PQ) / Reviewed: 21-Mar-2022 / QC No. JIDT-22-58151 / Revised: 28-Mar-2022 / Manuscript No. JIDT-22-58151(R) / Published Date: 04-Apr-2022
Abstract
Background: Long-term outcomes of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are now recognized as an emerging public health challenge-a condition termed Long-COVID. The pathophysiology of Long-COVID remains to be established. Functional P-selectin activity, implicated in COVID-19 sequelae, was measured between two convalescent COVID-19 subjects, one with (Long-COVID subject) and another without Long-COVID symptoms.
Methods: Flow adhesion of whole blood or isolated white blood cells to P-selectin (FA-WB-Psel and FA-WBC-Psel) was measured using a standardized microfluidics clinical assay; impedance aggregometry with a collagen agonist was measured using model 590 Chrono-Log impedance aggregometer; standard laboratory assays were performed to evaluate changes in blood chemistries.
Results: For both subjects, hemoglobin, WBC, platelet counts, electrolytes and ferritin were within normal reference ranges, with FA-WB-Psel significantly elevated compared to healthy controls (p< 0.01). In vitro treatment of whole blood samples with crizanlizumab (anti-p-selectin monoclonal antibody) within the clinical dose range (10 μg/ml) inhibited FA-WB-Psel only in samples from asymptomatic Post-COVID subject, with the Long-COVID subject sample requiring close to 5-fold elevated dose to achieve a response. Pronounced inhibition of P-selectin adhesion of isolated leukocytes was observed for both subjects in autologous platelet-poor plasma and buffer. Impedance aggregometry showed greater baseline platelet aggregation to collagen in the Long-COVID sample, although both samples responded similarly to aspirin-induced platelet inhibition.
Conclusion: Presented results suggest that elevated platelet activation in Long-COVID subject may be associated with increased P-selectin activity. The results are discussed in terms of possible use of P-selectin inhibition therapies in treating Long-COVID.
Keywords: P-Selectin; COVID-19; SARS-CoV-2; Infection
Abbreviations:
WBC: White Blood Cell; RBC: Red Blood Cell; MCV: Mean Corpuscular Volume; MCH: Mean Corpuscular Hemoglobin; MCHC: Mean Corpuscular Hemoglobin Concentration; RDW-CV: Red Cell Distribution Width; MPV: Mean Platelets Volume.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARSCoV- 2) causes the Coronavirus disease 2019 (COVID-19), which is characterized by severe vascular complications associated with endothelial dysfunction and overproduction of inflammatory mediators, with pathology extending to the cellular level inducing a pro-coagulant state. Research efforts have focused on the acute phase of the disease; however, long-term outcomes for people recovering from COVID-19 are now recognized as an emerging public health challenge. Although most people show complete recovery within a few weeks, some continue to experience a range of symptoms long after their initial recovery which is often referred to as “Long-COVID" or post-acute sequelae of COVID-19 (PASC). While the definition of Long-COVID is still evolving, it has been suggested to include persistence of symptoms or development of related pathologies beyond 3-4 weeks from the onset of acute symptoms of COVID-19 [1-3]. Specifically, it was proposed that at least 2 periods of illness appear to be contributing to morbidity beyond acute SARS-CoV-2 infection: A post-acute hyperinflammatory illness and late inflammatory and virological sequelae. The post-acute hyperinflammatory condition manifests approximately 2 to 5 weeks after the onset of SARS-CoV-2 infection and is characterized by systemic inflammation that can occur in organs distinct from those directly affected by the virus, after host clearance of the SARS-CoV-2 infection. The pathophysiology of this multisystem inflammatory syndrome likely reflects a dysregulated host immune response [4].
The late sequelae or Long-COVID includes a wide range of cardiovascular, pulmonary, neurological, and other physiological manifestations [5]. Typically, patients with Long-COVID report different combinations of symptoms: fatigue, difficulty thinking or concentrating (sometimes called “brain fog”), headache and migraines, loss of smell or taste, hypertension, dizziness on standing, heart palpitations, chest pain, difficulty breathing or shortness of breath, cough, joint or muscle pain, depression or anxiety, hair loss, fever, symptoms that get worse after physical or mental activities. Symptoms attributed to systemic inflammation and impaired microvascular blood flow are of particular concern. These symptoms can continue for an undetermined duration, and it remains unknown to what extent these symptoms can evolve into chronic or irreversible health conditions. There is limited information about the underlying pathophysiology, disease duration, or long-term prognosis of persons affected by Long-COVID. It seems likely that individual patients with Long-COVID would manifest the symptoms through different biological drivers with suggested contributions from viral-induced injury to one or multiple organs, persistent reservoirs of SARS-CoV-2 in certain tissues, re-activation of pre-existing pathogens stimulated by COVID-19 immune dysregulation, SARS-CoV-2 interactions with host microbiome/virome, ongoing activity of primed immune cells, and escalating autoimmunity among other possibilities [3].
The interest in Long-COVID continues to increase as new reports detailing the condition continue to emerge. A number of investigators reported different prevalence of persisting complications in patients recovered from COVID-19. A study published in JAMA presented cardiac magnetic resonance imaging data showing 78% of recently recovered from COVID-19 patients exhibiting cardiac involvement and 60% having an ongoing myocardial inflammation [6]. It was also reported that two-months post the infection among the adults with non-critical COVID-19, two thirds had complaints including severe dyspnea or asthenia, chest pain, palpitations, headache, myalgia, and fever [7], while a study published in BMJ reported about 10% of those who had COVID-19 experienced symptoms beyond three weeks, with an even smaller proportion experiencing them for months [2].
A recent FAIR Health study reviewed a total of 1,959,982 COVID-19 patients for the prevalence of Long-COVID condition 30 days or more after their initial diagnosis with COVID-19 and reported that about 23% had at least one Long-COVID symptom [8]. The five most common Long-COVID conditions across all ages, in order from most to least common, were pain, breathing difficulties, hyperlipidemia, malaise/fatigue, and hypertension. Long-COVID conditions were found to a greater extent in patients who had more severe cases of COVID-19, but also in a substantial share of asymptomatic patients. Of patients who were hospitalized with COVID-19, the percentage that had a Long-COVID condition was 50%, while for patients who were symptomatic but not hospitalized, 27.5%; and 19% for patients who were asymptomatic for COVID-19 [1]. Without a clear clinical picture of the underlying mechanisms, the treatment of Long-COVID syndrome is mainly limited to supportive care and symptomatic control, and its long-term impacts on quality of life of COVID-19 survivors remains unclear.
Commonly reported for COVID-19 positive patients are lymphocytopenia, as well as elevated levels of D-dimer, lactate dehydrogenase, ferritin, Von Willebrand factor, Factor VIII, and inflammatory markers like C-reactive protein, IL-6 and erythrocyte sedimentation rate [9-12]. Despite seeming trends, the reports do not present consistent picture of COVID-19-associated changes and can be hard to reconcile with existing patient-to-patient variability as the reportedly altered values in many cases remain within the normal physiological range. In other cases, both elevated and depleted biomarker values had been observed as is e.g., the case of platelet counts, where both thrombocytopenia and thrombocytosis have been reported [12]. The analysis is further complicated by biomarker significant dependence on disease severity and associated co-morbidities, as well as on wide range of possible patient clinical trajectories defined by an interplay of immunological, inflammatory, and coagulative processes.
COVID-19 association with platelet activation is well documented [13]. Typically, through binding to PSGL-1 on WBC, P-selectin overexpressed on platelets would enhance platelet aggregation and thrombus formation. Indeed, viral-induced coagulopathies including arterial thrombotic events like stroke and ischemic limbs as well as microvascular thrombotic disorders have been observed in SARSCoV- 2 infection with platelet hyperactivity suggested as the key element in disease thrombotic manifestations [14]. Clinically, in patients hospitalized with COVID-19, platelet hyperactivity had been associated with adverse events including thrombosis and death [15]. Such events are often, but not always, correlated with elevation of thrombosis biomarkers like D-dimer and fibrin/fibrinogen-degradation products. Interestingly, some studies did not observe a correlation between levels of D-dimers and platelet activation [16].
Inflammatory and coagulative sequelae are mediated by selectins including P, E, and L selectin and cell adhesion molecules (ICAM-1, VCAM-1), with P-selectin in particular gaining attention. Located within the a-granules of platelets and the Weibel-Palade bodies of endothelial cells, P-selectin rapidly translocates to the cell surface following activation. P-selectin in platelets interacts with P-selectin glycoprotein ligand-1 (PSGL-1) on leukocytes promoting plateletleukocyte aggregate formation, release of procoagulant microparticles, and upregulation of a several leukocyte cytokines. It is also involved in platelet-platelet aggregation, which is major factor in arterial thrombosis [17]. Most investigators report elevated levels of P-selectin in SARS-CoV-2 positive patients, with seemingly larger elevation of P-selectin in more severe disease states. However, a number of studies also report comparable levels of P-selectin in COVID-19 patients and healthy controls [18].
Studies also indicated elevated soluble P-selectin (sP-selectin) levels in plasma of COVID-19 patients [19], with such levels suggested as an early marker of thromboembolism [20]. sP-selectin predominantly originate through proteolytic cleavage of the transmembrane protein (shedding). Activated platelets were showed to be shedding P-selectin in hours after activation [21]. The process is mediated through leukocyte P-selectin glycoprotein ligand-1 (PSGL-1) interaction with transmembrane P-selectin on platelets or endothelial cells [22]. However, despite its elevated plasma concentration, mostly monomeric sP-selectin, by itself is expected to have limited impact on P-selectinassociated pathology, as its prior dimerization is likely required to promote inflammation and coagulation [22].
While some understanding seems to emerge regarding the active phase of COVID-19, the pathophysiology involved in persisting Long- COVID remain essentially unknown. Part of the reason is that longterm follow-up presently is too limited to reveal the full scope of all potential COVID-19 consequences. Another is the natural focus of the research community on understanding and combatting of the active phase of the infection. A recent review presents the multitude of mechanisms potentially involved in the development of Long-COVID symptoms [23]. Diversity of the proposed mechanisms underlines both the complexity of Long-COVID pathology and the need for further investigation on the resolution of this new health problem. Presently we report changes in platelet reactivity, blood cell adhesion on P-selectin and response to in vitro P-selectin inhibition therapy in subjects after SARS-CoV-2 infection with and without severe Long-COVID symptoms.
Methodology
Study subjects and sample collection
Two convalescent COVID-19 subjects, one with Long-COVID symptoms (LC) and the other asymptomatic Post-COVID (PC) were recruited according to the protocols FF-RBC-001 and FF-RBC- 003v2 approved by Institutional Review Board of the Institute for Regenerative and Cellular Medicine. Throughout this paper, the two subjects will be referred to as, the Long-COVID subject, (LC), and the asymptomatic Post-COVID subject (PC). The blood draws were obtained via venipuncture after a 12-h fast. Whole blood was collected into aggregometry, and flow adhesion assays. Sodium citrate, EDTA 3.2% sodium citrate tube and serum separation tubes for interleukins, COVID-19 antibodies and for calcium, LDH, iron, TIBC, CBC, D-dimer, and ferritin panel as appropriate transferred to Quest Diagnostic Lab for analysis. Reference ranges are those provided by the laboratory unless specified otherwise. Both subjects consented to take part in the present study and for the results to be published.
Flow adhesion assays
Flow adhesion of whole blood to P-selectin (FA-WB-Psel) and Flow adhesion of white blood cells to P-selectin (FA-WBC-Psel) were conducted as described previously [24]. Briefly, isolated white blood cell (i-WBC) suspensions were prepared according to a standardized protocol (HetaSepTM, StemCell Technologies). Whole blood samples (1:1 diluted with HBSS buffer) or i-WBC (5 × 106 cells/mL) were perfused through P-selectin-coated microfluidic channels at 1 dyne/cm2 for 10 or 6 minutes respectively, washed to eliminate non-adhering cells, with resultant adhesion quantified manually by an independent trained observer to generate an adhesion index (cells/mm²) using a previously described protocol [25]. For drug treatment conditions, samples were incubated for 5 minutes with crizanlizumab at the final concentrations as required before assessment by the flow adhesion assay. Crizanlizumab was from Creative Biolabs, Shirley, New York; P-selectin was a disulfide-linked homodimer from R & R systems, Minneapolis, MN.
Impedance aggregometry
Platelet aggregation in whole blood samples was tested with an impedance aggregometer (Model 590; Chrono-Log Corporation, Havertown, PA) and analyzed using Aggro/Link®. Whole blood samples were collected into 3.2% sodium citrate tubes, transferred at room temperature, and tested within 4 hours after the blood draw. The sample was split into control (1% DMSO treated) and aspirin treatment (100 μM). Briefly, after a stable baseline (steady state) had been established, the agonist collagen (Chrono-Par, Chrono-Log Corporation, Havertown, PA) was added to the sample to a final concentration of 2 μg/mL, and the aggregation was monitored for 10 minutes. Instrument normal control reference values (Table 1) are for normal donor with no previous exposure to COVID-19 tested same as Post-COVID subjects. Note, that sample collection in citrate tubes required for other assays could have resulted in lower overall values than would have been observed in EDTA [26].
| No ASA | + ASA2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Internal healthy control | Long-COVID subject | Post-COVID subject | Significance1 | Internal healthy control | Long-COVID subject | Post-COVID subject | Significance1 | |
| Amplitude, Ohm | 4.5 | 11.4 ± 0.2 | 8.6 ± 0.7 | p<0.05 | 2.3 | 1.5 ± 0.2 | 1.1 ± 0.2 | ns |
| Rate, Ohm/min | 1.5 | 8.1 ± 0.2 | 6.1 ± 0.2 | p<0.05 | 0.5 | 0.45 ± 0.05 | 0.35 ± 0.05 | ns |
| Lag time, min | 1.5 | 1.4 ± 0.1 | 1.3 ± 0.1 | ns | 3.5 | 2.3 ± 0.1 | 2.2 ± 0.1 | ns |
| AUC (at 10 min) | 35 | 80 ± 7 | 66 ± 5 | p<0.05 | 15 | 9 ± 3 | 5 ± 2 | ns |
ns: No significance
1Significance: T-test comparison between Long-COVID and asymptomatic Post-COVID subjects.
2Supplemented with 100 µM aspirin.
Note: Experimental error was evaluated based on signal to noise ratios of experimental data.
Table 1: Whole blood impedance aggregometry.
Statistical analysis
The data is presented as mean ± standard deviation (mean ± SD) with Student t-test paired or non-paired as appropriate used for assessment of statistical significance of the differences. Results were deemed significant for comparisons where two-tailed p<.05.
Results
Clinical sequelae
Post-COVID subject with severe long-COVID symptoms: LC is a 26-30 years old female with no history of chronic medical conditions, was not on any prescription medication, and had no prior health complaints. She was diagnosed with SARS-CoV-2 by PCR and presented with COVID-19 symptoms one week following a known exposure, including coughing, fever, difficulty breathing, cough, progressively worsening fatigue, brain fog, loss of taste and smell, and headache. In LC’s own words, “I had severe issues breathing unless I was laying down in a certain position. When I did need to sit or stand, I would have a coughing attack barely being able to breathe or be severely short of breath. My throat was extremely irritated. I was disoriented for about 3-4 days. I lost my taste and smell before the major symptoms presented but regained most of both shortly after I began to recover. I had a constant burning in my nose, almost like I had to sneeze. Headaches were nearly unbearable and remained consistent for weeks afterward, with no headache medication able to alleviate.” LC was not hospitalized, managing symptoms at home. At most severe (10-12 days after the infection), she was on bed rest, not responsive, refusing food or drink. The symptoms started to alleviate when the fever was broken and gradually receded over the course of another week. After the resolution of the infection, LC experienced a wide range of symptoms (Table 2) typical for those reported in Long-COVID cases. While some symptoms started to alleviate to different degrees over time, many of them remain quite severe even 5 months after the infection (Table 2).
| Symptom1 | Time after SARS-CoV-2 exposure, weeks | Comment | ||
|---|---|---|---|---|
| 11 | 20 | 28 | ||
| Brain fog | 8 | 8 | 6 | Frequent random moments of forgetfulness and fog that started only after the recovery from SARS-CoV-2 infection. |
| Headaches | 7 | 6 | 5 | Ongoing dull headaches: by 28 weeks after the exposure decreased in frequency to 2-4 times a week, but not responding to treatment by normal headache medication. |
| Fatigue | 8 | 8 | 7 | Subject commented that at 28 weeks “Fatigue has begun to wear off, but still hits hard at different times”. |
| Hair Loss | 9 | 8 | 1 | Was ongoing, but stopped at about around 24 weeks. |
| Burning in the nose | 8 | 7 | 3 | Added and highlighted by the subject. Described as an ongoing sensation ‘burning in the nose like I have to sneeze”. |
| Menstrual cycle | +++ | ++ | + | Cycle regular during the active infection with a 3-weeks additional delay after the recovery. About 5 weeks cycle afterwards (+++) slowly moving back to normal after 3 months post exposure (++), normalizing at about 28 weeks after the infection. |
1Self-reported severity of symptoms is shown with 0 being barely ever and 10 being very bad/frequent.
Table 2: Subject (LC) Long-COVID symptoms development.
Post-COVID subject with no long-COVID symptoms: PC, is a 16-20 years old male, with no history of chronic medical conditions. He was diagnosed with COVID-19 and presented mild symptoms during the active infection lasting approximately 3-4 days. He was asymptomatic three weeks before participating in the study and remained asymptomatic 20 weeks later. He achieved full recovery with no lingering Long-COVID symptoms present at any time since infection resolution.
Clinical chemistry
Long-COVID: Despite severe Long-COVID symptoms, clinical chemistry results of the Long-COVID subject (LC) show little deviation from the normal ranges (Table 3). As an exception, at 20 weeks after the infection with SARS-CoV-2, the subject shows slightly elevated RBC count (5.26*1012 cells/L), as compared to the normal reference range of 3.8-5.1*1012 cells/L. At normal blood hemoglobin value, this slight increase in RBC count is correlated with mean cell hemoglobin concentration (MCH) at the lower end of the normal range. In parallel, metabolic panel shows a decreased total iron and Transferrin-iron saturation percentage values on the background of normal Total Iron Binding Capacity (TIBC), that could be functionally related to increased RBC counts. The values for Ferritin and LDH, often reported as elevated in COVID-19 patients, as well as bilirubin, were within the normal reference range, with the subject thus presenting no evidence of extraordinary hemolysis that could be accompanying SARS-CoV-2 infection [12,27]. D-Dimer value was elevated compared to the reference range (1.02 vs. normal value of <0.5 mcg/mL). An increase in D-Dimer associated with increased in thrombosis risk has been well documented in SARS-CoV-2 infection [28] with significant, 3-4-fold rise in D-dimer levels (to 1.2 ± 1.4 mcg/mL) in the early stages of COVID-19 linked to poor prognosis. All other values are within the normal reference ranges at both 11 and 20-weeks post infection.
| Weeks past the resolution of SARS-CoV-2 infection | Post-COVID subject (male) | Long-COVID subject (female) | Reference range1 | Average reported values (mean ± SD) | ||
|---|---|---|---|---|---|---|
| 2.5 weeks | 11 weeks | 20 weeks | During active COVID-19 | |||
|
Complete Blood Count (CBC) |
||||||
| WBC, bil/L | 7.7 | 7.8 | 9.9 | 3.3-10.7 | 6.0 ± 2.0 | |
| RBC, tril/L | 5.07 | 4.92 | ↑ 5.26 | 3.8-5.1 | 4.2 ± 0.4 | |
| Hemoglobin, g/dL | 14.6 | 13.4 | 14.2 | 12.1-15.0 | 13 ± 1.1 | |
| Hematocrit, % | 0.436 | 0.415 | 0.436 | 35.4%-44.2% | 41.6% ± 2.7% | |
| MCV, fL | 86 | 84 | 82.9 | 80-100 | 91.5 ± 5.2 | |
| MCH, pg | 28.8 | 27 | 27 | 27-33 | 30.6 ± 2.1 | |
| MCHC, g/dL | 33.5 | 32 | 32.6 | 32.0-35.0 | 33.3 ± 1.1 | |
| RDW-CV, % | 0.117 | 0.14 | 0.13 | 11%-15% | 14.3% ± 1.7% | |
| Platelets, bil/L | 264 | 318 | 310 | 150-400 | 183 ± 39 | |
| MPV, fL | ↑ 12.8 | N/A | 10.2 | 7.5-12.5 | 7.8 ± 4.2 | |
|
Metabolic panel and electrolytes |
||||||
| Sodium, mmol/L | 144 | 140 | N/A | 135-145 | 138.7 ± 2.4 | |
| Potassium, mmol/L | 4.3 | 4.7 | N/A | 3.6-5.2 | 4.2 ± 0.5 | |
| Chloride, mmol/L | ↓ 97 | 104 | N/A | 98-111 | 102.8 ± 3.1 | |
| Carbon Dioxide (CO2), mmol/L | ↓ 16 | 23 | N/A | 20-29 | ↓ 12 ± 12.3 | |
| Ferritin, ng/mL | 134 | N/A | 26 | 11-172 | ↑↑ 1018 ± 533 | |
| Iron, Total, mcg/dL | 163 | N/A | ↓ 35 | 40-190 | 150 ± 55 | |
| Iron Binding Capacity (TIBC), mcg/dL | 394 | N/A | 358 | 250-450 | 390 ± 53 | |
| Transferrin-iron saturation percentage (TSAT), % | 0.41 | N/A | ↓ 10% | 16%-45% | 29% ± 10% | |
| Protein Total, g/dL | 7.7 | 7.1 | N/A | 6.4-8.3 | 6.8 ± 0.7 | |
| Albumin, g/dL | 4.8 | 4.4 | N/A | 3.5 – 5.1 | 3.9 ± 0.5 | |
| Globulin, g/dL | 2.9 | 2.7 | N/A | 2.2 – 4.0 | 2.7 ± 0.3 | |
| Alkaline Phosphatase, U/L | 79 | 82 | N/A | 33-120 | 84 ± 49.3 | |
| Aspartate Aminotransferase (AST), U/L | 25 | 22 | N/A | <35 | ↑ 44.2 ± 43.0 | |
| Alanine Aminotransferase (ALT), U/L | 15 | 24 | N/A | 13728 | ↑ 42.3 ± 34.9 | |
| Bilirubin Total, mg/dL | ↑ 1.3 | 0.6 | N/A | 0.3-1.2 | ↑ 1.3 ± 1.0 | |
|
Other parameters |
||||||
| D-Dimer, mcg/mL FEU | <0.19 | N/A | ↑↑ 1.02 | <0.50 | ↑↑ 1.2 ± 1.4 | |
| Total LDH, U/L | ↑ 278 | N/A | 180 | 100-200 | ↑↑ 370 ± 344 | |
1 The reference ranges are those provided by Quest Diagnostics.
Note: Values above the normal reference range are indicated by ↑ for moderate increase and by ↑ ↑ for large increase, and values below the normal reference range indicated by ↓ for moderate increase and by ↓↓for large increase. All other values are within the reference range.
Table 3: Clinical chemistry results of the Long-COVID subject (LC) show little deviation from the normal ranges.
Post-COVID: Clinical chemistry parameters were within the normal reference ranges for the asymptomatic Post-COVID subject (PC), except for slightly elevated total bilirubin (1.3 mg/dL, compared to the reference range 0.1-1.2 mg/dL) and total LDH (278 U/l, reference range 110-230 U/L). Simultaneous elevation of LDH and bilirubin may be indicative of slightly elevated rate of hemolysis on the background of compensating erythropoietic activity and thus with no impact on RBC count or total hemoglobin values. That supposition is supported by the concurrently decreased Red Blood Cell Distribution Width (RDW), with such a decrease being potentially associated with decreased average RBC lifespan in circulation resulting in smaller contribution of older and lower volume cells to RDW [29].
Interleukin 1 Beta (IL1β) and interleukin 4 (IL4) were in the normal reference ranges for both subjects (Table 3).
Flow adhesion on P-Selectin
Whole blood: Flow adhesion of whole blood on P-selectin (FAWB- PSel) was significantly elevated for both Long-COVID (LC) and asymptomatic Post-COVID (PC) subjects (590 ± 260 and 1,100 ± 250 cells/mm2 correspondingly) as compared to normal subjects (typically, 50 or less cells/mm2 if with no significant inflammation processes).
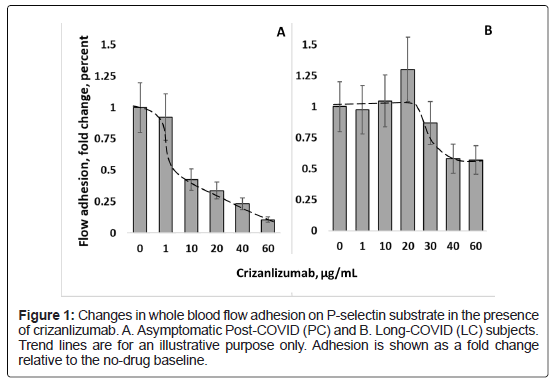
Post COVID: Measurements of flow adhesion of whole blood in the presence of crizanlizumab, a monoclonal antibody against P-selectin, resulted in a dose-dependent decline of adhesion to P-selectin for the asymptomatic Post-COVID subject’s sample. Such crizanlizumabinduced inhibition of adhesion to P-selectin reached about 60 percent at 10 μg/mL. This dose correlates to the reported mean steady-state serum crizanlizumab concentration at 10.5 to 15.0 μg/mL for high drug administration dose (5 mg/kg) [30]. At 60 μg/mL of crizanlizumab, 90% of inhibition of FA-WB-Psel was reached (Figure 1A).
Long COVID: For the Long-COVID subject with severe symptoms, supplementation of whole blood with crizanlizumab in doses up to 20 μg/mL range did not result in any measurable inhibition of cell adhesion to P-selectin. At the elevated drug doses (50-60 μg/mL) up to 50% inhibition of adhesion to P-selectin was observed, which is about half of that recorded for the asymptomatic subject at the same dose (Figure 1B).
Isolated white blood cells in buffer
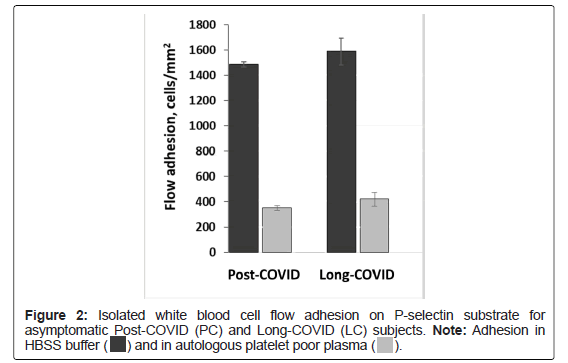
PC and LC: Flow adhesion of isolated White Blood Cells (i-WBC) to P-selectin (FA-WBC-Psel) was similar between Long-COVID and asymptomatic Post-COVID subjects, and in HBSS buffer was 1490 ± 30 and 1590 ± 110 cells/mm2 correspondingly. When i-WBCs were suspended in autologous plasma (see Material and Methods), adhesion on P-selectin was decreased by 4.2 and 3.8-fold to 350 ± 20 and 420 ± 50 cells/mm2 for asymptomatic Post-COVID and Long-COVID subjects correspondingly (Figure 2).
PC: For the asymptomatic Post-COVID subject, supplementation of buffer-suspended i-WBC with crizanlizumab resulted in a dosedependent inhibition of WBC adhesion with the magnitude of inhibition like that observed in whole blood (the difference lacked statistical significance).
LC: For the subject with Long-COVID symptoms, supplementation of i-WBC with crizanlizumab showed nearly complete (about 95%) inhibition of cell adhesion to P-selectin. Such unexpectedly strong adhesion inhibition had been observed even at 1 μg/mL dose of crizanlizumab, which is ten times lower than the standard clinical drug dose (Figure 3).
Isolated white blood cells in autologous platelet poor plasma
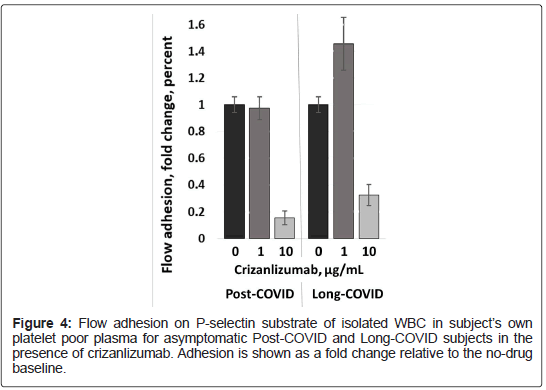
PC and LC: Crizanlizumab supplementation of same i-WBC suspended in autologous Platelet Poor Plasma (PPP) resulted in no significant inhibition at sub-clinical crizanlizumab dose corresponding to 1 μg/mL plasma concentration and in pronounced inhibition of adhesion to P-selectin (about 80% for asymptomatic and 65% for symptomatic subjects (Figure 4). Similar results with adhesion inhibited by about 70%-80% were obtained when crizanlizumabinduced inhibition of adhesion was measured on i-WBC in bloodtype complementary platelet poor plasma from healthy subjects (data not shown). For the Long-COVID subject similar results had been obtained on samples collected at 11 and 20 weeks after the infection (at approximately 8 and 16 weeks after the resolution of acute COVID-19).
Whole blood aggregometry
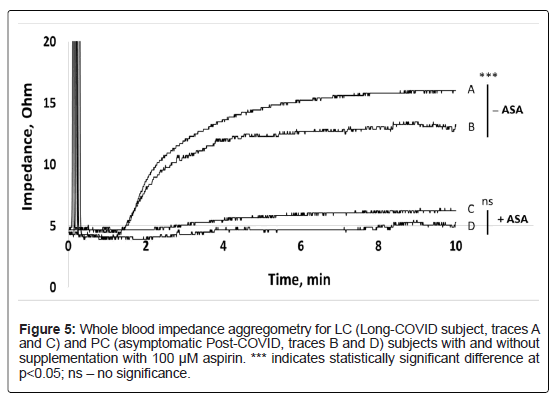
PC and LC: Whole blood impedance aggregometry showed that maximum amplitude, area under the curve (AUC) and maximum rate were all elevated in Post-COVID subjects, both with Long-COVID and asymptomatic, as compared to normal donors (see internal healthy control values, Table 1). Conversely, inhibition of activity induced by antiplatelet acetylsalicylic acid (ASA) was found to be more pronounced on both Post-COVID subjects as compared to the normal control.
As compared to asymptomatic Post-COVID subject, the Long- COVID subject further exhibited significantly elevated amplitude and AUC, and elevated maximum rate (Figure 5 and Table 1). The difference in lag time lacked significance. For both Long-COVID and asymptomatic Post-COVID subjects, in vitro ASA supplementation resulted in marked inhibition of aggregation with similar rates and lag times. Aggregation of platelets from both Post-COVID asymptomatic and Long-COVID subjects, as indicated by aggregometry, was significantly inhibited by ASA with the differences in resultant aggregation lacking statistical significance (Table 1).
Discussion
It was demonstrated that platelets from COVID-19 patients overexpress P-selectin both basally and upon activation, associated with faster platelet aggregation and increased circulating platelet– leukocyte aggregates [31]. Significantly higher levels of the platelet activation markers including P-selectin, had also been reported for stable hospitalized COVID-19 patients [32]. Interestingly, no changes in platelet reactivity or P-selectin expression were detected upon vaccination within 4 weeks of BNT162b2 administration, with overall activation marker expression, including P-selectin, being significantly lower than even for mild-COVID-19 patient cohort [33]. While the drivers behind platelet activation in COVID-19 remain to be determined, it is possible, that such activation occurs due to platelet interaction with the infected endothelium and/or because of the cytokine storm associated with SARS-CoV-2 infection [34].
Overall, the evidence suggests that SARS-CoV-2 infection is associated with platelet hyperreactivity, contributing to COVID-19 pathophysiology [35]. Despite the mounting evidence detailing possible involvement of platelets in COVID-19, the cohesive picture of their involvement remains to be developed. Some investigations report increased platelet activation and platelet-monocyte aggregates in severe COVID-19 patients, but not in patients with mild COVID-19 [13], while others document increased platelet activity in COVID-19 independent of disease severity [16]. Ex vivo exposure of healthy donor platelets to plasma from severe COVID-19 patients may increase platelet P-selectin expression and platelet–leukocyte aggregate formation, similar to that observed in vivo [36], and induce tissue factor (TF) expression by monocytes. TF expression was reduced by platelet pre-treatment with anti–P-selectin neutralizing antibodies [13].
Leukocyte adhesion to P-selectin on activated platelets and endothelial cells induces shedding of the P-selectin ectodomain into the circulation [22]. The sP-selectin circulates as a monomer in plasma, as opposed to dimeric and multimeric forms typical for membrane bound P-selectin with in vitro studies suggesting that sP-selectin must dimerize to induce signaling in leukocytes [37]. However, only the lectin and epidermal growth factor domains are necessary for P-selectin interaction with PSGL-1 [38]. Thus, monomeric sP-selectin was shown capable of binding to high affinity ligands on leukocytes even if its avidity for leukocytes is enhanced when P-selectin is present in an oligomeric transmembrane form [37]. P-selectin shedding does not seem to inhibit the function of circulating platelets, which retain aggregation, adhesion, and procoagulant activity [21]. However, P-selectin dimerization may be required for the protein to effectively promote inflammation and coagulation [22].
A recently completed Phase 2 clinical trial to test Crizanlizumab, an anti-P-selectin monoclonal antibody, in patients hospitalized with COVID-19 (NCT04435184) focused on sP-selectin reduction as the primary outcome measure [39]. However, despite 89 percent reduction in sP-selectin levels, crizanlizumab treatment did not improve the clinical endpoints (time to hospital discharge and change in patient clinical status as assessed by the World Health Organization (WHO) ordinal scale for coronavirus disease 2019 (COVID-19) trials) [40].
Despite previously reported correlation of sP-selectin with COVID-19 severity [18], the causation remains to be established. Thus, improvements in clinical status in COVID-19 and Long-COVID cases may not be directly associated or caused only by changes in sPselectin level as shown by the Phase 2 clinical trial to test Crizanlizumab as a therapy for treating active COVID-19. P-selectin antagonists, like crizanlizumab, affect not only sP-selectin, but also the protein expressed on both activated platelet and endothelial cells, with different compounds potentially presenting different activity profiles. Moreover, binding of P-selectin antagonist to sP-selectin could reduce the amount of antagonist available for blocking membrane-bound P-selectin on platelets and endothelial cells. Thus, assessing possible therapeutic effects of P-selectin antagonists it could be informative to evaluate changes in sP-selectin plasma levels, in conjunction with antagonists’ effect of the on platelets and endothelial cells P-selectin adhesion levels as all of these may be important for pathology resolution/of an ongoing pathology pathology amelioration [41-43].
We observed that in vitro treatment with clinical doses of crizanlizumab could reduce adhesion of whole blood from an asymptomatic Post-COVID subject to immobilized P-selectin, but blood from a subject with severe Long-COVID symptoms required significantly higher crizanlizumab dose to start inducing inhibition. Inhibition by crizanlizumab was, however, observed with isolated WBC, both in platelet poor plasma, which presumably contained sPselectin and in buffer missing both sP-selectin and platelets (Figure 2) in the Long-Covid subject. That would suggest that sP-selectin, unlike P-selectin on activated platelets, was not the dominant factor in reducing crizanlizumab activity in Long-COVID subject. Platelet activation with associated greater surface P-selectin expression in Long-COVID may reduce the crizanlizumab available to inhibit adhesion to immobilized P-selectin.
Platelet activation for the Long-COVID subject was further confirmed by significantly elevated platelet activity as shown by impedance aggregometry. Such activity was equally inhibited in the Post-COVID and Long-COVID subjects by ASA. As ASA inhibitory effect occurs through inhibition of cyclo-oxygenase, it would not be affected by levels of platelet P-selectin expression [44].
Platelet aggregation can be elevated in healthy women compared to men, with such sex-related difference present also after incubation with ASA [45]. A trend towards a higher platelet reactivity was also observed in women exhibiting elevated platelet counts (defined as >200 Billion cells/L) [46]. While gender-related factors cannot be conclusively excluded, the difference in platelet activity in this report is markedly larger than the 10% previously reported [45].
A higher platelets activation state would justify the higher sPselectin levels observed in autologous plasma from the Long-COVID subject[47]. However, a small amount of sP-selectin is derived from alternative messenger RNA splicing that removes the exon encoding the transmembrane domain, although sP-selectin is known to be primarily derived from the proteolytic cleavage of the transmembrane protein, which releases a fragment comprising most of the ectodomain in circulation [22]. Thus, most of the sP-selectin plasma pool would include sP-selectin originated from both platelets and vascular endothelial cells. The role and significance of sP-selectin in mediation of whole blood and plasma response to P-selectin inhibition therapy has not been directly assessed in this work, constituting a limitation of the study.
In vitro treatment with crizanlizumab of i-WBCs in autologous PPP resulted in comparable inhibition of adhesion to immobilized P-selectin between Long-COVID subject and asymptomatic Post- COVID subject (Figure 4). However, in the same assay performed in buffer, crizanlizumab shown higher inhibition effect on i-WBC resuspended in Buffer from the Long-COVID subject with respect to those from the Post-COVID subject, with nearly complete inhibition of P-selectin adhesion even at sub-clinical crizanlizumab dose (Figure 3). This suggests either decreased PSGL-1 expression or a decrease in post-translational modifications (PTM) by sialic acid (SiA) and fucose required to enable PSGL-1 binding to P-selectin with high affinity [48]. SiA-mediated interactions with the receptor-binding domain of SARSCoV- 2 spike protein had been suggested as the virus entry pathway to the cell [49]. Diverse clinical presentation of COVID-19 and specifically differences in age and gender associated severity may, in part, be explained through age and gender-related variability of sialome among the patients [50]. Overall, the clinical importance of SiA-mediated interactions between the host cell and virus certainly requires further investigation [51].
SiA receptor-destroying enzymes like Neuraminidase (NEU) in influenza or hemagglutinin-esterase (HE) in some coronaviruses, mediate cleavage of surface SiA-containing cell receptors promoting viral particles release from infected cells. Endogenous cell NA activity plays a role in shedding of cell surface SiA, plays a key role in activation of both platelets [52] and neutrophils [53]. While SARS-CoV-2 seem to be lacking HE gene [54], samples from COVID-19 patients revealed an overexpression of NEU in infected neutrophils and treatment with NEU inhibitors led to decreased host NEU-mediated shedding of cell SiA and reduced overactivation of neutrophils [55]. The limitation of this discussion is that the possibility of leukocyte-platelet aggregates in i-WBC isolation cannot be ruled out.
It remains uncertain whether SARS-CoV-2 can establish a persistent and stable chronic infection in the human tissues like other SARS coronaviruses [56]. Nevertheless, viral RNA is known to persist in human body well past the resolution of the active infection [3] and SARS-CoV-2 RNA has been identified in samples from patients long time after the resolution of the active infection [57-59]. Considering that SARS-CoV-2 RNA present in the blood stream was associated with platelet hyperactivity [60, 61], a similar mechanism for platelet activation can be suggested for Long-COVID. The key limitation of this hypothesis is that previous studies observed SARS-CoV-2 RNA up to a maximum of 3 months after the infection, while the Long-COVID subject in this study presented platelet hyperactivity at 20 weeks and experienced severe Long-COVID symptoms even at 28 weeks after the resolution of active COVID-19 disease. The potential for persistent SARS-CoV-2 or presence of active viral RNA for 28 weeks or more post-resolution has not been fully assessed. The uncertain potential for disease resurgence and/or spread gives this question extra importance and urgency.
The clear limitation of this work is the observation of a single Long- COVID subject, even if contrasted with an asymptomatic Post-COVID. Moreover, it is not known to what extent in vitro observations would translate to in vivo treatment efficacy, especially to its long-term effects, with the treatment-related changes to cell function accumulating over time. Additionally, interaction of P-selectin inhibitors with overexpressed P-selectin on platelets of Long-COVID patients may alter platelet function and lead to possible clinical effects not directly observable through or related to cell adhesion to P-selectin. There could be significant differences in efficacy of P-selectin antagonists in terms of their action on the protein expressed on blood cells (mostly platelets) as opposed to the impact on flow-mediated interaction with endothelially expressed immobilized P-selectin. However, relative significance of these two mechanisms of action to drug clinical efficacy remains to be elucidated.
It also remains unknown to what extent the observed cell function alterations in severe Long-COVID case are indeed a general feature of the condition or a feature of clinical presentation in that particular patient. Clinical presentation reported for SARS-CoV-2 infection is highly variable, and such is likely to extend to Long-COVID sequalae. Individual variability could be expected to play a significant role, likely on a background of certain common condition-associated factors, potentially including platelet hyperactivity with P-selectin overexpression and alteration in functionality of PSGL-1 leukocyte receptor reported here.
Where the results reported here represent general features of Long- COVID or a singular individual response, may be an important question that may clarify the potential utility of P-selectin inhibitors in Long- COVID therapy. Clinical assessment of P-selectin adhesion properties may expand our understanding of the underlying mechanisms of Long- COVID and give insight into novel therapeutic strategies.
Conclusion
Severe Long-COVID subject presented platelet hyperactivity associated with increased P-selectin activity and seemingly with alteration in functionality of PSGL-1 leukocyte receptor, while the asymptomatic Post-COVID subject did not present similar alterations of blood cell function.
The data suggests significantly higher levels of p-selectin activity in Long-COVID, suggesting P-selectin activity as a potential driver of Long-COVID pathology.
At similar concentrations, crizanlizumab, an anti-P-selectin monoclonal antibody, was less potent in whole blood from a Long- COVID subject as compared to a asymptomatic Post-COVID subject based on cell adhesion to immobilized P-selectin.
Compared to asymptomatic Post-COVID subject, leukocytes from the Long-COVID subject showed altered reactivity to immobilized P-selectin, potentially reflecting alterations in post-translational modifications of PSGL-1 receptor.
Assuming that adhesive and proinflammatory processes in Long-COVID can be mitigated through modulation of leukocytes reactivity with the endothelial cells, endothelial P-Selectin would the target of choice for P-selectin antagonist therapy. However, plasma concentration of P-selectin antagonists would be reduced after its interaction with elevated plasma sP-selectin and P-selectin on activated platelet membranes. Combining P-selectin antagonist treatment with e.g., plasmapheresis, may potentially reduce off-target interactions and improve the efficacy of treatment.
Conflict Of Interest Statement
B. Hannan, M. Ferranti, S. Mota and A. Zaidi are employees, and X. Gao, P. Hines, and M. Tarasev are employees and shareholders of Functional Fluidics Incorporated, a company developing and commercializing assays for assessment of blood cell functions.
References
- Datta SD, Talwar A, Lee JT (2020) A proposed framework and timeline of the spectrum of disease due to SARS-CoV-2 infection: illness beyond acute infection and public health implications. Jama 324:2251-2252.
[Crossref] [Google Scholar] [PubMed]
- Greenhalgh T, Knight M, Buxton M, Husain L (2020) Management of post-acute covid-19 in primary care. Bmj 370:3026.
[Crossref] [Google Scholar] [PubMed]
- Proal AD, VanElzakker MB (2021) Long COVID or post-acute sequelae of COVID-19 (PASC): An overview of biological factors that may contribute to persistent symptoms. Front Microbiol 12:698169.
[Crossref] [Google Scholar] [PubMed]
- Abrams JY, Godfred-Cato SE, Oster ME, Chow EJ, Koumans EH, et al. (2020) Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: A systematic review. J Pediatr 226:45-54.
[Crossref] [Google Scholar] [PubMed]
- Del Rio C, Collins LF, Malani P (2020) Long-term health consequences of COVID-19. Jama 324:1723-1724.
[Crossref] [Google Scholar] [PubMed]
- Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, et al. (2020) Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 5:1265-1273.
[Crossref] [Google Scholar] [PubMed]
- Carvalho-Schneider C, Laurent E, Lemaignen A, Beaufils E, Bourbao-Tournois C, et al. (2021) Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect 27:258-263.
[Crossref] [Google Scholar] [PubMed]
- Health F (2021) Detailed study of patients with long-haul COVID: An analysis of private healthcare claims.
- Mardian Y, Kosasih H, Karyana M, Neal A, Lau CY (2021) Review of current COVID-19 diagnostics and opportunities for further development. Front Med 8:562.
[Crossref] [Google Scholar] [PubMed]
- Deng X, Liu B, Li J, Zhang J, Zhao Y, et al. (2020) Blood biochemical characteristics of patients with coronavirus disease 2019 (COVID-19): A systemic review and meta-analysis. Clin Chem Lab Med 58:1172-1181.
[Crossref] [Google Scholar] [PubMed]
- Suklan J, Cheaveau J, Hill S, Urwin SG, Green K, et al. (2021) Utility of Routine Laboratory Biomarkers to Detect COVID-19: A Systematic Review and Meta-Analysis. Viruses 13:803.
[Crossref] [Google Scholar] [PubMed]
- Samprathi M, Jayashree M (2021) Biomarkers in COVID-19: an up-to-date review. Frontiers in pediatrics 972.
[Crossref] [Google Scholar] [PubMed]
- Hottz ED, Azevedo-Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, et al. (2020) Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood 136:1330-1341.
[Crossref] [Google Scholar] [PubMed]
- Battinelli EM (2020) COVID-19 concerns aggregate around platelets. Blood 136:1221.
[Crossref] [Google Scholar] [PubMed]
- Barrett TJ, Lee AH, Xia Y, Lin LH, Black M, et al. (2020) Platelet and vascular biomarkers associate with thrombosis and death in coronavirus disease. Circ Res 127:945-947.
- Zaid Y, Puhm F, Allaeys I, Naya A, Oudghiri M, et al. (2020) Platelets can associate with SARS-Cov-2 RNA and are hyperactivated in COVID-19. Circ Res 127:1404-1418.
[Crossref] [Google Scholar] [PubMed]
- Merten M, Thiagarajan P (2004) P-selectin in arterial thrombosis. Z Kardiol 93:855-863.
[Crossref] [Google Scholar] [PubMed]
- Agrati C, Sacchi A, Tartaglia E, Vergori A, Gagliardini R, et al. (2021) The role of P-selectin in COVID-19 coagulopathy: An updated review. Int J Mol Sci 22:7942.
[Crossref] [Google Scholar] [PubMed]
- Agrati C, Bordoni V, Sacchi A, Petrosillo N, Nicastri E, et al. (2021) Elevated P-Selectin in severe Covid-19: considerations for therapeutic options. Mediterr J Hematol Infect Dis 13.
[Crossref] [Google Scholar] [PubMed]
- Fenyves BG, Mehta A, COVID MGH, Kays K, Goldberg M, et al. (2021) Plasma P-selectin is an early marker of thromboembolism in COVID-19. Am J Hematol 96:468-471.
[Crossref] [Google Scholar] [PubMed]
- Michelson AD, Barnard MR, Hechtman HB, MacGregor H, Connolly RJ, et al. (1996) In vivo tracking of platelets: Circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci 93:11877-11882.
[Crossref] [Google Scholar] [PubMed]
- Panicker SR, Mehta-D’souza P, Zhang N, Klopocki AG, Shao B, et al. (2017) Circulating soluble P-selectin must dimerize to promote inflammation and coagulation in mice. Blood 130:181-191.
[Crossref] [Google Scholar] [PubMed]
- Castanares-Zapatero D, Chalon P, van den HEEDE K (2021) Pathophysiology of Long-COVID: a Preliminary Report. Federaal Kenniscentrum voor de Gezondheidzorg.
- Hines PC, Callaghan MU, Zaidi AU, Gao X, Liu K, et al. (2021) Flow adhesion of whole blood to P-selectin: a prognostic biomarker for vaso-occlusive crisis in sickle cell disease. Br J Haematol 194:1074-1082.
[Crossref] [Google Scholar] [PubMed]
- Kapileshwarkar Y, Gelmini L, Tseng YS, Jackson T, Gao X, et al. (2020) Assessment of antiplatelet therapy response in pediatric patients following cardiac surgery by microfluidic assay. Prog Pediatr Cardiol 56:101191.
- McShine RL, Das PC, SIBINGA CT, Brozovic B (1990) Differences between the effects of EDTA and citrate anticoagulants on platelet count and mean platelet volume. Clin Lab Haematol 12:277-285.
[Crossref] [Google Scholar] [PubMed]
- Mehta AA, Haridas N, Belgundi P, Jose WM (2021) A systematic review of clinical and laboratory parameters associated with increased severity among COVID-19 patients. Diabetes Metab Syndr 15:535-541.
[Crossref] [Google Scholar] [PubMed]
- Rostami M, Mansouritorghabeh H (2020) D-dimer level in COVID-19 infection: a systematic review. Expert Rev Hematol 13:1265-1275.
[Crossref] [Google Scholar] [PubMed]
- Malka R, Delgado FF, Manalis SR, Higgins JM (2014) In vivo volume and hemoglobin dynamics of human red blood cells. PLoS Comput Biol 10:1003839.
[Crossref] [Google Scholar] [PubMed]
- Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, et al. (2017) Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med 376:429-439.
[Crossref] [Google Scholar] [PubMed]
- Manne BK, Denorme F, Middleton EA, Portier I, Rowley JW, et al. (2020) Platelet gene expression and function in patients with COVID-19. Blood 136:1317-1329.
[Crossref] [Google Scholar] [PubMed]
- Bongiovanni D, Klug M, Lazareva O, Weidlich S, Biasi M, et al. (2021) SARS-CoV-2 infection is associated with a pro-thrombotic platelet phenotype. Cell Death Dis 12:1-0.50.
[Crossref] [Google Scholar] [PubMed]
- Klug ME, Lazareva O, Kirmes K, Rosenbaum M, Lukas M, et al. (2021) Platelet expression and reactivity after BNT162b2 vaccine administration. Thromb Haemost.
[Crossref] [Google Scholar] [PubMed]
- Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, et al. (2020) COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol 75:2950-2973.
[Crossref] [Google Scholar] [PubMed]
- Comer SP, Cullivan S, Szklanna PB, Weiss L, Cullen S, et al. (2021) COVID-19 induces a hyperactive phenotype in circulating platelets. PLoS Biol 19:3001109.
[Crossref] [Google Scholar] [PubMed]
- Canzano P, Brambilla M, Porro B, Cosentino N, Tortorici E, et al. (2021) Platelet and endothelial activation as potential mechanisms behind the thrombotic complications of COVID-19 patients. JACC Basic Transl Sci 6:202-218.
[Crossref] [Google Scholar] [PubMed]
- Ushiyama S, Laue TM, Moore KL, Erickson HP, McEver RP (1993) Structural and functional characterization of monomeric soluble P-selectin and comparison with membrane P-selectin. J Biol Chem 268:15229-15237.
[Crossref] [Google Scholar] [PubMed]
- Mehta P, Patel KD, Laue TM, Erickson HP, McEver RP (1997) Soluble Monomeric P-selectin Containing only the Lectin and EGF Domains Binds to PSGL-1 on Leukocytes. Blood 90:2381-2389.
[Crossref] [Google Scholar] [PubMed]
- Neri T, Nieri D, Celi A (2020) P-selectin blockade in COVID-19-related ARDS. Am J Physiol Lung Cell Mol Physiol 318:1237-1238.
[Crossref] [Google Scholar] [PubMed]
- Leucker TM, Osburn WO, Reventun P, Smith K, Claggett B, et al. (2021) Effect of crizanlizumab, a P-selectin inhibitor, in COVID-19: a placebo-controlled, randomized trial. JACC Basic Transl Sci 6:935-945.
[Crossref] [Google Scholar] [PubMed]
- Woollard KJ, Suhartoyo A, Harris EE, Eisenhardt SU, Jackson SP, et al. (2008) Pathophysiological levels of soluble P-selectin mediate adhesion of leukocytes to the endothelium through Mac-1 activation. Circ Res 103:1128-1138.
[Crossref] [Google Scholar] [PubMed]
- Karki NR, Kutlar A (2021) P-Selectin Blockade in the Treatment of Painful Vaso-Occlusive Crises in Sickle Cell Disease: A Spotlight on Crizanlizumab. J Pain Res 14:849-851.
[Crossref] [Google Scholar] [PubMed]
- Blann AD, Nadar SK, Lip GY (2003) The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J 24:2166-2179.
[Google Scholar] [PubMed]
- Taylor ML, Ilton MK, Misso NL, Watkins DN, Hung J, et al. (1998) The effect of aspirin on thrombin stimulated platelet adhesion receptor expression and the role of neutrophils. Br J Clin Pharmacol 46:139-145.
[Crossref] [Google Scholar] [PubMed]
- Ivandic BT, Giannitsis E, Schlick P, Staritz P, Katus HA, et al. (2007) Determination of aspirin responsiveness by use of whole blood platelet aggregometry. Clin Chem 53:614-619.
[Crossref] [Google Scholar] [PubMed]
- Ranucci M, Aloisio T, Di Dedda U, Menicanti L, de Vincentiis C, et al. (2019) Gender-based differences in platelet function and platelet reactivity to P2Y12 inhibitors. PLoS One 14:0225771.
[Crossref] [Google Scholar] [PubMed]
- Yatim N, Boussier J, Chocron R, Hadjadj J, Philippe A, et al. (2021) Platelet activation in critically ill COVID-19 patients. Ann Intensive Care 11:113.
[Crossref] [Google Scholar] [PubMed]
- Vachino G, Veldman GM, Kumar R, Sako D, Fouser LA, et al. (1995) P-selectin glycoprotein ligand-1 Is the major counter-receptor for P-selectin on stimulated T cells and Is widely distributed in non-functional form on many lymphocytic cells. J Biol Chem 270:21966-21974.
[Crossref] [Google Scholar] [PubMed]
- Sun XL (2021) The role of cell surface sialic acids for SARS-CoV-2 infection. Glycobiology 31:1245-1253.
[Crossref] [Google Scholar] [PubMed]
- Morniroli D, Giannì ML, Consales A, Pietrasanta C, Mosca F (2020) Human sialome and coronavirus disease-2019 (COVID-19) pandemic: An understated correlation? Front Immunol 11:1480.
[Crossref] [Google Scholar] [PubMed]
- Dhar C, Sasmal A, Diaz S, Verhagen A, Yu H, et al. (2021) Are sialic acids involved in COVID-19 pathogenesis?. Glycobiology 31:1068-1071.
[Crossref] [Google Scholar] [PubMed]
- Lauková L, Weiss R, Semak V, Weber V (2021) Desialylation of platelet surface glycans enhances platelet adhesion to adsorbent polymers for lipoprotein apheresis. Int J Artif Organs 44:378-384.
[Crossref] [Google Scholar] [PubMed]
- Cross AS, Wright DG (1991) Mobilization of sialidase from intracellular stores to the surface of human neutrophils and its role in stimulated adhesion responses of these cells. J Clin Invest 88:2067-2076.
[Crossref] [Google Scholar] [PubMed]
- Zandi M, Behboudi E, Soltani S (2021) Role of glycoprotein hemagglutinin-esterase in COVID-19 pathophysiology?. Stem Cell Rev Rep 17:2359-2360.
[Crossref] [Google Scholar] [PubMed]
- Formiga RO, Amaral FC, Souza CF, Mendes DA, Wanderley CW, et al. (2021) Neuraminidase inhibitors rewire neutrophil function in murine sepsis and ex-vivo in COVID-19 patients’ cells. bioRxiv.
[Crossref] [Google Scholar] [PubMed]
- Chan PK, To KF, Lo AW, Cheung JL, Chu I, et al. (2004) Persistent infection of SARS coronavirus in colonic cells in vitro. J Med Virol 74:1-7.
[Crossref] [Google Scholar] [PubMed]
- Liotti FM, Menchinelli G, Marchetti S, Posteraro B, Landi F, et al. (2021) Assessment of SARS-CoV-2 RNA test results among patients who recovered from COVID-19 with prior negative results. JAMA Intern Med 181:702-704.
[Crossref] [Google Scholar] [PubMed]
- Vibholm LK, Nielsen SS, Pahus MH, Frattari GS, Olesen R, et al. (2021) SARS-CoV-2 persistence is associated with antigen-specific CD8 T-cell responses. EBioMedicine 64:103230.
[Crossref] [Google Scholar] [PubMed]
- Sun J, Xiao J, Sun R, Tang X, Liang C, et al. (2020) Prolonged Persistence of SARS-CoV-2 RNA in Body Fluids. Emerg Infect Dis 26:1834-1838.
[Crossref] [Google Scholar] [PubMed]
- Zhang S, Liu Y, Wang X, Yang L, Li H, et al. (2020) SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol 13:120.
[Crossref] [Google Scholar] [PubMed]
- Zaid Y, Puhm F, Allaeys I, Naya A, Oudghiri M, et al. (2020) Platelets can associate with SARS-Cov-2 RNA and are hyperactivated in COVID-19. Circ Res 127:1404-1418.
[Crossref] [Google Scholar] [PubMed]
Citation: Tarasev M, Mota S, Gao X, Ferranti M, Zaidi AU, et al. (2022) Possible Role of P-Selection Adhesion in Long-COVID: A Comparative Analysis of a Long-COVID Case vs. an Asymptomatic Post-COVID Case. J Infect Dis Ther S3:005.
Copyright: © 2022 Tarasev M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2794
- [From(publication date): 0-2022 - Nov 25, 2025]
- Breakdown by view type
- HTML page views: 2311
- PDF downloads: 483