Pollution of the Environment by MSW Landfills in Developing Countries
Received: 26-Jan-2023 / Manuscript No. EPCC-23-88430 / Editor assigned: 28-Mar-2023 / PreQC No. EPCC-23-88430 (PQ) / Reviewed: 11-Feb-2023 / QC No. EPCC-23-88430 / Revised: 13-Feb-2023 / Manuscript No. EPCC-23-88430 (R) / Published Date: 20-Feb-2023 DOI: 10.4172/2573-458X.1000323
Abstract
Municipal solid waste (MSW) landfills in developing countries are a huge problem and threat to the environment and public health. To do this, within two years, 4 real MSW landfills (in fact, huge poorly equipped dumps) in an industrial Ukrainian city with 1 million populations were studied. These landfills operated for 15-50 years and accumulated 50- 150 thousand tons of MSW for each. Special attention was given to such problems as emission and spreading of greenhouse and toxic gases, seepage into the soil of toxic leachate, and also spreading toxic (heavy) metals which differently pollute air, the soil and underground water. In addition, the so-called “landfill gas” is a 99% greenhouse gas (its composition is a mixture of CO2 and CH4). That is, MSW landfills make a considerable contribution to global warming. Additionally, self-ignition of municipal waste in the MSW landfills and additional very dangerous pollution of the environment by fire gases have been studied. Thus, the purpose of this research is a comprehensive qualitative and quantitative study of all factors of real MSW landfills that can threaten the environment and health of the population.
Keywords
Municipal waste; Landfill; Environmental pollution; Ecological threats
Introduction
The municipal solid waste (MSW) management is a particularly critical problem for countries with “developing” economics [1]. From about 200 countries of the world, more than 50% of them do not have a modern waste management system. That is they don’t have preliminary sorting of MSW and collection of toxic and greenhouse “landfill gases” and also leachate; in addition, they have constant hotbeds of smoldering. Thus, poorly equipped MSW landfills pose a huge multilateral threat to the environment and health of residential areas. For example, approximately 5 billion m3 (over one billion tons) of MSW have been accumulated in Ukraine; it is disposed of at 800 large municipal landfills, and their total surface is more than 50 thousand hectares (including 500 m of sanitary zone for each). All they are 60-90% full, many of them are overfilled and should have been closed a long time ago. Also, there appeared thousands of unauthorized (“wild”) MSW dumps [2].
A lot of Ukrainian MSW dumps are permanently smoldering or even burning, especially during summer time. In addition to traditional toxic “fire gases”, it has been shown that the maximum concentration of dioxins in air can exceed the European Union standard of 0.1 nanogram/m3 [3-5].
Besides, when solid waste is disposed of, the available oxygen inside the body of the landfill may be quickly used up, so that the subsequent microbial activities go from aerobic to anaerobic [6,7]. Therefore, MSW can cause significant damage to the environment if they are not stored in a properly engineered system. Some of the problems that might occur are the following: emission of greenhouse biogas and other toxic gases, pollution of soil and ground water by highly toxic leachate [8], and also pollution of air by fire gases.
It would be desirable also to note: deep studying of REAL unequipped and semi-illegal large MSW landfill (containing tens of thousand tons of MSW) is accompanied by huge technical complexity and labor input and even some health hazards for researchers. Perhaps, it is why in scientific literature the quantitative studying of real large unequipped MSW dumps is deficiency. Thus, the purpose of this research was to provide a qualitative and quantitative estimation of the degree of environment pollution by poorly equipped real MSW landfills.
Materials and techniques
Note. Determination of the measurement error presented certain difficulties for us, since we were dealing with an indefinite mixture of components that changes in space (around the landfill) and time (due to biodegradation processes in the body of the landfill). Therefore, in addition to taking into account the “relative error” and the “error dispersion” of the results in the series of measurements, we also added “measurement error due to changes in measurement conditions” [9]. I want to emphasize that the real measurement error of such “undefined” mixtures as MSW is many times higher than the accuracy of the device used for measurements.
Measurement of biogas emission for real landfills in typical industrial city 1million population was fulfilled with the help of an individual multi-channel gas analyzer “MX-21-Plus” (France) and portable mobile ionic spectrometer “Multi-IMS” (Drōger, Germany). The most “young” landfill No. 4 was chosen by us for gases analysis (Table 1). The measurements of biogas were done in 8 boreholes 2 m deep and they were located at a distance of 10 m from each other. An average value was used on the basis of 3 measurements performed with an interval of 10 minutes. Analysis of the atmosphere above the real landfill was fulfilled on 1 m over the surface. The inaccuracy of all measurements did not exceed 8%.
| Landfills | Years of operation | Average quantity of MSW received each year (tons)* | Working area, hectares | Depth, m (aver-age) | Average composition (mass. %) |
|---|---|---|---|---|---|
| No. 1 | 47 | 115,000 | 11 | 25 | food-26; plastic-20; paper-11; glass-6; wood-8; metal-8; textiles-4; stones-6; sweepings**-11. |
| No. 2 | 37 | 51,000 | 4 | 12 | |
| No. 3 | 29 | 48,000 | 5 | 10 | |
| No. 4 | 15 | 155,000 | 24 | 18 | |
| *) The bulk density of incoming MSW is 0.25 t/m3, after landfill compaction it is 0.6 t/m3. **) Approximately 1/3 of sweepings is an organic matter. | |||||
In order to calculate the maximum theoretical biogas production at MSW landfills we used the following formula for first order reactions [10]:

where:
V0 – the theoretical MSW methane production potential, m3/t (for “average” Ukrainian MSW is equal 80);
Q – the average quantity of MSW received at a landfill, t/year (see Table 1);
k – the average constant of methane production, l/year (food – 0.35, paper – 0.12, textiles – 0.05, plastic – 0.01);
τ - the time of landfill operation, year (see Table 1).
The quantity of leachate (Vf) which might be produced at the working area of the landfill (dump) depends mainly on the amount of annual precipitation (P) of the region, evaporation (V), and water absorption by landfill wastes (W) (Qasim, 1995). However, we added to this formula another summand R:

where:
Р – precipitation for this area, mm/y-m2 (1 mm = 10 tons of precipitation per hectare; for East Ukraine P=500);
V - evaporation rate, mm/y•m2 (for East Ukraine V=200);
W - water absorbed by solid waste, mm/year•m2 (for East Ukraine W=100);
F - water drained, mm/year•m2 (for East Ukraine F=10);
S - landfill working area, m2;
R - water produced during MSW degradation, m3/year, which is 0.3 m3 (tons) of H2O for every 1000 m3 of natural biogas emitted.
Underground water samples for analysis were taken at the landfill border at the depth of 10-15 m. Altogether there were 8 wells: 2 at each of 4 sides. Three samples were taken from each well. The result of the analysis is an average value received for 3 samples. After that, an average value was obtained for all wells. Soil samples were taken at the distance of 500 m (sanitary zone) from the landfill border at the depth of 0.2-0.3 m also from four sides. From each side 3 samples were taken. After that all samples were averaged through quartering and the analysis was fulfilled. Atomic absorption spectrophotometer was used to measure toxic (heavy) metals in soil, water and ash (for that, samples of MSW were exposed to heat – see point 2.3). The inaccuracy of the analysis did not exceed 8%.
Derivatograph has been modified by us for heating of columns up to 325ºC, and was used to study thermal decomposition of MSW. MSW sample (225 g; composition is according to Table 1, right column; the speed of air supply into column was constant, being 1 liter/min; in fact, this is a slow burning of MSW with limited access to oxygen). The tests were conducted with MSW being heated (in the thermostat) by +70ºC, 120ºC, 170ºC, 220ºC, 270ºC, and 325ºC (when the temperature was higher than 300ºC some of MSW components started to burn - for instance, the temperature of self-ignition of pressed paper is about 250ºC).
We analyzed of soil and also toxic gases in air samples (1 m above ground) on border of a sanitary zone (SZ) of the of the smoldering MSW landfill No. 3 (500 m from edge of a landfill), with help portative analyzers “MX-21-Plus” and “Multi-IMS” (samples of air and soil were selected and delivered to laboratory for analysis of the heavy metals with help atomic absorption spectrophotometer).
We have measured concentrations of toxic gases produced after MSW smoldering (burning) and total concentrations of “heavy” (toxic) metals in the ash. We measured the part of heavy metals, which transforms in more “volatile” forms and is emitted into the atmosphere together with combustion gases as well as the part of heavy metals that enter the ash. Besides, we studied a separate part of heavy metals in the ash, which is “labile” and can migrate from ash into soil. The inaccuracy of all measurements did not exceed 6%.
Velocity of formation of toxic gases can be calculated using the Arrhenius equation (by means of building of diagrams lgK=ϕ(1/T):

where:
K - constants of velocity (we measure it on the device Figure 1);
A - pre-exponential factor (1013.5 s-1);
E – energy of activation;
R - gas constant R = 8,3 J/mol•K;
T - temperature, °K.
Results and discussion
Gas research of 4 real MSW landfills
In fact, there are not the classic landfills, there are the large unequipped dumps because the MSW are delivered there by dump trucks and then compacted by the tractors (up to density 0.6 t/m3). These dumps aren’t equipped with any technical means for collecting biogas and leachate. Besides, the wrong storing leads to self-heating and smoldering inside the MSW, and then to spontaneous ignition of separate sites of a dump (Table 1).
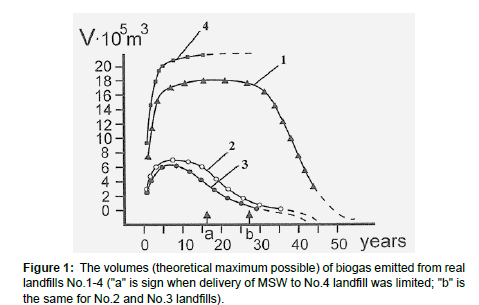
The volumes (theoretical maximum possible) of biogas emitted from real landfills No.1-4 were calculated according to formula (Eq.1). The results are illustrated at Figure 1.
As we can see from Figure 1, the biogas emitted from the No.1-4 landfills during biodegradation term reach their maximum at 1/4 – 1/3 of the full working period that is connected with activity of bacteria and also alterations of pH and temperature in a landfill body (similar curves like overturned parabola were described by de Bok, 2001, Gautam, 2012) [11,12]. Graph 1 also shows that, for example, landfills No.2 and No. 3, in fact, were already almost full 10 years ago but MSW delivery wasn’t stopped there (only were limited) as this zone of the city has no other place to store MSW.
At the depth of 25 m, from the bottom layers of most “old” No.1 landfill there have been taken samples of “residual” MSW. The age of these MSW layers corresponds to 45 years. The samples were tested for the share of organic components. The average result received on the basis of three samples is the following: the share of organic components - 13.5% (the initial share 45 years ago was about 75% - see Table 1). Thus, during 45 years MSW has been considerably mineralized as a result of a deep biodegradation of organic components of MSW.
In fact, these data have shown: at such landfills as No. 1-2 the process of biodegradation has almost finished, while at No. 3 and especially No. 4 «more young» landfills (see Table 1) it is still active.
Measurements of biogas (there are, basically, greenhouse gases) emissions at 4 real landfills (from 2 m deep boreholes) show the following composition of biogas (see Table 2). That is, MSW landfills make a considerable contribution to global warming. The global flow of methane from MSW landfills (mainly from poorly equipped landfills) reaches 30 million tons/year; accordingly, the CO2 flux reaches up to 20 million tons/year [13].
| No. | Biogas (vol. %) | |
|---|---|---|
| CO2 | CH4 | |
| 1 | 69 | 31 |
| 2 | 67 | 33 |
| 3 | 60 | 40 |
| 4 | 55 | 45 |
Gases sampled above 1m the real landfills surfaces were tested for dust, hydrogen sulfide (H2S), nitrogen dioxide (NO2), ammonia (NH3), sulfur dioxide (SO2) and carbon monoxide (CO) - see Table 3. These results show that the local atmospheric concentrations above the landfills were often more the norm (especially for dust and NO2). At landfills with smoldering waste - No. 1 and No. 2 - the share of carbon monoxide sharply increases.
| Parameter | No. 1 | No. 2 | No. 3 | No. 4 | MPC* |
|---|---|---|---|---|---|
| Dust | 0.8 | 0.5 | 0.6 | 0.3 | 0.15 |
| H2S | 0.01 | 0.053 | 0.05 | 0.003 | 0.005 |
| NH3 | 0.013 | 0.01 | 0.04 | 0.023 | 0.04 |
| NO2 | 0.09 | 0.05 | 0.06 | 0.052 | 0.04 |
| SO2 | 0.14 | 0.05 | 0.012 | 0.018 | 0.05 |
| CO | 3.1 (smoldering) | 5.6 (smoldering) | 1.6 | 0.7 | 3.0 |
| *)MPC - maximum permitted concentration in air of settlements (average daily) | |||||
However, additional research found that biogas also contains micro-amounts of highly toxic chlorides methane (less 5 ppm).
Leachate pollution
None of the four landfills has a leachate collection system. We have analyzed leachate composition at No. 3 landfill; the data are listed in Table 3. We have studied the composition of underground water the samples of which were taken from the wells surrounding No. 3 landfill. The sampling was done from the depth of about 5-10 m.
Data of Table 4 demonstrate that concentration of toxic substances in leachate is in hundreds, and sometimes thousand times more sanitary norms (MPC), i.e. leachate is highly toxic and a very dangerous liquid.
| Parameter | Concentration (mg/l) | MPC* | ||
|---|---|---|---|---|
| BOD** | 2130 | 350 | ||
| Oil products | 110 | 0.5 | ||
| Ammonia nitrogen | 512 | 10.0 | ||
| SSAM*** | 0.3 | 0.01 | ||
| Fe | 190 | 0.3 | ||
| Ni | 0.3 | 0.1 | ||
| Zn | 11.4 | 1.0 | ||
| Pb | 4.1 | 0.03 | ||
| Cd | 0.06 | 0.001 | ||
| Cr | 0.4 | 0.05 | ||
| Hg | 0.2 | 0.0005 | ||
| *) MPC- maximum permissible concentration; **) BOD - biochemical oxygen demand - is the amount of dissolved oxygen needed by aerobic biological organisms in a water; ***)SSAM - synthetic superficially-active materials. | ||||
The calculation of leachate volume produced at No. 3 landfill has been done by formula (Eq. 2). If to apply the equation to No. 3 landfill, which occupies 3.1 hectares (Table 1), using R = 200 m3/y and the values shown in Table 7, the expected annual leachate volume will be 298 m3/y:
Vf = [500 – 200 – 100 – 10] = 190 х 5 х 104 х 10-3 = 5890 + 300 = 298 m3/year.
Uncontrolled formation of such big volumes of toxic leachate should inevitably worsen ecological conditions of nearby underground water and soil.
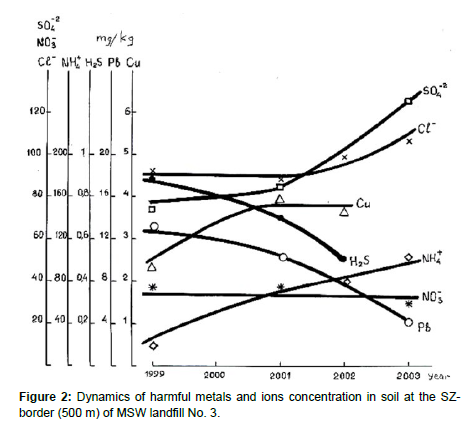
For check of possible soil pollution on the border of a sanitary zone (SZ-border) No. 3 landfill (a concentric circle of 500 m from edge of landfill) were analyzed samples of soil (see Table 5).
The data of Tables 4 and 5 and Figure 2 confirm the worst fears regarding high danger of leachate from unequipped MSW landfills.
| Parameter | MPC* (mg/kg) | Real concentration | Outreaching |
|---|---|---|---|
| Cd | 0.2 | 0.78 | 4 times |
| Ni | 4.0 | 3.3 | 7 |
| Pb | 6.0 | 1.9 | 3 |
| Hg | 0.05 | 0.3 | 6 |
| Nitrates | 10 | 82 | 8 |
| Oil products | 0.3 | 3.6 | 12 |
| *) MPC- maximum permissible concentration | |||
The danger of MSW smoldering processes
For studying of danger of self-heating and self-ignition of MSW stored on poorly equipped landfills, samples of MSW (in briquettes with density 0.6 t/m3) were exposed to thermal destruction in the laboratory device with using of derivatograph as adjustable furnace at temperatures of 70-325ºC. (I remind: air was supplied to the “furnace” of the derivatograph - 1 L/min; in fact, this is a slow burning of MSW with limited access to oxygen). Results of measurements - see in Tables 6 and 7.
| Concentration of toxic gases | |||||||
|---|---|---|---|---|---|---|---|
| CO | SO2 | H2S | C6H5OH (phenol) | NO2 | HCL | HCN | CH2O |
| 678 | 8.8 | 13.7 | 5.7 | 41 | 0,2 | 0,12 | 19.8 |
| Parameter | Concentration of toxic metals in MSW ash (mg/kg)* | |||||||
|---|---|---|---|---|---|---|---|---|
| Pb | Ni | Cr | Cu | Zn | Hg | Co | ||
| Sample of initial MSW | 511 | 140 | 190 | 1270 | 2410 | 3.2 | 46 | |
| Sample of MSW ash | 288 | 120 | 180 | 1100 | 2080 | 0 | 36 | |
| Quantity of toxic metals that was washed out from the ash – imitation of rain | 48.3 | 8.5 | 9.9 | 15.7 | 23.8 | 0 | 1.34 | |
| *) Pb – Lead; Ni – Nickel; Cr – Chromium; Cu – Copper; Zn – Zinc; Hg – Mercury; Co – Cobalt; | ||||||||
We have measured concentrations of toxic gases produced after MSW incineration (including such super-toxic ones as hydrogen cyanide - HCN, hydrogen chloride - HCl, formaldehyde - CH2O) and total concentrations of “heavy” (toxic) metals in the ash (with help of a mass-spectrometer). After that, we measured the part of heavy metals, which transforms in more “volatile” forms and is emitted into the atmosphere together with combustion gases as well as the part of heavy metals which enter the ash. Besides, we studied a part of heavy metals in the ash, which is “labile” (soluble) and can migrate into soils (if it will be washed out from ash by rain). The results of the measurements are provided in Tables 6,7.
By comparing the data of Table 7 we can see that ash accumulates all of toxic metals, excluding mercury and lead: mercury completely evaporates into air and lead – half-on-half. Therefore, the proposal to use ash after recycling MSW through incineration for building materials [14] causes concern.
So, we have established that during the incineration of MSW the vast emission of toxic gases in the atmosphere will take place. Some parts of each of the heavy metals are taken to the atmosphere together with combustion gases, the other parts enter the ash. At the same time, some parts of heavy metals that have passed into ash are in a soluble form, i.e. they might (in case of precipitation of ash on wet soil) enter in the soil. It is interesting to note that each heavy metal has its “own character”.
For check of air pollution on the border of a sanitary zone (SZborder) for smoldering No. 1 landfill (a concentric circle of 500 m from edge of landfill) samples of air were analyzed (see Table 8).
Evidently, combustion gases from the smoldering dumps have high toxicity (see Table 8) and high danger for environment and human health.
| Parameter | MPC*(mg/m3) | Amount | Exceeding |
|---|---|---|---|
| NO/NO2 | 0.035 | 0.55 | 16 (times) |
| H2S | 0.05 | 0.39 | 8 |
| HCl | 0.2 | 0.8 | 4 |
| Ash | 0.1 | 0.71 | 7 |
| *) MPC- maximum permissible concentration; | |||
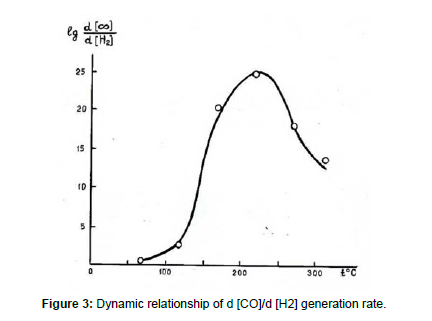
A typical phenomenon of pyrolysis processes of coal is the quantity and speed of CO and Н2 production start to grow rapidly when the temperature of coal mass is increasing. In this case, as a rule, at first CO is released faster than H2, however, after reaching a certain temperature, rapid emission of hydrogen begins. Scientists believe that this indicates the presence of spontaneous self-heating, smoldering, and burning inside the coal mass [15]. We tried to determine the temperature inside the body of the solid waste landfill, at which its “internal combustion” occurs.
The results of the experiments are provided in Table 9 and Figure 3. These data show that the curve in the ( coordinates has a “break” at a temperature of about 21ºC. This is probably the internal body temperature of a smoldering hearth in the body of the landfill. Such a high temperature is achieved due to the self-heating of the compressed mass of MSW at the landfill as a result of the activity of anaerobic bacteria [16].
| Temperature of the experiment, t °C | Gases generation rate, ml/g·s | |
|---|---|---|
| CO | H2 | |
| 70 | - | - |
| 120 | 3.7•10-7 | 1.5•10-7 |
| 170 | 5.8•10-5 | 8.8•10-6 |
| 220 | 5.0•10-4 | 2.0•10-5 |
| 270 | 1.5•10-3 | 4.7•10-3 |
| 325 | 0.5•10-3 | 5.5•10-2 |
The calculation of the maximum concentration limit Сml (g/s), i.e. the amount of harmful substances emitted by the source per unit of time, which in case of unfavorable weather conditions, being diffused in the atmosphere, will create at the surface layer (at the height of 2 m from the ground level) the concentration equal to a maximum allowable concentration of harmful particles in the atmosphere M (considering a background concentration Cb), can be calculated using the following formula where:
M - the mass of contaminant emitted into the atmosphere, g/s;
Cm - the maximum surface air concentration of contaminant, mg/ m3;
A - a coefficient which depends on atmospheric temperature stratification and defines conditions for vertical and horizontal dissipation of contaminants in the atmospheric air
(A = 140-250 depending on a geographic location);
F - a dimensionless factor reflecting a contaminant sedimentation rate in the air, its value for gases is 1 and for aerosols it is 2-3;
m and n - dimensionless factors reflecting conditions of the gasair mixture efflux and the emission source mouth form (typical values are m = 0.8-1.4, n = 1-2; the greater is the pipe diameter, the lower are m and n);
μ - a factor considering a relief of moorland (if it is “even”, i.e. the difference of levels at the distance of 1 km from the source of emission is not more than 50 m, μ = 1);
Н - the emission point (pipe) height above the ground level, m;
V - the gas-air mixture volume, m3/s;
ΔТ - the temperature difference between the emitted gas-air mixture Тd, and the ambient air Ta (ºС).
The calculation was done with the help of the computer software; the results are illustrated at Figure 4. At the border of the SZ (green circle with a red flag) the concentration of one of the most toxic components of fire-hazardous gases – nitrogen oxide – exceeds MPC 16, 59 times.
The problem of danger of poorly equipped MSW dumps is typical for the majority of the countries of South America, Asia, Africa and part of Europe. So, in Brazil from 2003 to 2011 1.5 million tons per year of CO2 (an average) were emitted into the atmosphere [17]. According to the Environmental Sanitation Technology Company (CETESB) study, the 6,000 waste sites in Brazil receive 60,000 tons of waste per day. 76% of this waste goes to dumps with no management, gas collection, or water treatment and 83% of Brazil methane gas emissions come from uncontrolled waste sites. But this problem also exists for economically developed countries. So, in Canada, landfill sites produce about 27 million tons of carbon dioxide and methane annually, and only 6.9 million tons (25%) from that are collected (Canada’s, 2019).
We don’t share an opinion [18] regarding “Significant amounts of biogenic carbon may still be stored within the landfill body after 100 year”. MSW sample from the real landfill of No.1 at a depth of 25 m and having age about 40 years - contained 13.5% organic components only.
Unfortunately, we didn’t study the smoldering dumps concerning dioxin due to the lack of access to reliable analyzers of dioxin. Therefore, the scientific paper [19] well fills up a gap in our studying. At research of influence of the illegal burning dumps in Italy (province of Campania) on health of local population, it was found high concentrations of dioxins (≥ 5.0 pg TEQ/g fat) in sheep and cow milk samples, and also dangerous contamination of dioxin and polychlorinated biphenyls in woman milk samples from those living in Campania (at 16.6 pg TEQ/g of fat).
In Table 4, the results of measurement of toxic metals concentrations in leachate are illustrated. But researchers [20] testify that the danger of toxic metals in MSW is underestimated. Baun and his colleagues evidently showed that colloids as well as organic and inorganic complexes additionally take place for all heavy metals in landfill leachate. Besides, standardized procedures for assessing the content of “associated” ions of heavy metals in leachate do not exist. Unequipped dumps are a powerful source of greenhouse gases and, therefore, it is one of “responsible” for negative climate change. In Figure 1 of our paper it is visible, what huge volumes of greenhouse gases (which are estimated by hundreds of thousands or millions of cubic meters) are emitted by each of these four dumps during the activity (20-40 years). According to calculations [21-23], world emission of biogas (which greenhouse gas is) from 1990 to 2050 will increase by 9 times (from the real 340 Mt in 1990 up to calculated 2900 Mt) – if we will not change relation to management of municipal waste).
Conclusions
1. In their present state, these researched MSW landfills don’t have any engineering infrastructure (gas collection, filtrate treatment, no smouldering, etc.) ensuring public health and environmental safety.
2. Our studies have provided qualitative and quantitative assessments of such danger factors from unequipped MSW landfills (dumps) as generation of greenhouse and toxic gases, toxic leachate, and also self-heating and smouldering MSW inside landfills due to bacteria activity.
3. It was established that each heavy metal during the incineration of MSW has its own “character”. For example, ash accumulates all of heavy metals, excluding mercury and lead; mercury completely evaporates into air and lead – half-on-half. Also, “soluble toxic metals” can migrate from ash into soil.
4. These problems are typical for many other countries with developing economies as their municipal budgets are not sufficient to solve problems of proper management of municipal solid waste.
References
- Baun DL, Christensen TH (2004) Speciation of heavy metals in landfill leachate: a review. Waste Management and Research 22: 3-23.
- de Bok F, Stams A, Dijkema C, Boone CD (2001) Diversity of Cellulolytic Bacteria in Landfill. J Appl Bacteriol 79: 73-78.
- Canada's Action on Climate Change (2019).
- Council of the European Communities, Council Directive on Waste Landfills (1999/31/EC). Official Journal 11: 182.
- Ferronata N, Torretta V (2019) Waste Mismanagement in Developing Countries: A Review of Global Issues. Int J Environ Res & Rublic Health 2019.
- Feldman E. Asymptotic analysis of the self-heating model coal. Possibilities of forecasting fire danger (Ukrainian language).
- Gautam SP, Bundela PS, Pandey AK (2012) Diversity of Cellulolytic Microbes and the Biodegradation of Municipal Solid Waste by a Potential Strain. Int J Microbiology 1-12.
- Gendebien A (1992) Landfill gas. Comission of the EU Communities. Brussel 865.
- Gomez MA, Baldini MD, Macros M (2012) Aerobic microbial activity and solid waste biodegradation in a landfill located in a semi-arid region of Argentina. Annals of Microbiology. 62: 1-24.
- Joseph AM, Snellings R, Van den Heede Ph (2018) The Use of Municipal Solid Waste Incineration Ash in Various Building Materials: A Belgian Point of View. Materials (Basel) Jan 11: 141-145.
- Krasnyansky M (2018) Ecological Safety (textbook, Ukrainian language). Publishing House "Condor", Kyiv, Ukraine. 177.
- Krasnyansky M (2022) Microbial Activity in the Unequipped Municipal Solid Waste Landfills. Acta Scientific Microbiology 5.
- Krasnyansky M., Belgasem A. (2005). Pollution of the Environment by Donetsk Landfills. The 4-th International Congress on Waste Management «WASTE-TECH», Moscow, 577-579.
- Lopes EJ, Okamura LA, Yamamoto CI (2015) Formation of dioxins and furans during municipal solid waste gasification. Braz J Chem Eng 32: 26-34.
- Manfredi S, Tonini D, Christensen T (2009) Landfilling of waste: accounting of greenhouse gases and global warming contributions. Waste Management & Research 27: 825-836.
- Mazza A, Piscitelli P, Neglia N (2015) Illegal Dumping of Toxic Waste and Its Effect on Human Health in Campania, Italy. Int J Environ Res Public Health 12: 6818-6831.
- Methane Emissions from Waste Treatment and Disposal (2011) First Brazilian Inventory of Anthropogenic Greenhouse Gas Emissions Background Report. Environmental Sanitation Technology Company (CETESB), Ministry of Science and Technology, Brazil.
- Monni S, Pipatti R, Lehtilla A (2006) Global climate change mitigation scenarios for solid waste management. Technical Research Centre of Finland 118.
- Møller J, Boldrin A, Christensen T (2009) Anaerobic digestion use: accounting of greenhouse gases and global warming contribution. Waste Management & Research 27: 813-824.
- Qasim S, Chiang W (1995) Sanitary Landfill Leachate. Technomic Lancaster-Basel. 339.
- Rabinovich SG (1978) Measurement error calculations. Leningrad. Energiya 262.
- Renou S, Givaudan JG, Poulain S (2008) Landfill leachate treatment: Review and opportunity. Journal of Hazardous Materials, 150: 468-493
- Sao Z, Ding X, Zung S (1990) Low-Temperature Dioxin Formation. Environmental Chemistry 9: 155-166.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Krasnyansky M (2023) Pollution of the Environment by MSW Landfills in Developing Countries. Environ Pollut Climate Change 7: 323. DOI: 10.4172/2573-458X.1000323
Copyright: © 2023 Krasnyansky M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1411
- [From(publication date): 0-2023 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 1158
- PDF downloads: 253