Research Article Open Access
Possible Involvement of Leptin in the Elevated Osteoblastic Activity Observed in High Turnover Type Osteoporosis of Ovariectomized Mice
| Mitsuki Tezuka, Seiko Tatehara, Takahiro Imamura, Ryusuke Tachibana, Yusuke Takebe, Reiko Tokuyama and Kazuhito Satomura* | ||
| Department of Oral Medicine and Stomatology, Second Department of Oral and Maxillofacial Surgery, Tsurumi University School of Dental Medicine, 2-1-3 Tsurumi, Tsurumi-ku, Yokohama 230-5801, Japan | ||
| Corresponding Author : | Kazuhito Satomura, DDS, PhD Department of Oral Medicine and Stomatology Second Department of Oral and Maxillofacial Surgery Tsurumi University School of Dental Medicine 2-1-3 Tsurumi, Tsurumi-ku, Yokohama 230-8501, Japan Tel: +81-45-580-8326 E-mail: satomura-k@tsurumi-u.ac.jp |
|
| Received July 25, 2014; Accepted October 30, 2014; Published November 04, 2014 | ||
| Citation: Tezuka M, Tatehara S, Imamura T, Tachibana R, Takebe Y, et al. (2014) Possible Involvement of Leptin in the Elevated Osteoblastic Activity Observed in High Turnover Type Osteoporosis of Ovariectomized Mice. J Autacoids 3:105. doi: 10.4172/2161-0479.1000105 | ||
| Copyright: © 2014 Tezuka M et al., This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Autacoids and Hormones
Abstract
Postmenopausal osteoporosis is a high turnover type of osteoporosis induced by estrogen deficiency following menopause. In this type of osteoporosis, the osteoblastic activity is known to be elevated even though bone resorption by osteoclasts eventually exceeds bone formation by osteoblasts, resulting in the deterioration of the bone structure. Although the mechanisms underlying the progression of bone resorption in this disease are relatively well understood, the mechanisms underlying the elevated osteoblastic activity are yet to be elucidated. The purpose of this study is to investigate the possibility that leptin, a 16 kDa circulating hormone secreted mainly by white adipose tissue, is involved in the development and/or progression of the high turnover type of osteoporosis. Immunohistochemical analysis and ELISA were used to examine the expression of leptin in bones of ovariectomized mice. To investigate the effect of leptin on proliferation and osteoblastic differentiation of bone marrow stromal cells, cell proliferation assay and real-time RT-PCR analysis were performed using a mouse bone marrow stromal cell line, MMSC3. Immunohistochemistry and ELISA revealed enhanced expression of leptin in bone marrows of ovariectomized mice. A cell proliferation assay detected no significant effect of leptin on the proliferation of MMSC3 cells. In contrast, real-time RT-PCR revealed that leptin promoted the osteoblastic differentiation of this cell line. Estrogen depletion caused by ovariectomy induces the pregulation of leptin expression in the bone marrow cavity,which leads to the elevated osteoblastic activity observed in the early phase of high turnover type osteoporosis of ovariectomized mice.
| Keywords |
| Leptin; Osteoporosis; Bone marrow stromal cells; Mesenchymal stem cells; Osteoblastic differentiation; Ovariectomy |
| Introduction |
| Postmenopausal osteoporosis, which is classified as a primary and high turnover type of osteoporosis, is induced by estrogen depletion following the menopause. In this type of osteoporosis, bone resorption by osteoclasts eventually exceeds bone formation by osteoblasts, even though osteoblastic activity is normal or even enhanced. Subsequently, bone resorption becomes relatively dominant, resulting in the deterioration of bone structure [1,2]. Accumulating evidence suggests that estrogen deficiency induces bone resorption by increasing levels of proinflammatory cytokines such as IL-1, IL-6, TNF-α and HIF1α which enhance the formation and activation of osteoclasts [3-7]. However, Jilka et al. [8] reported that the lack of estrogen not only stimulates the formation of osteoclasts but also inappropriately stimulates the formation of osteoblasts in mice. In addition, the lack of estrogen is also believed to modify bone formation and the responsiveness of bone tissue to mechanical stress through direct effects on osteoblasts and osteocytes [9]. However, the cellular and molecular mechanism underlying the elevated bone turnover in osteoporosis still remains unclear. |
| Leptin, a 16 kDa circulating hormone secreted mainly by white adipose tissue, was identified by Zhang et al. [10] in 1994 as the gene responsible for hereditary obesity in obese (ob/ob) mice. It crosses the blood-brain barrier and exerts weight-reducing effects including promotion of sugar and fat metabolism, inhibition of food intake and the enhancement of energy consumption [11]. In addition to these effects on the central nervous system, leptin has been reported to exert its influence on a variety of physiological activities including hematopoiesis [12], reproductive function [13,14] and thermoregulation [15]. Although an effect of leptin on bone formation has also been reported, its effects on bone metabolism remain controversial. Some studies have reported that it has anabolic effects on bone [16-24], while other studies reported suppressive effects on bone formation [25,26]. |
| In the present study, on the basis of the hypothesis that leptin is involved in development/progression of the high turnover type of osteoporosis, we examined the expression of leptin in the bone marrow of ovariectomized mice and also investigated the effects of leptin on cell proliferation and osteogenic differentiation of mouse bone marrow stromal cells. |
| Materials and Methods |
| Preparation of ovariectomized mice |
| ICR mice (8 weeks old, female) were used for the following experiments. The housing care and experimental protocol were approved by the Animal Care and Use Committee of Tsurumi University School of Dental Medicine (Permit number: 24A064). Ovariectomy was performed under pentobarbital anesthesia (40 mg/kg), and all efforts were made to minimize suffering as described previously [27]. These animals were designated as the ovariectomized group (OVX group, n=20). In the control group (n = 20), a sham operation was performed under the same conditions as the OVX group. To monitor the growth of the mice, body weight was measured every week. |
| Histology and microfocus X-ray CT |
| Mice were sacrificed by administration of an overdose of anesthetic at 2 or 4 weeks after operation, and then a laparotomy was performed on all OVX animals to confirm the complete removal of bilateral ovaries. |
| Both tibiae were removed and fixed with 10 N Mildform® (Wako Pure Chemical Industries, Osaka, Japan). Thereafter, the microstructure of the proximal epiphyses of the tibiae was examined using a Hitachi microfocus X-ray cone beam CT apparatus (μCT, Hitachi Medical Corporation, Tokyo, Japan). Four tibiae form each group were then decalcified with 10% formic acid/10% sodium citrate and embedded in paraffin. Sections 3 μm thick were cut and observed histologically after hematoxylin and eosin staining. |
| Immunohistochemistry |
| Ten sections from each group were immunostained for leptin. After inactivation of endogenous peroxidase, sections were incubated with 10% normal rabbit serum at room temperature (RT) for 10 minutes, and then with goat anti-mouse leptin antibody (Genozyme Teche, Cambridge, USA) diluted 1:500 in phosphate buffered saline (PBS, pH 7.4) containing 1% bovine serum albumin (BSA) at 4°C overnight. |
| The localization of leptin was visualized using a Histone SAB-PO (G) Kit (Nichirei, Tokyo, Japan) and a diaminobenzidine (DAB) Substrate Kit (Nichirei). The specificity of the immunoreaction was confirmed by preabsorption of the anti-mouse leptin antibody with recombinant mouse leptin (Diaclone Research, Besan, France). |
| Cell culture |
| Mouse bone marrow stromal cells (MMSC3) [28] and MC3T3-E1 cells, a mouse osteoblastic cell line, were cultured in alpha–modified Minimum Essential Eagle’s Medium (α-MEM: Sigma Chemical Co. St. Louis, MO) containing 10% fetal bovine serum (FBS, Filton, Brooklyn, Australia), 50 μg/mL L-ascorbic acid (Wako Pure Chemical Industries, Osaka, Japan), 1x Glutamax® (Invitrogen, Carlsbad, CA), 10 mM β-glycerophosphate (Sigma), 100 units/mL penicillin (Invitrogen) and 100 μg/mL streptomycin (Invitrogen) at 37°C in a humidified atmosphere of 5% CO2 in air. The medium was changed every three days. |
| Reverse transcription-polymerase chain reaction (RT-PCR) |
| MMSC3 and MC3T3-E1 cells were seeded in 100 mm dishes, and after reaching confluence total RNA was extracted using TRIzol® reagent (Invitrogen). cDNA was synthesized from 1 μg total RNA using the Superscript III First-Strand Synthesis System (Invitrogen) after DNase I treatment. PCR was carried out in a 50 μL reaction mixture using Thermo ReddyMix PCR Master Mix (Thermo Fisher Scientific). |
| The GAPDH gene was used as an internal control for the quantity and quality of cDNA. PCR products were analyzed by ethidium bromide staining after separation by electrophoresis through a 2% agarose gel. All primer sequences were determined using established GenBank sequences, and were listed in Table 1. |
| Alkaline phosphatase (ALPase) staining |
| Bone marrow was obtained from the dissected tibiae at 3 and 4 weeks after OVX or sham operation. The epiphyses were removed, and bone marrow was flushed from the shafts using 3 mL of α-MEM expelled from a syringe through a 26-gauge needle. A single cell suspension was obtained by gently aspirating the bone marrow tissue several times through 23-gauge needles, and finally filtering through a cell strainer (100 μm sieve size, Becton Dickinson Labware, Franklin Lakes, NJ) to remove tissue debris. All the cells from each tibia were washed with α-MEM and seeded into a 100 mm dish, then cultured in α-MEM containing 10% FBS, 50 μg/mL L-ascorbic acid, 1x Glutamax® (Invitrogen), 100 units/mL penicillin (Invitrogen) and 100 μg/mL streptomycin (Invitrogen). The cultures were maintained for 5 days without changing the medium, and thereafter the medium was changed every 2 days. To determine CFU-F, cells were fixed with 4% paraformaldehyde after 10 days of culture, and then stained using an ALPase Staining Kit (Primary Cell Co., Ltd. Hokkaido. Japan). Colonies containing a minimum of 20 cells were designated as CFU-F [8] and counted under a phase-contrast microscope (CK40, Olympus Corp., Tokyo, Japan). |
| Enzyme-linked immunosorbent assay (ELISA) for leptin |
| Femora were removed from OVX and sham-operated mice. After the soft tissue was completely removed from the surface of femora, they were immediately frozen in liquid nitrogen and ground using a vibratory mill (Retch Mixer Mill, Type MM400; Retch, Haan, Germany) with 5 mm diameter stainless steel balls at a frequency of 30/sec for 1 minute. Protein solubilization was performed with RIPA Lysis buffer containing protease inhibitor cocktail (Santa Cruz Biotechnology, Santa Cruz, CA). Femoral leptin levels were measured using a Quantikine® ELISA Mouse/Rat Leptin Immunoassay kit (R&D Systems, Minneapolis, MN). |
| Western blot analysis for the leptin receptor Ob-R |
| MMSC3 and MC3T3-E1 cells were cultured in growth medium until they reached confluence. The cells were lysed in RIPA buffer (10 mM Tris–HCl, 1% NP-40, 0.1% SDS, 150 mM NaCl and 1 mM EDTA) containing protease inhibitor cocktail (Thermo Scientific, Rockford, IL). The samples were separated by the NuPAGE System (Novex; San Diego, CA) using a 4–12% Bis-Tris Gel, and electrically blotted onto a nitrocellulose membrane (Bio-Rad, Laboratories, Hercules, CA). After blocking with 5% skimmed milk at 4°C overnight, the membranes were incubated with goat anti-Ob-R antibody diluted 1:1000 at 4°C for 3 hours. Membranes were then washed several times and incubated with horseradish peroxidase-conjugated anti-goat IgG antibody (Dako Denmark; Glostrup, Denmark) diluted 1:500 in the same buffer for 1 h at RT. After several washes, Ob-R was visualized using ECL Western Lighting Plus-ECL (Perkin Elmer, Inc., Waltham, MA) and an ECL mini-camera (Amersham Pharmacia Biotech, Little Chalfont, UK). |
| Cell proliferation assay |
| The effect of leptin on the proliferation of MMSC3 cells was analyzed using the crystal violet staining method as described previously [29]. In brief, cells were seeded into 24-well culture plates at a cell density of 3 x 103 cells/well and cultured in α-MEM containing 1% FBS and various concentrations (0, 1, 5, 10 and 100 ng/mL) of leptin. At 0, 3, 5, 7, 10 and 14 days of culture, cells were fixed with 1% glutaraldehyde in PBS and stained with 0.02% crystal violet in deionized water. After several rinses, crystal violet bound to cells was extracted by incubation with 200 μL/well of 70% ethanol at 4°C overnight. Absorbance was measured at 570 nm using a microplate reader (BIO-RAD Laboratories). |
| Real-time reverse transcription-polymerase chain reaction |
| The expression of mRNA encoding type I collagen (Col I), bone sialoprotein (BSP) and osteocalcin (OC) in MMSC3 cells was examined by real-time reverse transcription-polymerase chain reaction (real-time RT-PCR). At confluence, the cells were exposed to various concentrations (0, 1, 5, 10 and 100 ng/mL) of leptin. At 7 and 14 days of culture, total RNA was extracted using TRIzol® reagent (Invitrogen). PCR was performed with SYBR® Premix ExTaq™ (Takara Bio Inc., Shiga, Japan) using an Applied Biosystems StepOneTM Real-Time PCR System (Applied Biosystems Inc., Carlsbad, CA). The GAPDH gene was used as an internal control for the quantity and quality of cDNA. All primer sequences were determined using established GenBank sequences, and were listed in Table 1. |
| Statistics |
| All values are expressed as mean ± SE. For comparisons between two groups, data were analyzed by the Mann-Whitney U-test, and for multiple group comparisons, data were analyzed by one-way ANOVA. The significance of individual differences was evaluated using the Mann-Whitney U-test with Bonferroni correction. P<0.05 was considered statistically significant. |
| Results |
| Histological changes in tibiae of ovariectomized mice |
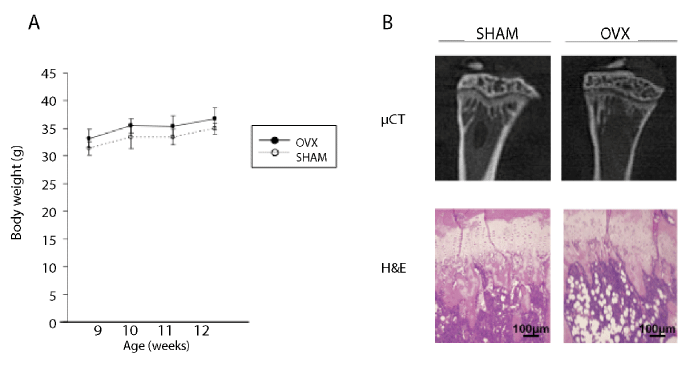
| Although OVX mice showed a slight increase in body weight compared to sham-operated mice, no statistically significant difference in body weight gain was detected between the OVX and the sham group (Figure 1A). The μCT analysis of tibiae at 4 weeks after ovariectomy revealed that cancellous bone volume in the proximal region of the tibiae was reduced in the ovariectomy group compared with the sham group. Histological observation also showed a reduction in trabecular bone beneath the growth plate in the OVX group. Moreover, the emergence of a large number of adipocytes was observed in the bone marrow cavity of OVX mice, leading to reduced cellularity in the bone marrow cavity (Figure 1B). These radiographic and histological findings confirmed that ovariectomized mice unfailingly suffered from high turnover type osteoporosis. |
| Expression of leptin in the bone marrow of OVX and sham-operated mice |
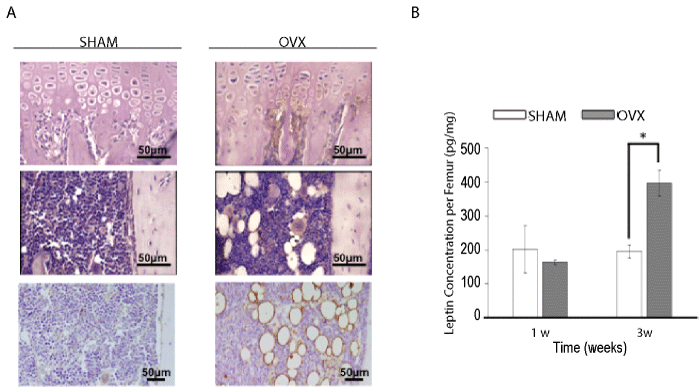
| Immunohistochemical analysis of leptin expression in tibiae showed that osteoblasts lining the primary spongiosa and hypertrophic chondrocytes expressed much more leptin in the OVX group compared with the sham group. In addition to these osteogenic/chondrogenic cells, adipocytes were also strongly positive for leptin, indicating that bone marrow adipocytes induced by estrogen depletion also strongly expressed leptin. Interestingly, megakaryocytes in the bone marrow cavity also showed slightly higher expression of leptin in ovariectomized mice (Figure 2A). The leptin protein concentration in whole femora at 1 and 3 weeks after ovariectomy was quantified by ELISA. The amount of leptin in the femora of OVX mice was approximately two-fold higher than that in the sham group at 3 weeks after operation, although no significant difference between the two groups was detected at 1 week after operation (Figure 2B). Semi-quantitative RT-PCR analysis also revealed higher expression of leptin mRNA in tibiae of ovariectomized mice at 2 and 4 weeks after operation compared with the sham group (data not shown). |
| Effect of ovariectomy on bone marrow stromal cells |
| In order to elucidate the effect of estrogen depletion on the bone marrow stromal cell population, a colony-forming assay and ALPase staining were performed using ex vivo bone marrow stromal cell cultures. The ALPase-positive colony forming ability of the bone marrow stromal cell population obtained from OVX mice showed an almost two-fold increase at 3 and 4 weeks after operation compared with that from sham-operated mice (Figure 3). Interestingly, very few colonies were observed to be completely negative for ALPase activity. |
| Expression of leptin and leptin receptor in mouse bone marrow stromal cells |
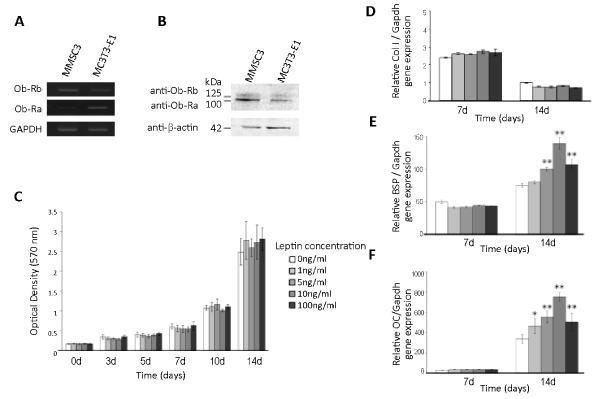
| RT-PCR analysis showed that MMSC3 cells expressed mRNA for two variant leptin receptors, Ob-Ra and Ob-Rb (Figure 4A). Western blot analysis also confirmed the expression of Ob-Ra and Ob-Rb in MMSC3 cells [30] (Figure 4B). |
| Effect of leptin on mouse bone marrow stromal cells |
| A cell proliferation assay detected no significant effect of leptin on the proliferation of MMSC3 cells at any concentration examined between 0 and 100 ng/mL (Figure 4C). In contrast, real-time RT-PCR analysis for some osteoblast differentiation markers revealed that exogenous leptin promoted the expression of some of these markers in bone marrow stromal cells. The expression of mRNA for both of bone sialoprotein, a midterm osteoblast differentiation marker and osteocalcin, a terminal osteoblast differentiation marker was enhanced by exogenous leptin in a dose dependent manner between 0 and 10 ng/mL. Interestingly, 100 ng/mL leptin showed a promoting effect equal to that of 5 ng/mL leptin (Figure 4E, F). The expression of mRNA for type I collagen, an early osteoblast differentiation marker, was not affected by exogenous leptin at any concentrations examined (Figure 4D). |
| Discussion |
| Postmenopausal osteoporosis, a high turnover type of osteoporosis induced by estrogen depletion in women, is characterized by a decrease in bone mass and density which can lead to an increased risk of fracture. In this type of osteoporosis, it is known that osteoclastic bone resorption and osteoblastic bone formation are both activated during the early stage of the disease [1,2]. Although the mechanisms underlying the activation of bone resorption are relatively well understood [1-7], the mechanisms underlying the increase in osteoblastic activity remain unclear. |
| In this study, on the basis of a hypothesis that leptin may be involved in the elevated osteoblastic activity observed during the early stage of estrogen depletion-triggered osteoporosis, we examined the expression of leptin in the bone marrow cavity of OVX mice and also investigated the cell biological effects of this molecule on mouse bone marrow stromal cells. Our results showed that hypertrophic chondrocytes, osteoblasts on the primary spongiosa and emerging adipocytes in the bone marrow cavity of OVX mice all expressed leptin more strongly than those in sham-operated mice. An ELISA also confirmed that the amount of leptin in the femora of OVX mice was much higher than that in the sham group. These findings clearly reveal that ovariectomy, i.e. estrogen depletion, could trigger enhanced expression of leptin in the bone marrow and also suggest the possibility that leptin could exert some local influence on the onset and/or progression of the high turnover type of osteoporosis, particularly in elevating osteoblastic activity, acting as a paracrine or autocrine factor. |
| Based on this outcome, we next performed a colony-forming assay to examine whether the osteoblastic differentiation of bone marrow stormal cells could be enhanced by exogenous leptin. We observed a two-fold increase in the number of ALPase-positive colonies, which presumably consisted of cells committed to the osteogenic cell lineage, in the OVX group compared with the sham group. Moreover, cell proliferation assay and real time RT-PCR for BSP and OC showed that leptin exerted no significant influence on the proliferation of bone marrow stromal cells, whereas the osteoblastic differentiation of bone marrow stromal cells was enhanced by exogenous leptin. These results are consistent with the results reported by Thomas et al. [20] and Chang et al. [22] using human bone marrow stromal cells. |
| Taken together, the results of our investigation strongly suggest that estrogen depletion by ovariectomy/menopause stimulates the production of leptin by some types of cells in the bone marrow cavity such as chondrocytes, osteoblasts and adipocytes, stimulating the osteoblastic differentiation of bone marrow stromal cells. We therefore hypothesize that the upregulation of leptin following estrogen depletion is, at least in part, involved in the elevated osteoblastic activity observed in the high turnover type of osteoporosis. The detailed molecular mechanisms connecting estrogen depletion and leptin upregulation are still unclear, but this issue will be addressed in a future study. |
References
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285: 785-795.
- Armas LA, Recker RR (2012) Pathophysiology of osteoporosis: new mechanistic insights. Endocrinol Metab Clin North Am 41: 475-486.
- Manolagas SC, Kousteni S, Jilka RL (2002) Sex steroids and bone. Recent Prog Horm Res 57: 385-409.
- Rogers A, Eastell R (2001) The effect of 17beta-estradiol on production of cytokines in cultures of peripheral blood. Bone 29: 30-34.
- Pfeilschifter J, Köditz R, Pfohl M, Schatz H (2002) Changes in proinflammatory cytokine activity after menopause. Endocr Rev 23: 90-119.
- Pacifici R (1996) Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res 11: 1043-1051.
- Miyauchi Y, Sato Y, Kobayashi T, Yoshida S, Mori T, et al. (2013) HIF1α is required for osteoclast activation by estrogen deficiency in postmenopausal osteoporosis. Proc Natl Acad Sci U S A 110: 16568-16573.
- Jilka RL, Takahashi K, Munshi M, Williams DC, Roberson PK, et al. (1998) Loss of estrogen upregulates osteoblastogenesis in the murine bone marrow. Evidence for autonomy from factors released during bone resorption. J Clin Invest 101: 1942-1950.
- Spelsberg TC, Subramaniam M, Riggs BL, Khosla S (1999) The actions and interactions of sex steroids and growth factors/cytokines on the skeleton. Mol Endocrinol 13: 819-828.
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, et al. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425-432.
- Bryson JM, Phuyal JL, Swan V, Caterson ID (1999) Leptin has acute effects on glucose and lipid metabolism in both lean and gold thioglucose-obese mice. Am J Physiol 277: E417-422.
- Gainsford T, Willson TA, Metcalf D, Handman E, McFarlane C, et al. (1996) Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc Natl Acad Sci U S A 93: 14564-14568.
- Zachow RJ, Magoffin DA (1997) Direct intraovarian effects of leptin: impairment of the synergistic action of insulin-like growth factor-I on follicle-stimulating hormone-dependent estradiol-17 beta production by rat ovarian granulosa cells. Endocrinology 138: 847-850.
- Spicer LJ, Francisco CC (1997) The adipose obese gene product, leptin: evidence of a direct inhibitory role in ovarian function. Endocrinology 138: 3374-3379.
- Hwa JJ, Ghibaudi L, Compton D, Fawzi AB, Strader CD (1996) Intracerebroventricular injection of leptin increases thermogenesis and mobilizes fat metabolism in ob/ob mice. Horm Metab Res 28: 659-663.
- Reseland JE, Syversen U, Bakke I, Qvigstad G, Eide LG, et al. (2001) Leptin is expressed in and secreted from primary cultures of human osteoblasts and promotes bone mineralization. J Bone Miner Res 16: 1426-1433.
- Holloway WR, Collier FM, Aitken CJ, Myers DE, Hodge JM, et al. (2002) Leptin inhibits osteoclast generation. J Bone Miner Res 17: 200-209.
- Kume K, Satomura K, Nishisho S, Kitaoka E, Yamanouchi K, et al. (2002) Potential role of leptin in endochondral ossification. J Histochem Cytochem 50: 159-169.
- Baldock PA, Sainsbury A, Couzens M, Enriquez RF, Thomas GP, et al. (2002) Hypothalamic Y2 receptors regulate bone formation. J Clin Invest 109: 915-921.
- Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, et al. (1999) Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. See comment in PubMed Commons below Endocrinology 140: 1630-1638.
- Gordeladze JO, Drevon CA, Syversen U, Reseland JE (2002) Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: Impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem 85: 825-836.
- Chang YJ, Shih DT, Tseng CP, Hsieh TB, Lee DC, et al. (2006) Disparate mesenchyme-lineage tendencies in mesenchymal stem cells from human bone marrow and umbilical cord blood. Stem Cells 24: 679-685.
- Scheller EL, Song J, Dishowitz MI, Soki FN, Hankenson KD, et al. (2010) Leptin functions peripherally to regulate differentiation of mesenchymal progenitor cells. Stem Cells 28: 1071-1080.
- Motyl KJ, Rosen CJ (2012) Understanding leptin-dependent regulation of skeletal homeostasis. Biochimie 94: 2089-2096.
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, et al. (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100: 197-207.
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, et al. (2005) Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434: 514-520.
- Kalu DN, Chen C (1999) Ovariectomized murine model of postmenopausal calcium malabsorption. J Bone Miner Res 14: 593-601.
- Satomura K, Krebsbach P, Bianco P, Gehron Robey P (2000) Osteogenic imprinting upstream of marrow stromal cell differentiation. J Cell Biochem 78: 391-403.
- Fedarko NS, D'Avis P, Frazier CR, Burrill MJ, Fergusson V, et al. (1995) Cell proliferation of human fibroblasts and osteoblasts in osteogenesis imperfecta: influence of age. J Bone Miner Res 10: 1705-1712.
- Lamghari M, Tavares L, Camboa N, Barbosa MA (2006) Leptin effect on RANKL and OPG expression in MC3T3-E1 osteoblasts. J Cell Biochem 98: 1123-1129.
Tables and Figures at a glance
| Table 1 |
 |
 |
 |
 |
|||
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14252
- [From(publication date):
November-2014 - Jul 05, 2025] - Breakdown by view type
- HTML page views : 9617
- PDF downloads : 4635
