Research Article Open Access
Polychlorinated Dibenzo-P-Dioxins (PCDD), Polychlorinated Dibenzofurans (PCDF) and Dioxin-Like Polychlorinated Biphenyls (Dl-PCB) in the Baltic and Arctic Fish and the Further Trophic Transfer of these Pollutants to Seabirds
Elżbieta Niemirycz1, Joanna Szlinder-Richert2, Ott Roots3,4, Lucyna Falkowska1, Ksenia Pazdro5, Agata Zaborska5, Matti Verta6, Grażyna Sapota7, Maria Witt1, Andrzej R Reindl1 and Marta E Kobusińska1*1University of Gdansk, Institute of Oceanography, Department of Marine Chemistry and Environmental Protection, 46 Marszalka Pilsudskiego Str., Gdynia 81-378, Poland
2National Marine Fisheries Research Institute, Department of Food and Environmental Chemistry, Kollataja Str, Gdynia 81-332, Poland
3Estonian Environmental Research Centre, Marja 4D, 10 617 Tallinn, Estonia
4University of Tartu, Estonian Marine Institute, Mäealuse 10A, 12 618 Tallinn, Estonia
5Institute of Oceanology of the Polish Academy of Sciences, 55 Powstancow Warszawy Str, Sopot 81-712, Poland
6Finnish Environment Institute (SYKE), P.O. Box 140 Helsinki, Finland
7Maritime Institute in Gdansk, Department of Environmental Protection, 41/42 Dlugi Targ Str, Gdansk 80-830, Poland
- *Corresponding Author:
- Marta E Kobusińska
University of Gdansk, Institute of Oceanography
Department of Marine Chemistry and Environmental Protection
46 Marszalka Pilsudskiego Str., Gdynia 81-378, Poland
Tel: +48 58 523 66 44
E-mail: marta.kobusinska@ug.edu.pl
Received date: November 24, 2016; Accepted date: February 08, 2017; Published date: February 16, 2017
Citation: Niemirycz E, Szlinder-Richert J, Roots O, Falkowska L, Pazdro K, et al. (2017) Polychlorinated Dibenzo-P-Dioxins (PCDD), Polychlorinated Dibenzofurans (PCDF) and Dioxin-Like Polychlorinated Biphenyls (Dl-PCB) in the Baltic and Arctic Fish and the Further Trophic Transfer of these Pollutants to Seabirds. J Marine Sci Res Dev 7:221. doi: 10.4172/2155-9910.1000221
Copyright: © 2017 Niemirycz E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
The study presents the analysis of polychlorinated dibenzo-p-dioxins, dibenzofurans (PCDD/F) and dioxin-like polychlorinated biphenyls (dl-PCB) in fish and seabirds from the Baltic Sea area and the Arctic region (the Svalbard Archipelago). Long-time studies revealed no significant temporal trends in PCDD/F and dl-PCB concentrations in fish from the Baltic Sea. Trace amounts of PCDD/F were detected in the Arctic fish as an evidence of their anthropogenic sources from the temperate zone, however congener profile in the cod was entirely different than in the Baltic fish. On the contrary, both concentrations and profiles of dl-PCB were comparable in cod from the Baltic Sea and from the polar region. PCDD/F`s congener profile in African penguin`s tissues breed in captivity and fed only by herring exhibited a clear resemblance to the profiles measured in herring. This indicates a relevant impact of diet (fish) on PCDD/F`s level in seabirds. Diet impact on the further transfer of these xenobiotics in the trophic chain, evidenced in the presented study, appears to be of the particular relevance due to the human health.
Keywords
Dioxins; Congener profiles; Marine organisms; Trophic chain; Diet impact; Climatic zones
Introduction
Properties of halogenated organic compounds, such as persistence, toxicity, bioaccumulation and potential for long range atmospheric transport on airborne particles pose a threat to marine organisms as well as human, so their emission must be reduced. The Stockholm Convention [1] and the Convention on Long-Range Transboundary Air Pollution (UNECE) [2] created a lists of hazardous substances and solutions aimed at mitigation of a global environmental risk Most of these compounds are produced commercially therefore, the control of their emission is controllable. However, polychlorinated dibenzop- dioxins and dibenzo-p-furans (PCDD/F) are a subject of particular concern since they are produced unintentionally in industrial and nonindustrial combustion processes or chemical reactions occurring in the environment, as well as in natural processes like volcanic eruptions or forest fires. The list of unintentionally produced persistent organic pollutants (UPOPs) has been doubled during recent years [3]. Due to the typical properties of dioxins and other POPs as hydrophobicity and low vapor pressure, these contaminants are characterized by relatively limited mobility in environmental compartments. Nevertheless, a vertical migration into groundwater, as well as horizontal transport by air can be achieved easily [4-6]. Increased mobility can be caused by the presence of other contaminants, e.g. the group of dense non-aqueous phase liquids (DNAPLs), including pesticides and organic solvents, but also creosote and soot. These impurities may result in the formation of colloidal particles of mobile POPs or facilitate the sorption process in the solid phases of atmospheric aerosols. Sites contaminated with halogenated organic compounds revealed presence of 70% of DNAPLs in a groundwater (US EPA) [7]. Thus, the ability of POPs to migrate in environmental compartments, particularly via atmosphere has become an important criterion for the environmental risk assessment. In aquatic ecosystems, pollutants originating from industrial activities, transport, and other human activities enter the sea through river inflow, direct discharge from the land, and atmospheric inputs. In the marine environment, organic pollutants such PCDD/F/dl-PCB exists as dissolved in the water, and as adsorbed on the suspended particles in the water column. Due to the high hydrophobicity, concentrations of organic pollutants in waters are relatively low, while the sediments constitute the significant reservoir for organic contaminants, posing a threat for the benthic organisms and for the water column due to the resuspension or remobilization processes, dependent on the particular compound properties and environmental conditions. These compounds are known to induce a number of adverse changes in the ecosystem while have been introduced to the trophic chain. Concentrations of pollutants in the sediments or water do not reflect their bioavailability. Therefore, biota is useful indicator for environmental risk assessment, representing a load of contaminants to the environment. Fish are a good indicator for assessment of the state of the environment, especially due to their trophic relationships to birds and other consumers as well as too human. Thus the risk assessment of aquatic ecosystems is very important.
Since the human remain exposed to PCDD/F/dl-PCB mainly via consumption, the EU has established their acceptable limits in several food groups, i.a. in fish. The latest European Commission Directive (EC) [8] establishes the maximum limit of PCDD/F in fish as 3.5 WHO-TEQ pg Ô?? g-1 w.w. and the maximum limit of the sum of PCDD/F and dl-PCB for 6.5 WHO-TEQ pg g-1 w.w.
The study aimed at comparison of levels and congener patterns of PCDD/F and dl-PCB in fish from the Baltic Sea- the basin particularly sensitive due to unique hydrological and geographical conditions, with the polar region, the Spitzbergen Island on the Arctic Ocean (the Svalbard Archipelago), distant from point sources of pollutants
The phenomenon of migration of pollutants is of the major relevance in the assessment of contamination of regions previously considered as unpolluted. Detection of hazardous substances in the Arctic region confirms a transboundary migration, suggesting a relevant threat to the organisms inhabiting there and also indicates insufficient decisionmaking in environmental protection of industrialized temperate zone. Furthermore, PCDD/F profiles in seabird (bred in captivity) and herring as its food was compared to assess the possible impact of diet.
Materials and Methods
Sampling
Fish: Concentrations of PCDD (7 congeners from tetra to octa), PCDF (10 congeners from tetra to octa) and 12 dl-PCB (non-ortho 77, 81, 126 and 169 and mono- ortho: 105, 114, 118, 123, 156, 157, 167 and 189) have been analysed in the muscle tissue of five fish species: herring (Clupea harengus membras), salmon (Salmo salar), sprat (Sprattus sprattus balticus), flounder (Platichthys flesus) cod (Gadus morhua callarias), collected in 2002-2014 from the Baltic Sea and a cod (Gadus morhua morhua) from the Kongsfiord on the Svalbard Archipelago (Figure 1), the Arctic.
Baltic fish were caught offshore by trawl and in the coastal area using gillnet tools. Arctic cod was caught using fishing rods from r/v “Oceania” board within “AREX 2013” campaign fishing area and the number of samples is given in Table 1. Some of the results presented have been yet published by Szlinder-Richert et al. [3,8-10].
Seabirds: PCDD/F were analyzed in muscle and liver tissues in three species of seabirds: wild living gulls- herring gull (Larus argentatus) and great great black-backed gull (Larus marinus), inhabiting the coastal zone of the Gulf of Gdansk (Poland) and the African penguin breed in captivity (Spheniscus demersus), from the culture the Zoological Garden in Gdansk. Three individuals of herring (Clupea harengus membras) from the Gulf of Gdansk were analyzed (whole fish as one sample) as a seabirds` diet component.
Sample Processing and Analysis: Each sample of herring, sprat and flounder consisted of a combined pooled muscle tissues from several individuals, classified by fish length. One individual of salmon, cod and bird, respectively, prepared as one sample Herring investigated as a diet component for African penguin was analyzed as a whole organisms. Homogenized, freeze-dried tissues were extracted by accelerated solvent extraction (ASE). The quantification of the studied compounds was based on the use of 13C labeled internal standards that were spiked into the sample extracts before extraction. Lipids were removed by passing the extract through a multilayer silica gel column eluting with n-hexane.
Analyses of 17 congeners of PCDD/F and 12 congeners of dl-PCB have been performed according to norm: PN-EN 1984, US EPA 1613, and US EPA 1668 in accredited laboratories (National Institute of Nutrition and Seafood Research in Copenhagen, Denmark, National Reference Laboratory for Analysis of POPs in Ostrava, Czech Republic, Eurofins GfA Gmbh Laboratory in Hamburg, Germany, National Institute for Health and Welfare in Helsinki, Finland, National Public Health Institute in Kuopio, Finland, Laboratory for Trace Organic Analyses at Krakow University of Technology, Poland).
The limits of detection (LOD) for PCDD/F and dl-PCB congeners were congeners dependent and varied in different samples between 0.1 and 1 pg g-1 w.w. for PCDD/F and from 0.5 to 45 pg g-1 w.w. for dl-PCB. Concentrations below the limits of quantification (LOQ) were equated to half of the LOQ. The uncertainty of measurements varied from 30% to 35% for sum of PCDD/F and dl-PCB, depending on the laboratory. The recoveries of the internal standards ranged between 74% and 126% for PCDD/F and 56–54% for dl-PCB.
Expression of results: PCDD/F and dl-PCB determined in fish muscle tissue were expressed in pg g-1 w.w. (wet weight). Total toxicity of PCDD/F and dl-PCB (TEQ) was calculated by multiplying the individual congener levels by the respective Toxic Equivalency Factor (TEF), (WHO 2005) [11] and expressed as a WHO-TEQ pg g-1 w.w. PCDD/F and dl-PCB in birds` muscle and liver tissues were expressed in pg g-1 l.w. (lipid weight).
Quality Assurance/Quality Control: QA/QC was performed through the analysis of procedural blanks, duplicate samples and standard reference materials for each sample set with a relative standard deviation (RSD) below 15% for all detected compounds.
Results and Discussion
Concentrations and congener profiles of PCDD/F and dl- PCB in Baltic and Arctic fish
The Baltic Sea, as a relatively shallow and small basin, characterized by limited water exchange through the Danish Straits, remains sensitive to the anthropogenic land pollution discharged via riverine outflow and via atmospheric deposition [12-17]. Currently, the concentration of the most hazardous halogenated organic substances such as PCDD/F, are higher in the Baltic Sea compared to other seas at the same latitude [18].
Anthropogenic pollution in the northern Europe increasingly affects the polar areas. Substances from the group of POPs unlikely undergo metabolic reactions and due to strong sorption capacity, are transported on aerosols over long distances. Due to their hydrophobicity they accumulate along trophic levels and induce multiple adverse effects in organisms, including developmental effects, immunotoxicity, and reproductive effects [19].
The level of POPs in marine organisms (zooplankton, fish) depends i.a. on seasonal changes in lipid content connected with physiological cycles (e.g. reproduction), age, feeding habits and the environmental conditions [18, 20-27] have been investigating the impact of seasonal changes in the concentrations of halogenated organic compounds in the Baltic salmon, due to the spawning run phenomenon, which remains insufficiently recognized.
The bioaccumulation and elimination of organochlorine contaminants in fish is complex and several routes for these processes have been proposed. The bioaccumulation defined as an increase in organisms/water chemical concentration ratio due to direct uptake from water (transport across respiratory surface and dermal absorption) and consumption. The elimination includes: respiration, egestion and metabolism (Mackay and Fraser, 2000). Development of analysis and synthesis of autocatalytic chemical systems, metabolomics and proteomics, facilitates identification of the possible migration routes of toxic substances in organisms [28,29]. Vuorinen et al. [30] proved that during vitellogenesis, estradiol-17β inhibits detoxification and influences accumulation of PCB in Baltic salmon roe to 68%.
Concentration of PCDD/F and dl-PCB in studied fish (331 samples) are presented in Table 1.
| Fish species |
Sampling locations | Year | WHO-TEQ pg Ô??g-1w.w. | |||||
|---|---|---|---|---|---|---|---|---|
ĂęPCDD/F ± SD ± SD |
Ăędl-PCB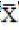 ± SD ± SD |
Total PCDD/F-dl-PCB | Ratio Ăędl-PCB/ PCDD/F |
Min.–Max. ĂęPCDD/F ± U |
Min.-Max. Ăędl-PCB ± U |
|||
| Salmon n=59 |
Polish Baltic fishing area | 2003 2004 2005 2006 2010-2011 2010-2011 |
2.62 ± 0.75 3.43 ± 0.66 2.10 ± 0.62 3.12 ± 0.7 3.26 ± 2.53 3.46 ± 1.27 |
5.12 ± 1.39 9.17 ± 2.84 3.96 ± 0.89 5.49 ± 0.86 7.05 ± 5.68 7.43 ± 3.43 |
7.74* 12.60* 5.97 8.62* 10.31* 10.89* |
1.95 2.67 1.89 1.76 2.16 2.15 |
0.75 ± 0.23 - 4.30* ± 1.29 2.48 ± 0.74 - 4.32* ± 1.30 4.27* ± 1.28 - 7.07* ± 2.12 1.20 ± 0.36 - 3.50* ± 1.05 0.86 ± 0.26 - 5.26* ± 1.58 1.35 ± 0.41 - 5.26* ± 1.58 |
1.39 ± 0.42 - 8.27 ± 2.48 5.86 ± 1.76 - 13.06 ± 3.92 4.27 ± 1.28 - 7.07 ± 2.12 2.60 ± 0.78 - 5.40 ± 1.62 2.67 ± 0.80 - 10.87 ± 3.26 3.85 ± 1.16 - 10.87 ± 3.26 |
| Gulf of Finland |
2005 | 3.13 ± 0.41 | 3.94 ± 0.68 | 7.07* | 1.26 | 2.67 ± 0.52 - 3.47 ± 0.69 | 3.50 ± 0.7 - 4.73 ± 0.95 | |
| Herring n= 134 |
Polish Baltic fishing area | 2002 2003 2004 2005 2006 2011 |
2.19 ± 0.91 1.23 ± 0.37 2.01 ± 0.55 1.75 ± 0.57 1.89 ± 0.47 1.01 ± 0.38 |
1.77 ± 0.57 1.51 ± 0.38 2.11 ± 0.54 1.83 ± 0.53 2.12 ± 0.49 1.51 ± 0.57 |
3.96 2.74 4.12 3.58 4.01 2.52 |
0.81 1.23 1.05 1.05 1.12 1.50 |
0.34 ± 0.10 - 3.77 ± 1.13 0.76 ± 0.23 - 2.05 ± 0.62 1.34 ± 0.40 - 3.28 ± 0.98 0.98 ± 0.29 - 2.60 ± 0.78 0.96 ± 0.29 - 2.60 ± 0.78 0.52 ± 0.16 - 2.01 ± 0.60 |
0.45 ± 0.14 - 2.69 ± 0.81 1.16 ± 0.35 - 2.05 ± 0.62 1.03 ± 0.31 - 3.02 ± 0.91 1.16 ± 0.35 - 2.61 ± 0.78 1.32 ± 0.40 - 3.01 ± 0.74 0.25 ± 0.08 - 2.47 ± 0.74 |
| Gulf of Riga | 2003 2004 2007 2008 2009 |
3.23 ± 2.81 1.63 ± 0.57 3.42 ± 0.60 1.94 ± 0.14 1.83 ± 1.16 |
2.61 ± 1.00 1.03 ± 0.38 1.91 ± 0.31 1.30 ± 0 0.04 2.15 ± 1.97 |
5.84 2.66 5.33 3.24 3.98 |
0.81 0.63 0.56 0.67 1.17 |
1.19 ± 0.30 - 8.61 ± 0.34 1.23 ± 0.31 - 2.04 ± 0.55 2.83 ± 0.71 - 4.03 ± 0.98 1.83 ± 0.46 - 2.14 ± 0.53 1.11 ± 0.28 - 3.19 ± 1.23 |
1.34 ± 0.34 - 3.41 ± 0.85 0.75 ± 0.19 - 1.30 ± 0.33 1.63 ± 0.41 - 2.25 ± 0.56 1.27 ± 0.32 - 1.35 ± 0.34 0.97 ± 0.24 - 4.42 ± 1.11 |
|
| Gulf of Finland | 2005 2007 2009 2010 |
0.85 ± 0.24 1.35 ± 0.08 0.95 ± 0.36 1.51 ± 0.33 |
0.72 ± 1.11 0.94 ± 0.72 0.99 ± 0.45 1.01 ± 0.25 |
1.57 2.29 1.94 2.52 |
0.85 0.70 1.04 0.67 |
0.20 ± 0.05 - 1.16 ± 0.29 1.29 ± 0.32 - 1.44 ± 0.36 0.58 ± 0.15 - 1.37 ± 0.34 1.31 ± 0.33 - 2.00 ± 0.50 |
0.48 ± 0.12 - 0.80 ± 0.20 0.86 ± 0.22 - 1.01 ± 0.25 0.57 ± 0.14 - 1.54 ± 0.39 0.89 ± 0.22 - 1.27 ± 0.32 |
|
| Northern Baltic | 2003 2006 |
2.77 ± 1.00 1.59 ± 0.24 |
2.86 ± 1.00 1.41 ± 0.23 |
5.63 3.00 |
1.03 0.89 |
2.07 ± 0.52 - 3.46 ± 0.91 1.35 ± 0.30 - 1.79 ± 0.41 |
2.08 ± 0.51 - 3.63 ± 0.92 1.18 ± 0.28 - 1.63 ± 0.43 |
|
| Sprat n= 104 |
Polish Baltic fishing area | 2002 2003 2004 2005 2006 2011 |
2.56 ± 0.47 2.11 ± 0.89 2.59 ± 0.65 1.88 ± 0.56 2.77 ± 0.35 1.34 ± 0.51 |
3.28 ± 0.65 2.94 ± 1.08 3.52 ± 0.91 2.73 ± 0.59 3.60 ± 0.51 2.28 ± 0.91 |
5.84 5.05 6.11 4.61 6.37 3.62 |
1.28 1.39 1.36 1.45 1.30 1.70 |
0.47 ± 0.14 - 3.19 ± 0.99 0.74 ± 0.22 - 2.80 ± 0.84 1.35 ± 0.41 - 3.14 ± 0.94 0.56 ± 0.17 - 2.04 ± 0.61 0.56 ± 0.17 - 3.01 ± 0.90 0.59 ± 0.18 - 2.18 ± 0.65 |
0.65 ± 0.20 - 4.23 ± 1.27 1.00 ± 0.30 - 3.98 ± 1.19 1.79 ± 0.54 - 4.69 ± 1.41 0.58 ± 0.17 - 3.80 ± 1.14 0.59 ± 0.18 - 4.30 ± 1.29 0.97 ± 0.29 - 0.51 ± 0.15 |
| Gulf of Finland |
2003 2004 2007 |
2.89 ± 0.46 1.15 ± 0.47 1.60 ± 0.09 |
2.79 ± 0.22 1.77 ± 0.72 1.65 ± 0.26 |
5.68 2.92 3.25 |
0.97 1.54 1.03 |
2.49 ± 0.50 - 3.56* ± 0.89 0.76 ± 0.27 - 1.85 ± 0.46 1.45 ± 0.36 - 1.76 ± 0.44 |
2.48 ± 0.62 - 3.07 ± 0.77 0.84 ± 0.21 - 2.60 ± 0.65 1.36 ± 0.34 - 2.07 ± 0.52 |
|
| Northern Baltic | 2004 2006 |
1.80 ± 0.62 1.51 ± 0.08 |
2.27 ± 1.23 1.57 ± 0.09 |
4.07 3.08 |
1.26 1.04 |
1.18 ± 0.30 - 2.61 ± 0.65 1.42 ± 0.36 - 1.57 ± 0.39 |
0.96 ± 0.24 - 3.35 ± 0.84 1.47 ± 0.37 - 1.65 ± 0.41 |
|
| 2010 | 1.86 ± 0.15 | 1.64 ± 0.17 | 3.50 | 0.88 | 1.73 ± 0.43 - 2.02 ± 0.51 | 1.47 ± 0.37 - 1.81 ± 0.45 | ||
| Flounder n=28 |
Polish Baltic fishing area | 2011 | 0.26 ± 0.15 | 0.63 ± 0.29 | 0.89 | 2.42 | 0.15 ± 0.05 - 0.77 ± 0.23 | 0.22 ± 0.07 - 1.55 ± 0.47 |
| Northern Baltic | 2004 | 0.35 ± 0.14 | 0.70 ± 0.47 | 1.05 | 2.00 | 0.19 ± 0.04 - 0.45 ± 0.09 | 0.33 ± 0.07 - 1.22 ± 0.31 | |
| Cod n=6 |
Polish Baltic fishing area | 2008-2009 | 0.18 ± 0.08 | 0.97 ± 0.45 | 1.05 | 5.39 | 0.03 ± 0.01 - 1.16 ± 0.35 | 0.65 ± 0.31 - 1.53 ± 0.51 |
| The Arctic Sea (the Svalbard Archipelago) |
2014 | 0.03 ± 0.03 | 0.59 ± 0.15 | 0.62 | 16.83 | 0.002 ± 0.001 -0.063 ± .0126 | 0.30 ± 0.06 - 0.59 ± 0.118 | |
Table 1: PCDD/F and dl-PCB (WHO-TEQ pg g-1 w.w.) in marine fish caught in the Baltic Sea and the Arctic region during 2002-2014. Total number of samples (n) equal of 311, average concentration (![]() ) and standard deviation (SD), range (Min.-Max.) with expanded uncertainty (U).
) and standard deviation (SD), range (Min.-Max.) with expanded uncertainty (U).
Any noticeable decreasing trend of PCDD/F/dl-PCB concentrations in Baltic fish during the decade (2002-2014) has been observed, despite the implementation of legal restrictions on the POPs emissions. This fact may suggest a relevance of uncontrolled diffuse sources and low emission (municipal combustion, traffic etc.) contribution, not to discard the physiological processes (e.g. spawning run process) as well as individual characteristics (age and sex).
PCDD/F and dl-PCB, as well as dl-PCB/PCDD/F ratio in the study revealed significant interspecies and spatial differences. Twelve of the non-ortho-PCB and mono-ortho-PCB (dl- PCB), have been calculated together with PCDD/F for establishing the TEQ (WHO 1998) and frequently considered as a one group. However, PCDD/F and PCB are characterized by different source of origin, thus PCDD/F concentration remains higher in a winter season, while dl-PCB predominates in the summer [31] and consequently, environmental fate of these chemicals proceeds differently, what was also observed in this study.
Minor interspecies differences in PCB concentration were observed in contrast to PCDD/F. Among the Baltic fish, the highest contribution of dl-PCB in PCDD/F/dl-PCB was noticed in cod, salmon and flounder. However the most evident difference was observed in the Baltic and the Arctic cod. Concentration of dl-PCB was about 5-fold higher than PCDD/F in the cod from the Baltic, whereas in the cod from the polar region concentrations of dl-PCB were 16-fold higher than PCDD/F. The highest concentrations of investigated compounds, particularly dl-PCB, were observed in salmon, which may be explained by the intensified bioaccumulation due to the high lipid content and biomagnification, considering the trophic chain level that is represents. The highest average concentration (12.6 WHO-TEQ pg g-1 w.w.) in salmon`s muscle tissue has been observed in the individuals from the southern part of the Baltic Sea (Table 1). Vuorinen et al. [18], observed halved level of contamination in the northern regions such as the Aland Sea and the Bothnian Sea about ten years later (6.7 WHO-TEQ pg g-1 w.w.and 6.6 WHO-TEQ pg g-1 w.w., respectively). These results exceed the acceptable level for both PCDD/F (3.5 WHO-TEQ pg Ô?? g-1 w.w.) and the sum PCDD/F/dl-PCB (6.5 WHO-TEQ pg g-1 w.w.) [8].
Concentrations determined in herrings and sprats were prominently lower. All averaged sums remain compliant with the acceptable limits for fish with one exception of elevated concentration in herring caught in 2008 and the maximum values have been exceeded occasionally (Table 1). Individuals of Baltic flounder and cod revealed the lowest PCDD/F contamination, lower than 1.05 WHO-TEQ pg g-1 w. w. of PCDD/F/dl-PCB sum.
Levels of the tested xenobiotics revealed correlation with fat content and the body weight of fish. Herring, belonging to the fatty fish that live only in marine waters is the most representative for the Baltic Sea, compared with fatty salmon which life cycle assumes the existence in both salt and fresh waters.
It was confirmed, that the biological factor as age, plays a relevant role for the contamination of the fish with toxic organohalogenated compounds such as PCDD/F. It was observed that fish older than 6 years and more than 17 cm in length had higher PCDD/F concentration than the internationally permitted threshold [4,32,33].
Uptake of contaminants by fish is strictly related to their diet, therefore, spatial variation of PCDD/F and dl-PCB level observed in organisms reflect the quality of their habitats. The best foraging conditions for fish prevail in the Baltic Proper and the Northern Quark, where their major diet components, species-dependent, are zooplankton and sprats. According to Peltonen et al. [18,27] the sum of PCDD/F and dl-PCB concentrations in the fish muscle did not exceed 4.5 WHO-TEQ pg g-1 w.w. The less preferred conditions are observed in the northern Baltic Sea (the Åland Sea, the Bothnian Sea, the Bothnian Bay) and in the southern Baltic Sea (the Bornholm Basin), which are dominated by fatty herring and sprats, major diet component for salmon and cod.
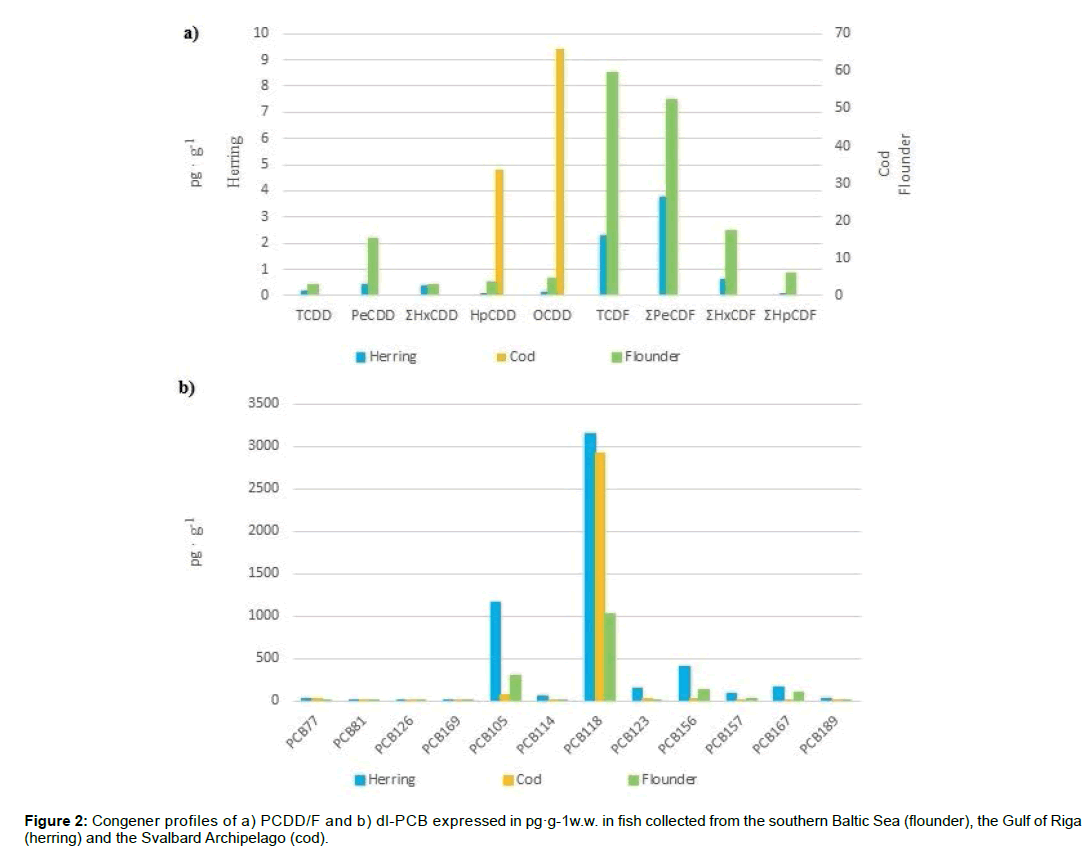
The theory of congener profiles of PCDD/F was created and introduced by Rappe thirty years ago as a method for presentation of PCDD/F congeners (isomers) associated with different sources of emissions, so the particular congener profile pattern may be applied for emission source identification (Figure 2) [34].
The congener profiles in fish from the different parts of the Baltic Sea area revealed resemblance in most studied cases [18,25]. Among congeners of PCDD/F, in Baltic fish, TCDF and PeCDF predominated, what was confirmed by the results of presented study (Figure 3a). The 1,2,3,7,8-PeCDD followed by 2,3,4,7,8-PeCDF are the main contributors to the sum of toxicity (WHO-TEQ) in Baltic fish and at the same air deposition indicators [12,25]. In Atlantic cod, only HpCDD and OCDD were identified but surprisingly, at comparable levels as the Baltic fish (Figure 2a). In terms of environmental risk, it is relevant, that PCDD/F congeners found in the Atlantic cod represents the lowest TEF, consequently- the lowest toxicity. This phenomenon could be explained by more efficient air transfer of highly chlorinated compounds, which are known to absorb on particulate matter to the higher extent than less chlorinated PCDD/F congeners [35-37]. On the contrary, the profile of dl-PCB was similar in the Baltic fish and the Atlantic cod from the Arctic region, what potentially may pose a threat to organisms from the higher trophic levels, e.g. polar bears. In both Baltic and Arctic fish, the PCB118 mono-ortho congener dominates among dl-PCB (Figure 2b). All non-ortho congeners (PCB77, PCB126, PCB169) have been detected in Baltic herring and Atlantic cod.
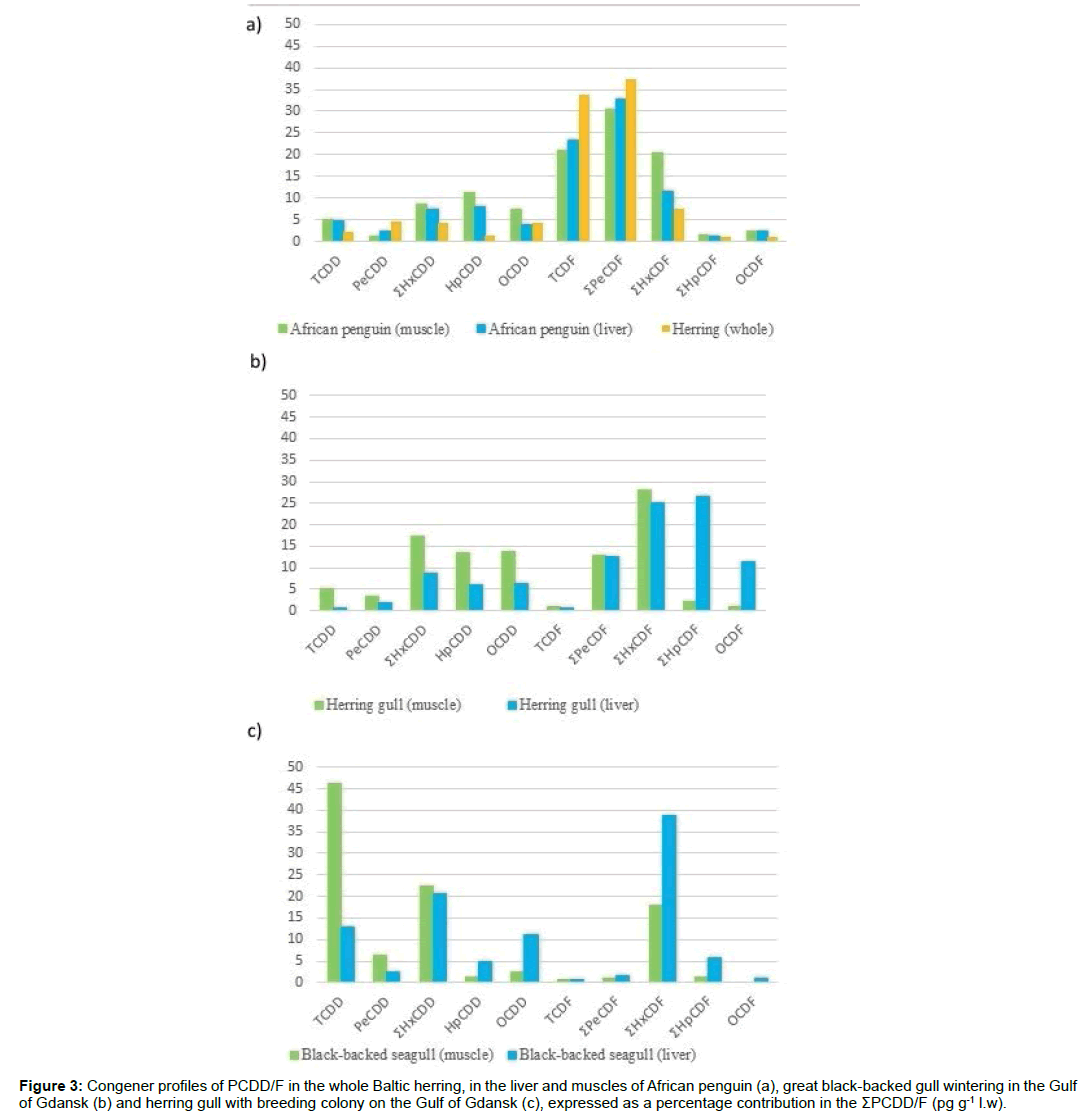
Fish as a source of pollutants to the higher trophic levels: Fish due to the high content of protein, minerals, fatty acids and vitamins are extremely valuable food for birds and other predators, but they are also a source of hazardous chemicals accumulating through trophic chains. In 2012, the University of Gdansk performed a study of the African penguin inhabiting the Zoological Garden in Gdansk, fed only by studied herring, as well as herring gull and great black-backed gull fed in the natural environment. The aim of the research was to determine the effects of the diet on the PCDD/F contamination of studied birds. PCDD/F congener profile in the whole herring was similar to those measured in herring muscles tissue. Profiles of PCDD/F in the whole herrings have a clear resemblance to the muscle tissue and the liver glandular of penguin (Figure 3a), which proves that diet determines the load of toxic substances to the consumer tissues. The highest concentration of congeners PeCDF and TCDF in herring dominated also in the liver and muscles of penguin.
Figure 3: Congener profiles of PCDD/F in the whole Baltic herring, in the liver and muscles of African penguin (a), great black-backed gull wintering in the Gulf of Gdansk (b) and herring gull with breeding colony on the Gulf of Gdansk (c), expressed as a percentage contribution in the ĂęPCDD/F (pg g-1 l.w).
Herring gulls are the most abundant bird (approx. 70% of individuals) in the southern area of the Baltic Sea (the Gulf of Gdansk) [37] while black-backed gulls represent the largest group of gulls occurring on islands and coastal areas of the northern Atlantic hemisphere (from the Great Lakes to the White Sea). The typical gulls` diet consists of fish, fish guts delivered from fishing boats, invertebrates, eggs and chicks of other birds, while the penguin`s diet includes only herring. The differences in the obtained congener profiles in the penguin`s tissues and the both species of seagulls indicate the influence of the specific diet`s composition on the birds` contamination (Figure 3).
The sum of PCDF congeners in liver is higher than the sum of these congeners in muscles, while the sum of PCDDs remains higher in muscles than in liver. Particularly high concentrations of TCDD- the most toxic congener (TEF=1), have been recorded in the black-backed gull, which may be related to the randomly acquired, significantly contaminated food.
Conclusion
Toxic substances from the POPs` group are spread over the long distances originating from the point sources located in the industrialized countries of the temperate latitudes, up to the Arctic region. The low mobility, nonetheless the strong chemical bonding with other pollutants from the DNAPL group, enhances their potential to be transported.
Presented paper includes an extensive and comprehensive data on PCDD/F and dl-PCB concentrations in various fish species from the Baltic Sea sub- basins and comparatively, the Arctic region. Concentrations of PCDD/F and dl-PCB in fish, in the majority, have met the stringent requirements established by European Commission in 2011 (3.5 WHO-TEQ pg g-1 w.w. for PCDD/F and the sum of PCDD/F and dl-PCB for 6.5 WHO-TEQ pg g-1 w.w.) and do not exhibit any noticeable fluctuations in the recent decade. The exceedances have been observed mostly in fatty individuals as salmon.
Dl-PCB/PCDD/F ratio in investigated Baltic fish remains comparable to the literature`s reports, however, the remarkably raised ratio observed in the Arctic cod may indicate more efficient long range transport of dl-PCB. Nevertheless, the observed phenomenon requires a further examination.
Comparative analysis of PCDD/F congener profiles in the selected seabirds, the African penguin fed on the homogenous diet as herring and the two species of seagulls, fed on the randomly acquired food, proved the apparent trophic transfer of selected halogenated contaminants from fish to seabirds.
The diet impact on the further transfer of these xenobiotics in the trophic chain, evidenced in the presented study, appears to be of the particular relevance due to the human health.
References
- The Stockholm Convention on Persistent Organic Pollutants (2001) United Nations Environment Programme (UNEP), Stockholm Sweden.
- The Convention on Long-Range Transboundary Air Pollution: The Protocol on Persistent Organic Pollutants (1998) United Nations Economic Commission for Europe (UNECE), Aarhus, Denmark.
- Weber R, Gaus C, Tysklind M, Johnston P, Forter M, et al. (2008) Dioxin- and POP-contaminated sites-contemporary and future relevance and challenges. Environ Sci Pollut Res Int 15: 363-393.
- Roots O, Lahne R, Otsa E, Simm M, Schramm KW (2003) Dioxins in the Baltic herring and sprat in Estonian coastal waters. Organohalogen Compounds 62: 201-203.
- Persson Y, Shchukarev A, Oberg L, Tysklind M (2008) Dioxins chlorophenols and other chlorinated organic pollutants in colloidal and water fractions of groundwater from a contaminated sawmill site. Environ Sci and Pollut Res 15: 463-471.
- Venier M, Ferrario J, Hites R (2009) Polychlorinated dibenzo-p-dioxins and dibenzofurans in the atmosphere around the Great lakes. Environ Sci and Tech 43: 1036-1041.
- US EPA (United States Environmental Protection Agency) (2014) Estimating exposure to dioxin-like compounds, 1-3. Office of Research and Development, Washington.
- EC (2011) European Commission Directive 2011/516/EU amending Regulation (EC) 1881/2006 as regards maximum levels for dioxins dioxin-like PCB and non-dioxin-like PCB in foodstuffs and Commission Recommendation of 23 August 2011 on the reduction of the presence of dioxins furans and PCB in feed and food. Official Journal of the European Union, I 218: 823-825.
- Szlinder-Richert J, Barska I, Usydus Z, Ruczynska W, Grabic R (2009a) Investigation of PCDD/F and dl-PCB in fish from the southern Baltic Sea during the 2002–2006 period. Chemosphere 74: 1509-1515.
- Szlinder-Richert J, Barska I, Mazerski J, Usydus Z (2009b) PCB in fish from the southern Baltic Sea: levels bioaccumulation features and temporal trends during the period from 1997 to 2006. Marine Pollution Bulletin 58: 85-92.
- Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, et al. (2006) The 2005 World Health Organization Re-evaluation of Human and Mammalian Toxic Equivalency Factors for Dioxins and Dioxin-like Compounds. Toxicol Sci 93: 223-241.
- Verta M, Salo S, Korhonen M, Assmuth T, Kiviranta H, et al. (2007) Dioxin concentrations in sediments of the Baltic Sea – a survey of existing data. Chemosphere 67: 1762-1775.
- Roots O, Zitko V, Kiviranta H, Rantakokko P, Ruokojarvi P (2009) Polybrominated diphenyl ethers in Baltic herring from Estonian waters 2006-2008. Russian J of General Chem 80: 2721-2730.
- Falkowska L, Be┼?dowska M (2011) Deposition of chemical substances from the atmosphere. In Sz U┼?cinowicz (Ed), Geochemistry in the Surface Baltic Sediments, Warsaw, Ministry of Environment.
- Geeraerts C, Belpaire C (2010) The effects of contaminants in European eel: a review. Ecotoxicology, 19: 239-266.
- Niemirycz E (2011) River discharges of chemical substances. In Sz. U┼?cinowicz (Ed). Geochemistry in the Surface Baltic Sediments, Warsaw, Ministry of Environment.
- Niemirycz E, Jankowska D (2011) Concentration and profiles of PCDD/F in sediments of major polish rivers and the Gdansk Basin – Baltic Sea. Chemosphere 85: 525-532.
- Vuorinen PJ, Kiviranta H, Koistinen J, Pöyhönen O, Ikonen E, et al. (2014) Organohalogen concentrations and feeding status in Atlantic salmon (Salmo salar L) of the Baltic Sea during the spawning run. Sci of the Total Environ 468-469: 449-456.
- Geeraerts C, Focant JF, Eppe G, De Pauw E, Belpaire C (2010) Reproduction of European eel jeopardized by high levels of dioxins and dioxin-like PCB. Science of the Total Environment 209: 4039-4047.
- Hites RA (2006) Persistent Organic Pollutants in the Great Lakes Part N Handbook. In Environ Chem, DC: Springer-Verlag Berlin and Heidelberg.
- Szlinder-Richert J, Barska I, Usydus Z, Grabic R (2010) Polybrominated diphenyl ethers (PBDEs) in selected fish species from the southern Baltic Sea. Chemosphere 78: 695-700.
- Szlinder-Richert J, Usydus Z, Malesa-Cie─?wierz M, Polak-Juszczak L, Ruczynska W (2011) Marine and farmed fish on the Polish market: Comparison of the nutritive value and human exposure to PCDD/F and other contaminants. Chemosphere 85: 1725-1733.
- Szlinder-Richert J, Usydus Z, Drgas A (2012) Persistent organic pollutants in sediment from the southern Baltic: risk assessment. J of EnvironMonitoring 14: 2100-2107.
- Piskorska-Pliszczynska J, Maszewski S, Warenik-Bany M, Mikolajczyk S, Goraj L (2012) Survey of persistent organochlorine contaminants (PCDD, PCDF and PCB) in fish collected from the Polish Baltic fishing areas. The Scientific World Journal 973292: 1-7.
- Vuorinen PJ, Keinanen M, Kiviranta H, Koistinen J, Kiljunen M, et al. (2012) Biomagnification of organic halogen in Atlantic salmon (Salmo salar) from its main prey species in three areas of the Baltic Sea. Sci of the Total Environ 421-422: 129-143.
- Strucinski P, Piskorska-Pliszczynska J, Maszewski S, Górlaczyk K, Warenik-Bany M, et al. (2013) PCDD/F and dl-PCB intake from fish caught in Polish fishing ground in the Baltic Sea- characterizing the risk for consumers. Environ Sci and Technol 30: 2294-2304.
- Peltonen H, Ruokojarvi P, Korhonen M, Kiviranta H, Flinkman J, et al. (2014) PCDD/F PCB and PBDEs in zooplankton in the Baltic Sea- spatial and temporal shifts in the congener specific concentrations. Chemosphere 114: 172-180.
- Mackay D, Fraser A (2000) Bioaccumulation of persistent organic chemicals: mechanisms and models. Environ Poll 110: 375-391.
- Jakóbkiewicz-Banecka J, Gabig-Ciminska M, Banecka-Majkutewicz Z, Banecki B, W─?grzyn A, (2014) Factors and processes modulating phenotypes in neuronopathic lysosomal storage diseases. Metabolic Brain Disease 29: 1-8.
- Vuorinen PJ, Vuorinen M (1985) Effect of bleached kraft mill on effluent on reproduction of brown trout (Salmo trutta L) on a restricted diet. Finnish Fisheries Research 6: 92-105.
- Niemirycz E, Witt M, Kobusi┼?ska M (2016) Polychlorinated Dibenzo-P-Dioxins and Dibenzofurans (PCDD/F) Measurements in Ambient Air over the Northern Poland. Intern J of Chemical Eng and App 7: 32-35.
- Roots O, Schramm K-W, Simm M, Henkelmann B, Lankov A (2006) Polychlorinated dibenzo-p-dioxins and dibenzofurans in Baltic herring and sprat in the north-eastern part of the Baltic Sea, Proc. Proceedings of the Estonian Academy of Sciences, Biol and Ecol 55: 51-60.
- Pandelova M, Henkelmann B, Roots O, Simm M, Jarv L, et al. (2008) Levels of PCDD/F and dioxin-like PCB in Baltic fish of different age and gender. Chemosphere 71: 369-378.
- Rappe C, Bergqvist PA, Kjeller LO, Swanson S, Belton T, et al. (1991) Levels and patterns of PCDD and PCDF contamination in fish brabs and lobsters from Newark Bay and New York Bright. Chemosphere 22: 239-266.
- Falandysz J (1999) Polichlorowane bifenyle (PCB) w ┼?rodowisku: chemia analiza toksyczno┼?─? st─?┼╝enia i ocena ryzyka. Gda┼?sk, DC: Fundacja Rozwoju Uniwersytetu Gdanskiego (in polish) 54: 657-668.
- Lohmann R, Belkin I (2014) Organic pollutants and ocean fronts across the Atlantic Ocean: A review. Progress in Oceanography 128: 172-184.
- Meissner W, Betleja J (2007) Sk┼?ad gatunkowy liczebno┼?─? i struktura wiekowa mew Laridae zimuj─?cych na sk┼?adowiskach odpadów komunalnych w Polsce. Notatki Ornitologiczne 48: 11-27.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 5389
- [From(publication date):
February-2017 - Apr 01, 2025] - Breakdown by view type
- HTML page views : 4518
- PDF downloads : 871