Review Article Open Access
Polyp Size Estimation Strategies during Endoscopy Procedures: A Review
Fabio Martínez, Josué Ruano, Martín Gómez and Eduardo Romero*
Computer Imaging and Medical Applications Laboratory, CIM@Lab, Universidad Nacional de Colombia, Colombia, USA
- *Corresponding Author:
- Eduardo Romero
Carrera 30 45-03, Ciudad Universitaria Facultad de Medicina-Edificio 471. Bogotá D.C
Colombia, USA
Tel: +57-1-3165000
Fax: 15025; E-mail: edromero@unal.edu.co
Received date: May 25, 2015 Accepted date: August 12, 2015 Published date: August 18, 2015
Citation: Martínez F, Ruano J, Gómez M, Romero E (2015) Polyp Size Estimation Strategies during Endoscopy Procedures: A Review. J Gastrointest Dig Syst 5:329. doi:10.4172/2161-069X.1000329
Copyright: © 2015 Martínez F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Colorectal cancer is the seventh cause of death and a relevant public health problem. This aggressive cancer is commonly identified during a routine endoscopy examination by characterizing a set of polyps that may appear along the colon and rectum. The polyps are usually described by the tissue appearance, shape, rigidity, surface irregularity, resectability, among others. In particular, the polyp size is an important index since large polyps are labeled as advanced adenomas with strong evidence that such adenomas may turn into cancer. Additionally, this index may also determine the procedure and surgical polyp management because of the bleeding risk. In some protocols, the polyp size indicates a closer surveillance, with additional genetic analyses. Currently, polyp size estimation is a challenge because several limitations such as the endoscopy camera distortions, illumination and exacerbated physiological conditions, abrupt motion and expert subjectivity. This work presents a state-of-the-art review of the current semi-automatic computational approaches for estimating the polyp size, aiming to reduce the subjectivity while exploring the gastrointestinal tract.
Keywords
Polyp size estimation; Endoscopy; Colorectal cancer
Introduction
Digestive diseases prevail in most public health reports as a major century concern, affecting in 2010 more than 70 million people [1]. Among these diseases, the colorectal cancer is the seventh cause of death [2], with around 1.2 million new cases worldwide and about 5000 in Colombia during 2009 [3-5]. This cancer is basically an asymptomatic illness, being usually the first evidence an abnormal mucosal growth at any place of the digestive tract, known as polyps [6,7].
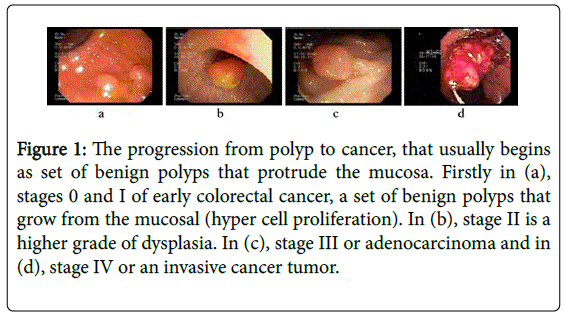
Currently, the colorectal cancer is diagnosed and followed up by examining and searching relevant features of the gastrointestinal tract during an endoscopy procedure. The expert typically looks for salient facets of the gastrointestinal mucosa such as abnormal tissue appearance, atypical polyp shape and morphology, altered stiffness of specific tract regions, and surface irregularity and abnormal protruding mucosal growth. These clinical signs, complemented by the histopathological study, define the the malignancy [8,9]. For instance, Figure 1 illustrates some polyp states for different cancer stages. In the early phases (Figure 1, subplot a), polyps are characterized by hyperproliferation (<5 mm) in the lamina propria of the tract mucosa, appearing sessile, smooth and glassy. For a level 2, (Figure 1, subplot b) an adenomatous lesion grows out the intestinal lumen and usually invades the muscularis propria (6-10 mm). In a more advanced phase (Figure 1 subplot c), polyps looks pedunculated (11-30 mm) and the dysplastic cells invade the smooth muscle layer and the serosa, generally with lymph nodes involvement. Finally, in the most advanced stage of the disease, as illustrated in Figure 1 subplot (d), the lesion is an adenocarcinoma with typical sizes larger than 30 mm and with high metastasis risk. In fact, several studies have shown that if a polyp is in average growing more than 3 mm per year, there exists a high probability of colorectal cancer [9-12]. This strong correlation between the polyp growing velocity and the lesion malignancy has been also described in different reports and for different populations studies [8,12,13]. For instance, around 83% of cancer incidence in 345 removed polyps with size larger than 10 mm has been reported [14], samples that were additionally histologically analyzed to determine advanced adenomas [15].
Figure 1: The progression from polyp to cancer, that usually begins as set of benign polyps that protrude the mucosa. Firstly in (a), stages 0 and I of early colorectal cancer, a set of benign polyps that grow from the mucosal (hyper cell proliferation). In (b), stage II is a higher grade of dysplasia. In (c), stage III or adenocarcinoma and in (d), stage IV or an invasive cancer tumor.
The polyp size is useful to support some clinical decisions, for instance patient surveillance protocols are typically determined by following the polyp growing, the hereditary susceptibility (genetic testing) and a complete morphological characterization [9]. On the other hand, the resection decision is performed during a colonoscopy procedure, being fundamental an appropriate selection of different instruments and clinical protocols. In this case the resectability factor is estimated by taking into consideration different variables such as the polyp size, the surface irregularity, fragility, bleeding trend and how invasive is the lesion into the underlying bowel wall [6,7,15-19]. In some cases, microscopical characteristics of sessile polyps must be examined [20], case in which polyps are categorized as hyperplastic, inflammatory, fundic and adenomatous, being the two last the two most prone to evolve to cancer, usually showing exaggerated cellular proliferation, few differentiation, senescence trend and increased programmed cell death [18,19,21,22].
During the endoscopy, a monocular video-camera with specialized wide angle lens facilitates the visualization and estimation of much of the polyp geometrical and appearance variables [19]. This videosequence is however distorted by many physiological factors, which many times occlude or distort the estimated measures. Therefore, additional instruments support the different measures, for instance, special micro-rules and biopsy forceps, devices that an expert uses to compare a lesion width with previously standardized dimensions [19,23]. This routine size estimation is however highly subjective and prone to errors because of the dependence on the observer expertise to manipulate the measure tools while estimating the polyp size, but also since the environment is variable due to changing optical conditions and variable physiological states. These factors make a gastroenterologist needs specialized and intense training to achieve reliable polyp measures [24]. Given the importance of the polyp size estimation, this review is dedicated to further describe the state-of-theart concerning current clinical strategies and semi-automatic computational approaches for estimating the polyp size during endoscopy, aiming to reduce the expert estimation variability.
Dealing with Observational Polyp Size Variability
A diagnosis of gastric cancer has been established in about a 94% of patients by using typical endoscopy examination [25]. After diagnosis is reached, preoperative evaluation aims to establish and classify the polyp malignancy based on the shape, size, color and location characteristics, after the Borrmann classification [26]. Additionally, polyp morphological characteristics are fundamental at correlating the endoscopic diagnosis with histological analysis. However, after polyps are excised, most morphological features are importantly altered because of the dehydration and formalin fixation procedures [27,28]. As a result, in spite of the necessity of quantifying polyp morphological features, estimation of such characteristics is fully dependent on the gastroenterologist experience and the endoscopy video characteristics [25,29].
In clinical routine, the polyp sizes can be estimated: 1) by an exhaustive expert examination, 2) by comparing the polyp size with open biopsy forceps that span a known measure or 3) by probe tools. The most common methodology is a simple endoscopic examination fully. However, this technique is highly variable and prone to errors since polyps may be hidden by some physiological conditions and a reference to compare is really difficult to establish. This lack of ground truth results in inaccurate estimation and has been systematically studied, reporting around a 6.4% of error between the real and estimated sizes [23]. Interestingly, the expert estimates the polyp size by using a direct comparison with biopsy forceps that are usually opened near to the lesion. Nevertheless, this method has been reported as introducing a 12.3% of error w.r.t real measures because of the variability at locating the biopsy forceps, the irregular perspective for the polyp observation and the limited range of the device aperture. Currently, the best polyp size estimation is obtained by supporting the polyp examination with probe tools. This device is a flexible grid that is introduced by a channel of the endoscope and allows a direct comparison with the lesion. By studying 100 polyps with a wide range of size, real measures and estimations with the probe tools showed a mean difference of 3.4% [23]. Yet probe tools estimate the polyp size with less error that other classical approaches, in practice these methodologies remain limited because they are highly dependent on the expert aptness to find a proper perspective angle, they are in most cases invasive and of course their accuracy is dependent on the appropriate localization of the supporting tools with respect to the lesion. Additional techniques and devices complement or search more specific characteristics of the pathology, for instance, extramural polyps are usually identified by the echo-endoscopy device [19]. Some new sophisticated endoscopes zooms out the lesion up to highlight important histological characteristics in vivo [30,31]. Also, there exist stereo-endoscopy devices that reconstruct three dimensional surfaces, for a complete evaluation of the digestive tract [32]. Other strategy is the virtual endoscopy from CT images that obtains three dimensional re-construction but at the cost of the side effect of largely exposing the patient to ionizing irradiation [33-36].
Characterization of Polyps by Computational Video- Strategies
Currently, image and video processing strategies have been adapted for characterization of polyps that are non-invasive, reduction of subjectivity and estimations that better predict and follow the pathology [31,32,34]. In general, these strategies search predominant primitives such as the appearance, contrast, or motion among others, which might be correlated with the lesion so that polyp classification may be improved. In spite of the advantages of these computational strategies, there exist many limitations and challenges for characterization of polyps, mainly because: 1) certain zones of the image look completely saturated due to specular highlights [37,38], 2) the fuzzy boundary between the polyp and the tract [38] and 3) abrupt movements of the endoscope device while performing the navigation.
Currently, some computational strategies have been proposed for characterization of lesions along the digestive tract, most of them based on intensity or geometrical appearance at each frame [39]. Liu et al. [40] reconstructs a 3D intestinal tract using flow deformation maps computed during the endoscopy sequence. In this strategy, a set of salient points are also matched and propagated [23], but consecutive correspondence between these points is hardly established in case of homogeneous textures as the intestinal tract. As a result, the apparent motion estimation is easily contaminated by the noise introduced by abrupt motions. Although the available probe tools estimate the polyp size with less error that the other classical approaches, these methods require a manual initialization to identify the presence/absence of polyps. In contrast, Bernal et al. [37] and Condessa et al. [41] approximate the polyp shape by using per-frame static features such as the local binary maps and a set of local derivatives which are mapped by a typical support vector machine that finally obtains the polyp segmentation. The former investigation [37] uses the intensity and edges that follow a polyp appearance while the latter [41] is based on a set of classical geometrical and color descriptors. Nevertheless this kind of characterizations is strongly sensible to intensity polyp changes occurred during the sequence while the fuzzy edges can distort the segmentation by the high illumination variation.
Other strategies highlight 3D polyp shape characteristics by using geometric and brightness depth assumptions as well as manual interventions. For instance, Hong et al [39] propose a strategy to semiautomatically reconstruct the tract by introducing tubular prior shapes while using a sequence of images as observations. This strategy is however limited when representing the tract as simple geometrical primitives while local deformations are not considered. An additional work was proposed by Kaufman et al. [24] who achieved a 3D representation by a local strategy in which the tract is reconstructed by sub-regions and then partially integrated. In this approach, shape observations are refined with the characterization of the shadows. This strategy is nevertheless computationally highly expensive and the reconstruction may take long time. Additionally, Alcantarilla et al. [42] use a set of salient points as information to reconstruct the digestive tract. All these strategies may fail because the presence of noise, similar texture pattern and the abrupt changes of the camera during the sequence.
Regarding the polyp size estimation, several computational strategies have been proposed to cope with identification, characterization and measure of polyps. Ganz et al. [43] used a multispectral endoscopic imaging to highlight the region that bounded the polyp, expecting certain histological properties are present. Then, the boundary is detected using a prior shape term as regularizer. This method requires a special device to characterize the polyp, that is to say, to define the set of histological characteristics that might be associated to the lesion. In addition, this approach results dependent on a very large database that can store the high shape variability. Besides, Chadebecq et al. [44] proposed a semi-automatic method that started by manually placing a bounding box surrounding the polyp, followed by a conventional affine registration that propagates such initial guess to the whole sequence and estimates the best focused region by a depth learning procedure. However, in real conditions, the camera movements may be so rapid that the RoI easily losses the polyp and the break focus is determined for the entire bounding box, with the consequent error coming from calculating the polyp distance as a linear function of the estimated depth within the box.
Conclusions
This review has presented and analyzed the problem of estimating the polyp size during endoscopy procedures as one of the potential indicators of colorectal cancer. Overall, the polyp size is estimated during an actual procedure and the automation process is a very challenging task because of the different nature of noises that may be present and mixed up in a complex manner. Some of the presented strategies deal with the estimation of polyp size. The results of these studies demonstrate the importance of measuring polyps during the endoscopic procedure while they also indicate that novel approaches are required for these measurements reach proper levels of confidence. Likewise, these techniques should be integrated with the actual workflow of the endoscopic procedure while they also should be computationally optimal.
References
- Eckel, Robert H, Barouch, Winifred W, Ershow, et al. (2002) Report of the National Heart, Lung, and Blood Institute National Institute of Diabetes and Digestive and Kidney Diseases Working Group on the pathophysiology of obesity-associated cardiovascular disease. In: Circulation 105: 2923-2928.
- (2008) Organization, World H. Ten leading causes of death worldwide.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics.CA Cancer J Clin 61: 69-90.
- (2008) Organization, World H. Cause-specific mortality, 2008: World Bank income group N 1542-4863.
- de la Salud, Organización P (2007) OPS (Ed.): Salud en lasAméricas.
- Frazier AL, Colditz GA, Fuchs CS, Kuntz KM (2000) Cost-effectiveness of screening for colorectal cancer in the general population.JAMA 284: 1954-1961.
- Ramsey SD1, Yoon P, Moonesinghe R, Khoury MJ (2006) Population-based study of the prevalence of family history of cancer: implications for cancer screening and prevention.Genet Med 8: 571-575.
- Shinya H, Wolff W (1979) Morphology, anatomic distribution and cancer potential of colonic polyps.Ann Surg 190: 679-683.
- Church JM (2003) Experience in the endoscopic management of large colonic polyps.ANZ J Surg 73: 988-995.
- Hofstad B, Vatn MH, Andersen SN, Huitfeldt HS, Rognum T, et al. (1996) Growth of colorectal polyps: redetection and evaluation of unresected polyps for a period of three years. In: Gut Nr. 3: 449-456.
- Morson B (1974) President's address. The polyp-cancer sequence in the large bowel.Proc R Soc Med 67: 451-457.
- Potet F, Soullard J (1971) Polyps of the rectum and colon.Gut 12: 468-482.
- Tribukait B, Hammarberg C,Rubio C (1983) Ploidy and proliferation patterns in colorectal adenocarcinomas related to dukes ‘classification and to histopathological differentiation. ActaPathologicaMicrobiologicaScandinavica Series A: Pathology 91: 89-96.
- Sakamoto T, Matsuda T, Nakajima T, Saito Y (2013) Clinicopathological features of colorectal polyps: evaluation of the 'predict, resect and discard' strategies.Colorectal Dis 15: e295-300.
- Hiroyasu L, Tatsuta M, Narahara H, Iseki K, Sakai N (1996) Endoscopic resection of large pedunculated colorectal polyps using a detachable snare, Gastrointestinal Endoscopy, Volume 44: 594-597.
- Hassan C, Repici A, Sharma P, Correale L, Zullo A, et al. (2015) Efficacy and safety of endoscopic resection of large colorectal polyps: a systematic review and meta-analysis.Gut .
- Summers RM (2010) Polyp size measurement at CT colonography: what do we know and what do we need to know?Radiology 255: 707-720.
- Muto T, Bussey HJ, Morson BC (1975) The evolution of cancer of the colon and rectum.Cancer 36: 2251-2270.
- Luis R, Albis H, Diego A, Luis S, Fabio G, et al. (2007) (Ed.) ; Restrepo, Angela (Ed.): Técnicas en EndoscopiaDigestiva. ACED - AsociaciónColombiana de EndoscopiaDigestiva.
- Christie JP (1976) Colonscopic excision of sessile polyps.Am J Gastroenterol 66: 23-28.
- Mark F, Lawrence FS, Lawrence JB (2010) Sleisenger and Fordtran’s gastrointestinal and liver disease: pathophysiology, diagnosis, management, expert consult premium edition-enhanced online features. Vol. 1. Elsevier Health Sciences.
- Yaron N, Georges D, Ami DS, Judith S,Howard Z (2003) Hyperplastic gastric polyposis, hypergastrinaemia and colorectal neoplasia: a description of four cases. En: European journal of gastroenterology &hepatology 15: 1361-1366.
- Gopalswamy, Narasimh, Shenoy, Vishwanath N,Choudhry, et al. (1997) Is in vivo measurement of size of polyps during colonoscopy accurate? In: Gastrointestinal Endoscopy 6: 497-502.
- Kaufman, Arie, Wang, Jianning (2008) 3D Surface Reconstruction from Endoscopic Videos. In: Linsen, Lars, Hagen, Hans,Hamann, Bernd(eds): Visualization in Medicine and Life Sciences. Springer Berlin Heidelbergp. 61-74.
- Wanebo HJ, Kennedy BJ, Chmiel J, Steele G Jr, Winchester D, et al. (1993) Cancer of the stomach. A patient care study by the American College of Surgeons.Ann Surg 218: 583-592.
- Larraga A, Cossio J, Guerrero S, Pluma AC, Jesus VC (2003) The Borrmann classification. Interobserver and intraobserver agreement of endoscopists in an oncological hospital. In: Clinical and Translational Oncology 5: 345-350.
- Morales TG, Sampliner RE, Garewal HS, Fennerty MB, Aickin M (1996) The difference in colon polyp size before and after removal.GastrointestEndosc 43: 25-28.
- Schoen RE, Gerber LD, Margulies C (1997) The pathologic measurement of polyp size is preferable to the endoscopic estimate.GastrointestEndosc 46: 492-496.
- Francis AF, Jerome DW, Maria M, Timothy CH, Robert DO (2007) Variability in the diagnosis and management of adenoma-like and non-adenoma-like dysplasia-associated lesions or masses in inflammatory bowel disease: an Internet-based study. In: Gastrointestinal Endoscopy 3: 519-529.
- Hurlstone DP, Cross SS, Adam I, Shorthouse AJ, Brown S (2004) Efficacy of high magnification chromoscopic colonoscopy for the diagnosis of neoplasia in flat and depressed lesions of the colorectum: a prospective analysis. In: An International Journal of Gastroenterology and Hepatology 54: 284-290.
- Bruno MJ (2003) Magnification endoscopy, high resolution endoscopy, and chromoscopy; towards a better optical diagnosis.Gut 52 Suppl 4: iv7-11.
- Karargyris A, Karargyris O, Bourbakis N (2010) 3D Representation of the Digestive Tract Surface in Wireless Capsule Endoscopy Videos. In: BioInformatics and BioEngineering (BIBE), 2010 IEEE International Conference on p. 279-280.
- de Vries AH, Bipat S, Dekker E, Liedenbaum MH, Florie J, et al. (2010) Polyp measurement based on CT colonography and colonoscopy: variability and systematic differences.EurRadiol 20: 1404-1413.
- Dijkers JJ, van Wijk C, Vos FM, Florie J, Nio YC, et al. (2005) Segmentation and size measurement of polyps in CT colonography.Med Image ComputComput Assist Interv 8: 712-719.
- Dachman AH, Yoshida H (2003) Virtual colonoscopy: past, present, and future.RadiolClin North Am 41: 377-393.
- Yao J, Miller M, Franaszek M, Summers RM (2004) Colonic polyp segmentation in CT colonography-based on fuzzy clustering and deformable models.IEEE Trans Med Imaging 23: 1344-1352.
- Bernal J, Sánchez J, VilarioF (2012)Towards automatic polyp detection with a polyp appearance model. In: Pattern Recognition 45 (2012), Nr. 9, p. 3166 - 3182. - Best Papers of Iberian Conference on Pattern Recognition and Image Analysis (IbPRIA’2011). ISSN 0031-3203.
- Grossman P (1987) Depth from Defocus. In: Journal Pattern Recognition Letters 5: 63-69.
- Ho HD, Tavanapong W, Wong J, JungHwanO, Groen P (2009) 3D Reconstruction of Colon Segments from Colonoscopy Images. In: Bioinformatics and BioEngineering, 2009. BIBE ’09. Ninth IEEE International Conference p. 53-60.
- Liu J, Subramanian KR, Yoo TS (2013) A robust method to track colonoscopy videos with non-informative images.Int J Comput Assist RadiolSurg 8: 575-592.
- Filipe C, Bioucas-Dias, José (2012) Segmentation and Detection of Colorectal Polyps Using Local Polynomial Approximation. In: Campilho, Aurélio; Kamel, Mohamed (eds): Image Analysis and Recognition Vol. 7325. Springer Berlin Heidelberg 188-197.
- Alcantarilla PF, Bartoli A, Chadebecq F, Tilmant C, Lepilliez V (2013) Enhanced imaging colonoscopy facilitates dense motion-based 3D reconstruction. In: Engineering in Medicine and Biology Society (EMBC), 2013 35th Annual International Conference of the IEEE 7346-7349.
- Ganz M, Xiaoyun Yang, Slabaugh G (2012) Automatic segmentation of polyps in colonoscopic narrow-band imaging data.IEEE Trans Biomed Eng 59: 2144-2151.
- Chadebecq F, Tilmant C, Bartoli A (2013) Using the Infocus-breakpoint to estimate the scale of neoplasia in colonoscopy. In: Biomedical Imaging (ISBI). IEEE 10th International Symposium p. 354-357.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 21218
- [From(publication date):
October-2015 - Jul 15, 2025] - Breakdown by view type
- HTML page views : 16492
- PDF downloads : 4726