Pollution Removal Efficiency of Micro Algae Chlorella Vulgaris from Hyper Eutrophic Lake
Received: 14-Apr-2022 / Manuscript No. jee-22-60801 / Editor assigned: 15-Apr-2022 / PreQC No. jee-22-60801(PQ) / Reviewed: 28-Apr-2022 / QC No. jee-22-60801 / Revised: 02-May-2022 / Manuscript No. jee-22-60801(R) / Accepted Date: 09-May-2022 / Published Date: 09-May-2022 DOI: 10.4172/2157-7625.1000333
Abstract
Water quality of many lakes in Chennai is deteriorating due to fast urbanization and industrialization. Urban wastewater contains both organic and inorganic nutrients and discharge of untreated water increases nitrogen and phosphorous content in water bodies leading to eutrophication problem. This study firstly aims to assess physicochemical characteristics of Velachery Lake water for effective management of the lake. Water samples were collected during the post monsoon season. The sampling and analytical methodologies were followed for the estimation of selected parameters as per ‘Standard methods of water and wastewater analysis’ (APHA 2005). This study also investigates the growth of micro algae Chlorella vulgaris in Velachery Lake water and also the reduction of pollution at the end of growth phase. The biomass density, biomass productivity and the lipid content were estimated as 0.722 ± .028 g/l, 0.0589g/l/d, 21 ± .06%. The results emphasized that polluted lake water as a cost-effective growth medium for higher biomass and lipid production accompanied with the nutrient removal efficiency of micro algae to reduce eutrophication. The removal efficiencies were assessed as 91.63 ± .054% for TN, 96.31 ± .022 for TP, 67.93 ± 2.78% for COD, 80.95 ± 2.01% for BOD. The TDS reduction was estimated as 32 ± 1.28%. The results of this study demonstrated that even the lake water with less concentration of nutrients is a suitable growth medium for microalgae, since C. vulgaris could adapt well with the environmental stress factors.
Keywords: Chlorella vulgaris; Nutrient Removal; Biomass; Biomass Productivity; Lipid; Eutrophication
Keywords
Chlorella vulgaris; Nutrient Removal; Biomass; Biomass Productivity; Lipid; Eutrophication
Introduction
Rapid industrialization around lakes, tanks and other water bodies became irresistible due to various anthropogenic compulsions, resulting in the gradual deterioration of the water bodies. Encroachment and pollution aggravated the degeneration of water by letting domestic waste water, industrial effluents, nutrient rich agricultural runoff resulting in the loss of morphometry. The rural, suburban lakes were subjected to invasive and aggressive burden of expansion that brought ecological stress. Pollution of every sort started endangering the physio-eco environment system. Dumping of solid waste, negligence, deface the lake causing considerable loss to ground water recharge. Encroachment, trespassing, pollution pilferages and misappropriation of lake resources caused damage to the biodiversity bringing the lake to the brim of extinction. Therefore water pollution is a serious threat to the human population. The cities like Chennai facing acute shortage of water. So, as a safety measure, a monitoring program that will provide a representative and reliable estimation of the quality of surface waters is necessary. Thus, monitoring programs including frequent water sampling at various locations and determination of physicochemical parameters are carried out. Many water bodies like lakes, river, ponds are polluted due to urbanization beyond their self-purification capacity. Dumping of garbage and used waters into the water bodies leads to the accumulation of various organic compounds, phosphates and nitrates which in turn deteriorate the water quality. The nutrients like phosphate and nitrate lead to eutrophication of water bodies algal blooming interferes with penetration of sunlight and also reduce the dissolved oxygen concentration in the water bodies. Now the question is how do we bring a eutrophic body back to life? The answer is- Restoration and Rejuvenation. This method includes structural and land treatment measures, interception of nutrients and sediments, lake deepening or dredging, Dilution/Flushing, Aeration of Water, and much more. Bioremediation is the one among many restoration techniques [1]. Treating the polluted water with microorganisms biologically has lot of advantages which includes the low-cost of treatment, easy operation methods, can be done any type water body, reduce toxicity of water resource, easily breakdown the organic pollutants, simple oxidation of most of the organic compounds, even these micro-organisms are capable of handling inorganic compounds and heavy metals [2]. Among biological treatment rather bacteria algae found to be better candidate in treating water and the method is widely known as Phycoremediation. Among the wide variety of algal population the Chlorella vulgaris is selected for the phycoremediation of lake water due to its competent characteristics like absorbing the elements easily, withstanding wide temperature ranges, easy cultivation in the uncontrolled open environment.
Experimental Methods
Inoculation of micro algae
The pure culture of alga Chlorella vulgaris sp., made available to us from the algal bank. The alga was frequently sub cultured in Bold’s Basal Medium (BBM) for our experiment in conical flask. The preparation of the medium was done under aseptic conditions. The culture was kept in the culture room fitted with photoperiodic culture racks and it was periodically agitated. The culture room temperature was maintained at 27 ±1°C and the chamber was illuminated 12 hours light and 12 hours dark. After ten days the culture was used as mother inoculum to carry out the Phycoremediation process to treat Velachery Lake water [3].
Growth of algae in different environment conditions
To compare the algal growth and biomass production under controlled environment, experiment was done under lab condition. In this experiment undiluted raw Velachery Lake water was used as medium. For this one litre Borosil flask was used to culture the microalgae in 500 ml of Lake water and inoculated with 5 ml algal culture Chlorella vulgaris. In closed system 10 litre capacity sterile plastic bottles were utilized and in each bottle 5 litre of raw lake water was used. Lake water in all the bottles were inoculated with 50 mL algal cultures in each bottle. The bottles were kept in the outdoor condition under sunlight. The open pond system was made using the Thermacoal tub of 25 l capacity. Velachery lake water was used as the culture medium and 100 ml algal culture was used as mother inoculum. The water in the open pond was frequently agitated manually. The open pond system was kept in the outdoor condition under direct sunlight. Growth rate was calculated in alternative days. The experiment was kept for 16 days. The optical density was measured and tabulated for the samples. Also samples were analyzed in triplicates to estimate the Algal biomass density, Biomass productivity, and the Lipid content [4].
Growth rate determination
The algal growth rate was determined using the culture density. To find the culture density photo-colorimeter was used and the optical density was observed at 650nm. Every alternative day at 11 am the optical density of culture was measured to find the growth rate.
Biomass harvesting and determination
After the incubation period the algal culture was harvested. For harvesting the culture was centrifuged using a cooling centrifuge to make algal pellet at 3000 rpm for 10 minutes. Biomass pellet was collected in pre-weighed petri-plates and the weight of the wet biomass was taken. The biomass was shade dried in a sterile environment within the lab and the weight of the dry biomass was taken. The supernatant obtained from the culture bottle in which raw lake water was used for culturing algae was used for physical and chemical analysis [5].
Biomass density and biomass productivity
At first weight of filter paper (f1) was measured in grams. Then samples (v in ml) were collected from the bio reactors and then filtered. Filtration left only wet algae on filter paper; the filter paper with wet micro algae was then dried with a solar drier. The weight of the dried filter paper with microalgae (f2) was measured in grams. Mass of microalgae found by subtracting f1 from f2.
Algal density in g/l = (f2-f1)/v
The biomass productivity (g/l/d) was calculated according to following Equation
Biomass productivity = (Bi-B t)/d where Bi was the initial biomass concentration, Bt is the final biomass concentration and d is the cultivation time.
Lipid extraction
The harvested biomass was dried and 100 mg of each biomass were taken and lipid was extracted using Bligh and Dyer method [6]. The lipid extraction method by Bligh and Dyer is one of the standard procedures to isolate total lipid fractions from biological matrices. Dried algal biomass was grinded into fine powder using Mortar and pestle or blender. Lipid (algal oil) was extracted using bligh and dyer’s method .The solvents used in this method were chloroform: methanol: water in the ratio of 2:2:1.8 [v/v/v]. The mixture was kept undisturbed at refrigerator for overnight and during this the mixture was covered with aluminum foil to prevent the solvent evaporation and any type of contaminations [7]. The next day the mixture was centrifuged at 6000 rpm for 10 minutes. After separation the lower aqueous phase was carefully transferred into a sterile and clean container. The empty weight of the sterile container was noted to calculate the amount of lipid. The percentage of lipid was calculated using the following formula
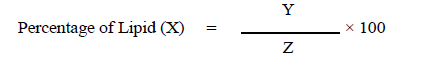
Where,
Y= weight of the lipid extracted (g)
Z= weight of biomass used in extraction (g)
Pollution removal efficiency
Velachery lake water under open pond natural environment was analyzed to determine the nutrient and other contaminant removal efficiency of Chlorella vulgaris in batch experiment for a period of 12 days. The concentrations of the elements after the cultivation period were determined by centrifuging a 10 ml liquid culture sample at 8000 rpm for ten minutes.
Results and Discussions
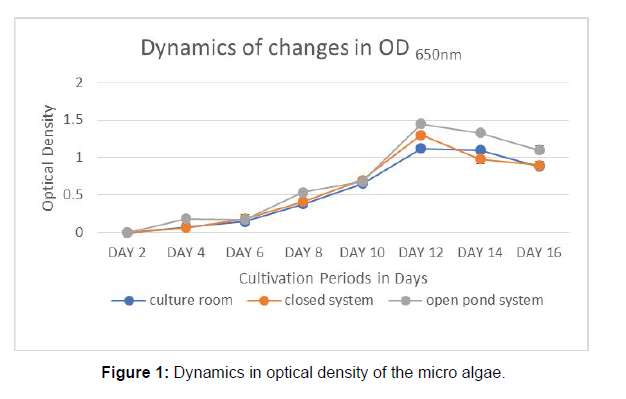
The Lake water was characterized for its physicochemical properties. pH was observed between the ranges 7.31 to 8.07. The dissolved oxygen ranged between 4.8 mg/l to 5.65 mg/l . The maximum value of other parameters were observed as, electrical conductivity 2034 μS/cm, TDS 1769 mg/l, BOD 17 mg/l, COD 216 mg/l Total Nitrogen 3.12mg/l, and Total Phosphorus 0.84 mg/l. The values of Electrical Conductivity (EC), Total Dissolved Solids (TDS), Biochemical Oxygen Demand (BOD), Chemical Oxygen Demand (COD), Total Nitrogen (TN), and The Total Phosphorus (TP) levels exceed the permissible limit of surface water quality standards mentioned in IS 2296. So, these parameters were checked, by treating with Chlorella vulgaris in order to reduce the pollution load in the lake water. The results showed that growth of algae in lake water medium in three different environments showed slight variation in the pattern. The Optical Density (OD) measured alternate days were plotted to generate the growth curve illustrated in Figure 1. In all the three cases, the OD reaches the maximum in the 12th day after that it declines. Which shows the algal biomass density gradually increases and reaches its maximum density on the 12th day and shows the decline trends in 14th and 16th days. So it was inferred that the harvesting and treatment period of Chlorella vulgaris in Velachery lake water medium is 12 days. The algal density, Biomass productivity and the lipid content were estimated at the end of incubation period. The results were listed in Table 1. The lipid content of microalgae is usually in the range of 20-50% of the cell dry weight and can be as high as 80% under certain conditions. Micro algae can over produce lipids under stress conditions such as high salt or high light or nutrient limitation. This investigation shows high lipid in room culture environment (23%) and 21% in open pond system.
| Culture Environment | Biomass density (g/l) | Bio mass productivity (g/l/d) | Lipid content (%) |
|---|---|---|---|
| Room Culture | 0.557 |
0.045 ± .01 | 23 ± .12 |
| Closed setup | 0.693 |
0.0565 ± .000 | 19 ± .23 |
| Open pond setup | 0.722 |
0.0589 ± .000 | 21 ± .06 |
Table 1: Biomass density, biomass productivity, lipid content of C.vulgaris at the end of 12 days in different
environmental conditions.The open pond system was closely monitored for pollution removal efficiency since it almost stimulates the lake system.
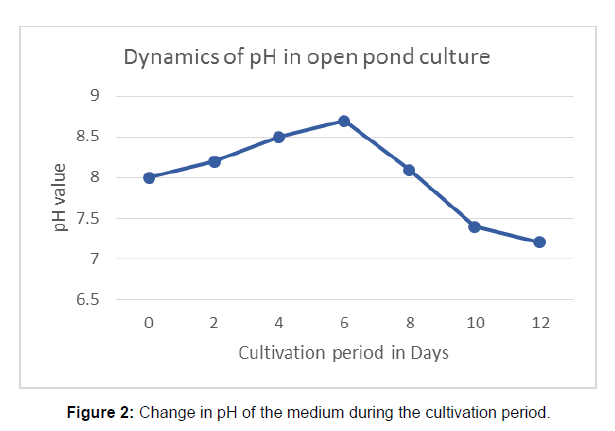
The open bond condition exhibited moderate fluctuation in pH and DO, and the highest reduction of TN and TP concentration in the medium. The Figure 2 shows the dynamics in pH in all the three systems. The pH of the lake water was increased to 8.7 in the 6th day but after that it attained the value of 7.2 at the end of cultivation period.
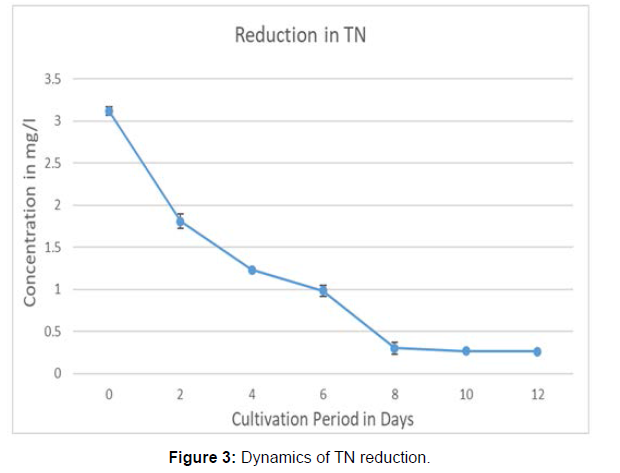
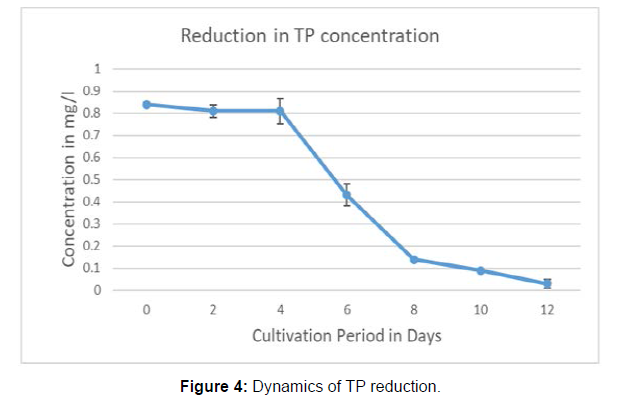
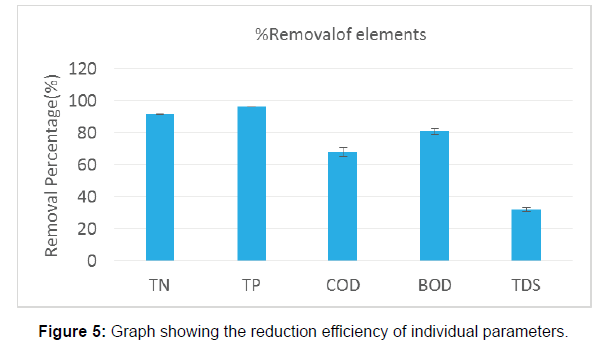
The concentration of DO increased from 5.3 mg/l at the first day of inoculation to 8.2 mg/l at the end of the cultivation with 60.37% increment. (Measured at 12 noon both the days). The dynamics in the reduction of Total Nitrogen and Total Phosphorus during the incubation period are in the Figure 3 and Figure 4. The removal efficiencies after 12 days incubation were assessed as 91.63 ± .054% for TN, 96.31 ± .022 for TP, 67.93 ± 2.78% for COD, 80.95 ± 2.01% for BOD .The TDS reduction was estimated as 32 ± 1.28%. The results were tabulated in Table 2. The Figure 5 shows the bar graph of individual reduction efficiency of TN, TP, COD, BOD, and TDS.
| Elements | Initial concentration (mg/l) | Final concentration (mg/l) | % Removal |
|---|---|---|---|
| TN | 3.12 ± .054 | 0.261 ± .014 | 91.63 ± .054 |
| TP | .84 ± .008 | .031 ± .02 | 96.31 ± .022 |
| COD | 184 ± 2.2 | 59 ± 1.7 | 67.93 ± 2.78 |
| BOD | 21 ± 2 | 4 ± .2 | 80.95 ± 2.01 |
| TDS | 958 ± 1.3 | 651.44 ± 05 | 32 ± 1.28 |
Table 2: The percentage removal of elements from the lake water treated by C.vulgaris.
The reduction in the concentration of total nitrogen was gradual up to eight days after that no considerable reduction was noticed in the open pond system. But the pattern was different in total phosphorus reduction curve. The removal is less for the initial 4 days then the reduction was noticed with sudden decline and the gradual trend up to 12th day. In a previous research by Wang it was reported that, cultivating Chlorella sp. in wastewater from a local municipal wastewater treatment plant, achieved removal rates of NH4-N, phosphorus and COD of 74.7%, 90.6% 56.5% respectively, before the primary settling stage. It was also reported a high removal efficiency of NH4-N and phosphorus from secondary waste water. Hongyang reported the removal of nitrogen (77.8%) and phosphorous (88.8%) from soybean processing wastewater using Chlorella pyrenoidosa. In other studies, removal rate of total nitrogen was observed between 61.1%-91.8% by Chlorella sp. grown in municipal wastewater whereas 87.9% removal was recorded in this study. It was observed that 98.4% of total phosphorous was removed which is higher than removal rate reported in previous studies [8].
Conclusion
The study shows inoculation of outdoor ponds with a monoculture can lead to high nutrient removal .The results of this study demonstrated that even the lake water with less concentration of nutrients is a suitable growth medium for microalgae, since C. vulgaris could adapt well with the environmental stress factors. Microalgae-based systems are a promising and sustainable solution to change contaminated water with the use of sun energy and natural carbon source (CO2 from atmosphere) into valuable products while protecting water from pollution. The nutrient removal efficiency by C. Vulgaris in lake water proved that microalgae are highly efficient in removing nitrogen and phosphorous from hyper eutrophic lake water. This study highlights the more effective, promising, alternative eco-friendly approach for lake water treatment using microalgae to lessen eutrophication.
Acknowledgement
None
Conflict of Interest
None
References
- Jay IM, Kawaroe M, Effendi H (2018) Lipid and fatty acid composition microalgae Chlorella vulgaris using photo bioreactor and open pond. IOP Conf Ser Earth Environ Sci 141: 012015.
- Gouveia L, Oliveira AC (2009) Micro algae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36: 269-274.
- Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31: 1043-1049.
- Widjaja A, Chien CC, Ju YH (2009) Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. J Taiwan Inst Chem Eng 40: 13-20.
- Yeh KL, Chang JS, Chen W (2010) Effect of light supply and carbon source on cell growth and cellular composition of a newly isolated microalga Chlorella vulgaris ESP-31. Eng Life Sci 10: 201-208.
- Ru KIT, Sung YY, Jusoh M, Wahid MEA, Nagappan T (2020) Chlorella vulgaris: a perspective on its potential for combining high biomass with high value bioproducts. Appl Phycol 1: 2-11.
- Durai G, Rajamohan N, Karthikeyan C, Rajasimman M (2010) Kinetics studies on biological treatment of tannery wastewater using mixed cultures. Int J Chem Biol Eng 3: 105-109.
- Ranjithkumar R, Rao PH, Arumugam M (2015) Lipid extraction methods from microalgae: a comprehensive review, front. Energy Res 8.
Indexed at Google Scholar, Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Citation: Raji PK, Abraham M (2022) Pollution Removal Efficiency of Micro Algae Chlorella Vulgaris from Hyper Eutrophic Lake. J Ecosys Ecograph 12: 333. DOI: 10.4172/2157-7625.1000333
Copyright: © 2022 Raji PK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2717
- [From(publication date): 0-2022 - Nov 23, 2025]
- Breakdown by view type
- HTML page views: 2099
- PDF downloads: 618