Review Article Open Access
Pneumocystis Pneumonia in Inflammatory Bowel Disease: The Costs of Immunosuppression
Ersilia M. DeFilippis1,2* and Ellen J. Scherl1,2
1Jill Roberts Center for Inflammatory Bowel Disease, Division of Gastroenterology and Hepatology, USA
2Weill Cornell Medical Center-New York Presbyterian Hospital, 1315 York Avenue, New York, NY 10021, USA
- *Corresponding Author:
- Ersilia M. DeFilippis
1315 York Avenue, Mezzanine Level
New York, NY 10021, USA
Tel: 212-746-5077
Fax: 212-746-8144
E-mail: emdefilippis@partners.org
Received date: October 26, 2015 Accepted date: November 04, 2015, Published date: November 11, 2015
Citation: DeFilippis EM, Scherl EJ (2015) Pneumocystis Pneumonia in Inflammatory Bowel Disease: The Costs of Immunosuppression. J Gastrointest Dig Syst 5:352. doi:10.4172/2161-069X.1000352
Copyright: © 2015 DeFilippis EM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Patients with inflammatory bowel disease (IBD) are subject to a spectrum of immunosuppressive agents including corticosteroids, immunomodulators, and biological therapies. Despite the benefits of these therapies and their ability to induce remission, they increase the risk of infectious complications with various organisms including Pneumocystis jiroveci pneumonia (PCP), which is associated with significant morbidity and mortality. Although PCP infection is typically associated with HIV-infected populations, the risk is increased in patients with IBD. Inflammatory autoimmune diseases like IBD account for as high as 20% of PCP infection in HIV-negative patients with greater than 50% mortality. Despite this, there are no clear guidelines for PCP prophylaxis in IBD. PCP should be considered in the differential when an IBD patient on immunosuppression presents with fever and respiratory symptoms. Here we review the existing literature regarding PCP risk in inflammatory bowel disease, the role of tumor necrosis alpha-inhibitors, and considerations for prophylaxis.
Keywords
Opportunistic infections; Inflammatory bowel disease; Immunosuppression; Pneumonia
Introduction
Patients with inflammatory bowel disease (IBD) are subject to a spectrum of immunosuppressive agents including corticosteroids, immunomodulators, and biologic therapies [1,2]. Despite the benefits of these therapies, they increase the risk of infection by intracellular pathogens, granulomatous infections, mycobacteria, histoplasmosis and others including Pneumocystis jiroveci (PCP) [3].
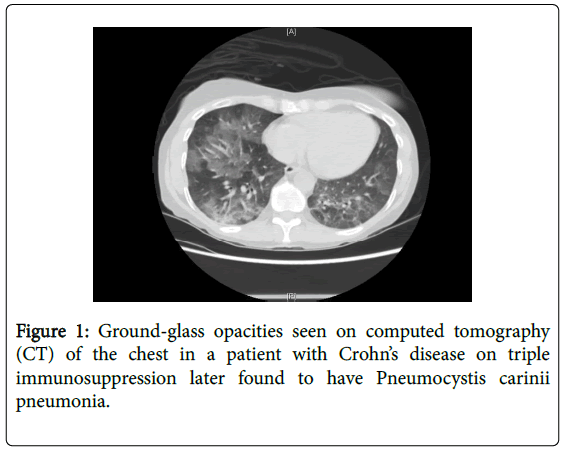
Pneumocystis jiroveci pneumonia (also known as pneumocystis carinii pneumonia) is an atypical unicellular fungus that produces local inflammatory reactions and alveolar infiltrates in the lung [4]. These lead to widened alveolar-arterial oxygen gradient and impaired gas exchange [1]. The infection is associated with significant morbidity and mortality. Symptoms may be non-specific leading to difficulties in diagnosis. Typical radiographic features include bilateral perihilar interstitial infiltrates. When chest radiographs are normal, highresolution computed tomography (CT) of the chest may show groundglass opacities or cystic lesions (Figure 1) [5,6]. It is considered an opportunistic infection since the organism takes advantage of a weakened immune system and would typically cause mild illness or no disease in the immunocompetent host [7]. The treatment of choice is trimethoprim-sulfamethoxazole (TMP-SMX). In patients who are sulfa-allergic or intolerant, second line therapies include primaquine, pentamidine, and dapsone [8].
Although PCP is typically associated with human immunodeficiency virus (HIV)-infected populations, a range of patient populations and medications have been associated with increased risk of PCP [9]. Other non-HIV populations at risk include solid-organ transplant recipients, connective tissue and rheumatological disorders, hematologic and solid malignancies, and IBD [10-21]. Inflammatory autoimmune diseases like IBD account for as high as 20% of PCP infection in HIV-negative patients with greater than 50% mortality [3].
In HIV patients, PCP presents as a slow and indolent process characterized by fever, cough, and pulmonary infiltrates [4]. In non- HIV populations, patients with PCP infection typically present with abrupt onset of respiratory insufficiency that may correlate with a tapered dosage of immunosuppressant agents [5]. One study found that patients with HIV had symptoms for 37.8 days on average before diagnosis compared to 7.6 days in non-HIV patients [8]. The non-HIV patients have more neutrophils and fewer pneumocystis organisms in their lungs as well as higher C-reactive protein and lower beta-D glucan levels [8]. Mortality in patients without AIDS may be 30 to 60 percent [5]. Given the lower number of organisms in their lungs, sputum induction may have less diagnostic yield. Therefore, if the initial specimen is negative, bronchoscopy with bronchoalveolar lavage should be performed [5]. The use of simultaneous serum beta-D glucan and respiratory sample polymerase chain reaction (PCR) testing for PJP may help with diagnosis [8].
The remainder of this review will focus on the risk of PCP in IBD, the role of tumor necrosis factor alpha inhibitors and new biologics, and considerations for prophylaxis.
Increased Risk of PCP in Inflammatory Bowel Disease
PCP has been reported in IBD patients on various medication regimens including cyclosporine, tacrolimus, infliximab, adalimumab, and azathioprine [3,22-39]. In one cohort study, patients with IBD were found to have significantly increased risk of PCP compared to the general population with an adjusted hazards ratio of 2.96 [4]. The annual incidence of PCP was 10.6 cases per 100,000 in the IBD population compared to 3 cases per 100,000 in the non-IBD population [4]. The risk appeared greater in the IBD patients with Crohn’s disease (CD) compared to those with ulcerative colitis [4].
The risk of infection depends on the host as well as the specific therapies, including whether a patient is on dual or even triple immunosuppression. One study from the Mayo Clinic examined risk factors for opportunistic infections in IBD patients [40]. They found that age greater than 50 was a significant risk factor as well as Crohn’s colitis as compared to isolated Crohn’s ileitis [40]. Additional risk factors for PCP infection include concomitant use of corticosteroids for greater than 8 weeks, advanced age greater than 65 years, lung disease, leucopenia, or hypoalbuminemia [3,4,41]. Likewise, in patients on monotherapy with azathioprine or 6-mercaptopurine, their risk of infection is increased 2-3 fold compared to the general population [40]. If these patients are also on corticosteroids, this risk increases to 15-fold [40].
Corticosteroids
Corticosteroids are a major risk factor for development of PCP and infection more generally [4,42,43]. Over fifty percent of the affected individuals in the above cohort study were on corticosteroids alone or in combination with other medication. Fifty percent had been hospitalized in the previous 60 days [4]. In one study by Yale and Limper of PCP in non-HIV patients, they found that regardless of the underlying condition, corticosteroids had been administered systemically in 105 of 116 patients within 1 month before the diagnosis of P. carinii pneumonia [44]. The median daily corticosteroid dose was equivalent to 30 mg of prednisone; however, 25% of patients had received as little as 16 mg of prednisone daily. P. carinii pneumonia developed after 8 weeks or less of corticosteroid therapy in 25% of these patients [44].
Many patients with IBD are treated with corticosteroids for acute disease flares. This may paradoxically mask the symptoms of PCP in these populations since high-dose steroids (greater than 60 mg of prednisone) are used in the management of severe PCP infection with hypoxia [1]. Therefore, physicians must be acutely aware of complaints of dyspnea in this population of patients.
Cyclosporine and Tacrolimus
Cyclosporine and tacrolimus have been the backbone of treatment for organ rejection in transplant patients. However, both have also been used in ulcerative colitis patients (UC) who are unresponsive to intravenous steroids [23,32]. Cases of fatal PCP as complications of these therapies have been reported, two patients receiving tacrolimus and one receiving cyclosporine [23,32]. In one case, the patient developed respiratory insufficiency requiring mechanical ventilation only 23 days after initiating tacrolimus therapy [23]. IBD patients treated with high dose cyclosporine for acute UC flares receive a dose roughly comparable to that used initially in renal transplant patients (4 mg/kg/day) [32]. It is standard practice for renal transplant patients to receive prophylaxis against PCP for three to six months after transplantation, however, the same is not currently true for IBD patients.
Tumor Necrosis Factor and Host Response to PCP
The host response to pneumocystis infection specifically involves many of the cytokines and molecules that are inhibited by inflammatory bowel disease therapies including TNF alpha. TNFalpha helps recruit neutrophils, lymphocytes, and monocytes as well as production of other chemokines like interleukin-8 and interferon gamma [45,46]. When this factor is blocked by therapies like infliximab and adalimumab, clearance of pneumocystis is delayed. Anti-TNF therapy may also decrease CD4 lymphocytes, the same cell population that makes HIV-infected patients susceptible to PCP infection [47].
Tumor necrosis factor alpha (TNF-alpha) inhibitors, such as infliximab, are used for IBD patients with moderate-to-severe disease [48]. Often these patients have been refractory to other immunomodulatory therapy. Infliximab is associated with various opportunistic infections, most commonly histoplasmosis [40]. One study in patients with rheumatoid arthritis found that patients treated with 10 mg/kg of infliximab were three times more likely to have infection compared to the placebo group [40,49].
Infliximab was approved for use in CD in 1998. Three years after, the first reported cases of PCP in patients on infliximab were reported in the literature [8]. Subsequent reports were reported over the following years. Although the studies which led to FDA approval of infliximab for IBD did not report PCP, Kaur and Mahl published a study of 84 cases of PCP associated with infliximab that were reported to the Adverse Event Reporting System (AERS) of the FDA between 1998 and 2003 [47]. Typically, the interval identified between the first infusion or dose and the start of infection is between 9 and 14 weeks [15,47]. Cases of PCP have also been reported in patients receiving adalimumab [3,50]. There are currently no published cases of PCP in association with certolizumab therapy.
Overall, studies in the United States and Japan have differing estimates of incidence of PCP in patients on TNF-alpha inhibitors [15,51-55]. These studies are largely based on studies of patients with rheumatoid arthritis. They report incidence of pneumocystis ranging from 1 to 8.8 cases per 1000 person-years [8,15,51-54,56,57]. This data is currently lacking in IBD-only populations. These discrepancies in results have made it difficult to support broad recommendations for prophylaxis.
The Role of Vedolizumab
Vedolizumab is a newer biologic agent available for patients with UC and CD who have failed or not responded to anti-TNF therapy [58,59]. Vedolizumab is a humanized monoclonal antibody that recognizes the alpha-4 beta-7 integrin molecule, selectively blocking gut lymphocyte trafficking [58]. Given that the drug is specific to the migration of leukocytes into the gut, it is thought to be safer than other options. In the GEMINI trials of vedolizumab in UC, serious infections were not more common in the study group than with placebo [58]. However, in patients with CD, serious infections occurred in 5.5% of the vedolizumab group compared to 3.0% of the placebo group [59]. Nevertheless, it has been postulated that the specificity of the drug may be safer than other therapies, including the anti-TNF medications which may have more global effects. In PCP specifically, tumor necrosis factor plays a role in clearing the infection. Thus, this pathway may not be affected in anti-integrin therapy.
Loftus et al. studied infection rates in patients treated with vedolizumab alone compared to those on concomitant corticosteroids and/or immunosuppressants using the GEMINI data [60]. The authors found that the percentages of those with infectious adverse events and infectious serious adverse events were similar among the groups, regardless of concomitant steroids or immunomodulatory therapy [60]. More long-term data is needed to determine the safety of vedolizumab and its associated risk of PCP.
The Question of Prophylaxis
There are no consistent guidelines or consensus regarding the need for prophylaxis for PCP in the IBD. According to the European Crohn’s and Colitis Organization (ECCO), patients with IBD on triple immunosuppression where one agent is a calcineurin inhibitor or anti- TNF-alpha therapy should receive standard prophylaxis with trimethoprim/sulfamethoxazole [7]. However, there is no consensus in patients with dual immunosuppression [7]. Furthermore, some consider administration of prednisolone at more than 16 mg/day over 8 weeks or 20 mg/day over 4 weeks as an indication for prophylaxis (Table 1) [30].
| Prior PCP infection |
|---|
| Triple immunosuppression where one agent is calcineurin inhibitor or TNF-alpha inhibitor |
| Prednisolone > 16 mg/day over 8 weeks or 20 mg/day over 4 weeks |
Table 1 Indications for Prophylaxis against Pneumocystis jiroveci. TNF: tumor necrosis factor.
Long et al. performed a retrospective cohort study examining over 100,000 patients with IBD [4]. They determined the number needed to treat of 3750 to prevent one case of PCP, which must be balanced by the side effects of prophylaxis including Stevens Johnson’s syndrome, aplastic anemia. These authors suggest considering prophylaxis on an individual basis where the benefits of prophylaxis outweigh the risks [4].
The lack of official guidelines appears to influence the decision to prescribe PCP prophylaxis in IBD patients. Okafor et al. studied practice patterns among gastroenterologists for PCP prophylaxis [61]. Eighty percent of respondents had patients who had developed the infection on immunosuppressive therapy yet only 11% reported initiating prophylaxis, largely for patients on triple immunosuppressive therapy [61]. Furthermore, those gastroenterologists with patients who had developed PCP were 7.4 times more likely to prescribe prophylaxis [61].
Some have suggested that one means of identifying the most at-risk patients could involve testing for colonization by P. jiroveci [62]. Oral washes are a specific and sensitive procedure for detecting colonization through the use of polymerase chain reaction (PCR) [62]. However, existing data is limited and further trials are needed to determine the role of this in prevention of PCP in IBD patients.
Conclusion
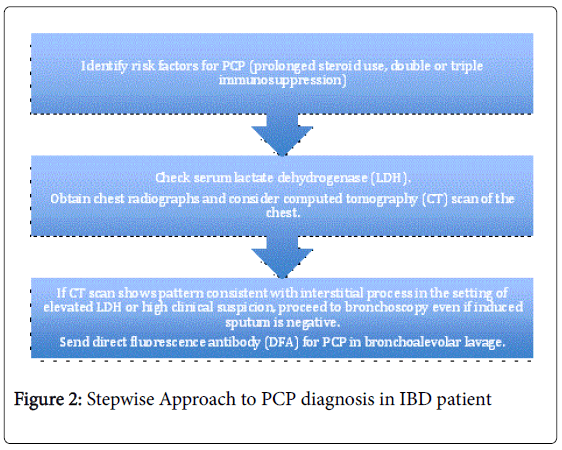
Pneumocystis carinii pneumonia is not a wholly uncommon infection in patients with IBD and has significant associated morbidity and mortality. PCP should be considered in the differential diagnosis in IBD patients on infliximab as well as other immunosuppressants who present with respiratory symptoms [22]. An algorithmic approach to the diagnosis of PCP is presented in Figure 2.
Bronchoalveolar lavage should be performed rapidly in patients with IBD presenting with fever and pulmonary infiltrates even if induced sputum samples are negative. Furthermore, prophylaxis should be strongly considered in patients receiving combination therapy with corticosteroids, especially in those of older age. The absolute risk of opportunistic infection in IBD patients and the potential benefit of preventive strategies remainS to be determined. As more and more biologic therapies become available for this patient population, one must constantly weigh the risks and benefits of immunosuppression especially when multiple agents are required for treatment. More research is required in order to form more definitive guidelines for PCP prophylaxis in order to reduce morbidity and mortality associated with this disease.
References
- Poppers DM, Scherl EJ (2008) Prophylaxis against Pneumocystis pneumonia in patients with inflammatory bowel disease: toward a standard of care. Inflamm Bowel Dis 14: 106-113.
- Lichtenstein GR, Abreu MT, Cohen R, Tremaine W, American Gastroenterological Association (2006) American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 130:935–939.
- Desales AL, Mendez-Navarro J, Méndez-Tovar LJ, Ortiz-Olvera NX, Cullen G, et al. (2012) Pneumocystosis in a patient with Crohn's disease treated with combination therapy with adalimumab. J Crohns Colitis 6: 483-487.
- Long MD, Farraye FA, Okafor PN, Martin C, Sandler RS, et al. (2013) Increased risk of pneumocystis jiroveci pneumonia among patients with inflammatory bowel disease. Inflamm Bowel Dis 19: 1018-1024.
- Thomas CF Jr, Limper AH (2004) Pneumocystis pneumonia. N Engl J Med 350: 2487-2498.
- Gruden JF, Huang L, Turner J, Webb WR, Merrifield C, et al. (1997) High-resolution CT in the evaluation of clinically suspected Pneumocystis carinii pneumonia in AIDS patients with normal, equivocal, or nonspecific radiographic findings. AJR Am J Roentgenol 169: 967-975.
- Dave M, Purohit T, Razonable R, Loftus EV Jr (2014) Opportunistic infections due to inflammatory bowel disease therapy. Inflamm Bowel Dis 20: 196-212.
- Grubbs JA, Baddley JW (2014) Pneumocystis jirovecii pneumonia in patients receiving tumor-necrosis-factor-inhibitor therapy: implications for chemoprophylaxis. CurrRheumatol Rep 16: 445.
- Reid AB, Chen SC, Worth LJ (2011) Pneumocystis jirovecii pneumonia in non-HIV-infected patients: new risks and diagnostic tools. CurrOpin Infect Dis 24: 534-544.
- Wang EH, Partovi N, Levy RD, Shapiro RJ, Yoshida EM, et al. (2012) Pneumocystis pneumonia in solid organ transplant recipients: not yet an infection of the past. Transpl Infect Dis 14: 519-525.
- Crayton HE, Sundstrom WR (1991) Pneumocystis carinii pneumonia following corticosteroid therapy for giant cell arteritis. Wis Med J 90: 170-171.
- Gupta D, Zachariah A, Roppelt H, Patel AM, Gruber BL (2008) Prophylactic antibiotic usage for Pneumocystis jirovecii pneumonia in patients with systemic lupus erythematosus on cyclophosphamide: a survey of US rheumatologists and the review of literature. J ClinRheumatolPract Rep Rheum Musculoskelet Dis 14:267–272.
- Stern A, Green H, Paul M, Vidal L, Leibovici L (2014) Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst Rev 10:CD005590.
- Zhang Y, Zheng Y (2014) Pneumocystis jirovecii pneumonia in mycophenolatemofetil-treated patients with connective tissue disease: analysis of 17 cases. RheumatolInt 34: 1765-1771.
- Komano Y, Harigai M, Koike R, Sugiyama H, Ogawa J, et al (2009) Pneumocystis jiroveci pneumonia in patients with rheumatoid arthritis treated with infliximab: a retrospective review and case-control study of 21 patients. Arthritis Rheum 61:305–312.
- Tokuda H, Sakai F, Yamada H, Johkoh T, Imamura A, et al (2008) Clinical and radiological features of Pneumocystis pneumonia in patients with rheumatoid arthritis, in comparison with methotrexate pneumonitis and Pneumocystis pneumonia in acquired immunodeficiency syndrome: a multicenter study. Intern Med Tokyo Jpn 47:915–923.
- Vananuvat P, Suwannalai P, Sungkanuparph S, Limsuwan T, Ngamjanyaporn P, et al. (2011) Primary prophylaxis for Pneumocystis jirovecii pneumonia in patients with connective tissue diseases. Semin Arthritis Rheum 41: 497-502.
- Gerhart JL, Kalaaji AN (2010) Development of Pneumocystis carinii pneumonia in patients with immunobullous and connective tissue disease receiving immunosuppressive medications. J Am AcadDermatol 62:957–961.
- Neuwelt AJ, Nguyen TM, Fu R, Bubalo J, Tyson RM, et al. (2014) Incidence of Pneumocystis jirovecii pneumonia after temozolomide for CNS malignancies without prophylaxis. CNS Oncol 3: 267-273.
- De Castro N, Neuville S, Sarfati C, Ribaud P, Derouin F, et al. (2005) Occurrence of Pneumocystis jiroveci pneumonia after allogeneic stem cell transplantation: a 6-year retrospective study. Bone Marrow Transplant 36: 879-883.
- Mathew BS, Grossman SA (2003) Pneumocystis carinii pneumonia prophylaxis in HIV negative patients with primary CNS lymphoma. See comment in PubMed Commons below Cancer Treat Rev 29: 105-119.
- Estrada S, García-Campos F, Calderón R, Delgado E, Bengoa R, et al. (2009) Pneumocystis jiroveci (carinii) pneumonia following a second infusion of infliximab in a patient with ulcerative colitis. Inflamm Bowel Dis 15: 315-316.
- Escher M, Stange EF, Herrlinger KR (2010) Two cases of fatal Pneumocystis jirovecii pneumonia as a complication of tacrolimus therapy in ulcerative colitis--a need for prophylaxis. J Crohns Colitis 4: 606-609.
- Lee JC, Bell DC, Guinness RM, Ahmad T (2009) Pneumocystis jiroveci pneumonia and pneumomediastinum in an anti-TNFalpha naive patient with ulcerative colitis. World J Gastroenterol 15: 1897-1900.
- Tschudy J, Michail S (2010) Disseminated histoplasmosis and pneumocystis pneumonia in a child with Crohn disease receiving infliximab. J PediatrGastroenterolNutr 51: 221-222.
- Sharma K, Rao P, Krishnamurthy P, Ali SA, Beck G (2007) Pneumocystis cariniijiroveci pneumonia following infliximab infusion for Crohn disease: emphasis on prophylaxis. South Med J 100: 331-332.
- Itaba S, Iwasa T, Sadamoto Y, Nasu T, Misawa T, et al. (2007) Pneumocystis pneumonia during combined therapy of infliximab, corticosteroid, and azathioprine in a patient with Crohn's disease. Dig Dis Sci 52: 1438-1441.
- Seddik M, Melliez H, Seguy D, Viget N, Cortot A, et al (2005) Pneumocystis jiroveci (carinii) pneumonia after initiation of infliximab and azathioprine therapy in a patient with Crohn’s disease. Inflamm Bowel Dis 11:618–620.
- Velayos FS, Sandborn WJ (2004) Pneumocystis carinii pneumonia during maintenance anti-tumor necrosis factor-alpha therapy with infliximab for Crohn's disease. Inflamm Bowel Dis 10: 657-660.
- Takenaka R, Okada H, Mizuno M, Nasu J, Toshimori J, et al. (2004) Pneumocystis carinii pneumonia in patients with ulcerative colitis. J Gastroenterol 39: 1114-1115.
- Khatchatourian M, Seaton TL (1997) An unusual complication of immunosuppressive therapy in inflammatory bowel disease. Am J Gastroenterol 92: 1558-1560.
- Quan VA, Saunders BP, Hicks BH, Sladen GE (1997) Cyclosporin treatment for ulcerative colitis complicated by fatal Pneumocystis carinii pneumonia. BMJ 314: 363-364.
- Scott AM, Myers GA, Harms BA (1997) Pneumocystis carinii pneumonia postrestorativeproctocolectomy for ulcerative colitis: a role for perioperative prophylaxis in the cyclosporine era? Report of a case and review of the literature. Dis Colon Rectum 40:973-976.
- Smith MB, Hanauer SB (1992) Pneumocystis carinii pneumonia during cyclosporine therapy for ulcerative colitis. N Engl J Med 327: 497-498.
- Kaur N, Mahl TC (2004) Pneumocystis carinii pneumonia with oral candidiasis after infliximab therapy for Crohn's disease. Dig Dis Sci 49: 1458-1460.
- Stratakos G, Kalomenidis I, Papas V, Malagari K, Kollintza A, et al. (2005) Cough and fever in a female with Crohn's disease receiving infliximab. EurRespir J 26: 354-357.
- Lawrance IC, Radford-Smith GL, Bampton PA, Andrews JM, Tan P-K, et al (2010) Serious infections in patients with inflammatory bowel disease receiving anti-tumor-necrosis-factor-alpha therapy: an Australian and New Zealand experience. J GastroenterolHepatol 25:1732-1738.
- Oshitani N, Matsumoto T, Moriyama Y, Kudoh S, Hirata K, et al (1998) Drug-induced pneumonitis caused by sulfamethoxazole, trimethoprim during treatment of Pneumocystis carinii pneumonia in a patient with refractory ulcerative colitis. J Gastroenterol 33:578-581.
- Bernstein CN, Kolodny M, Block E, Shanahan F (1993) Pneumocystis carinii pneumonia in patients with ulcerative colitis treated with corticosteroids. Am J Gastroenterol 88: 574-577.
- Toruner M, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Orenstein R, et al. (2008) Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology 134: 929-936.
- Okafor PN, Nunes DP, Farraye FA (2013) Pneumocystis jiroveci pneumonia in inflammatory bowel disease: when should prophylaxis be considered? Inflamm Bowel Dis 19: 1764-1771.
- Kelly DM, Cronin S (2014) PCP prophylaxis with use of corticosteroids by neurologists. PractNeurol 14: 74-76.
- Plakke MJ, Jalota L, Lloyd BJ (2013) Pneumocystis pneumonia in a non-HIV patient on chronic corticosteroid therapy: a question of prophylaxis. BMJ Case Rep 2013.
- Yale SH, Limper AH (1996) Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo ClinProc 71:5-13.
- Chen W, Havell EA, Harmsen AG (1992) Importance of endogenous tumor necrosis factor alpha and gamma interferon in host resistance against Pneumocystis cariniiinfection. Infect Immun 60:1279-1284.
- Hoffman OA, Standing JE, Limper AH (1993) Pneumocystis carinii stimulates tumor necrosis factor-alpha release from alveolar macrophages through a beta-glucan-mediated mechanism. J Immunol 150:3932-3940.
- Kaur N, Mahl TC (2007) Pneumocystis jiroveci (carinii) pneumonia after infliximab therapy: a review of 84 cases. Dig Dis Sci 52: 1481-1484.
- Järnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlén P, et al. (2005) Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 128: 1805-1811.
- Westhovens R, Yocum D, Han J, Berman A, Strusberg I, et al (2006) The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo-controlled trial. Arthritis Rheum 54:1075-1086.
- Watanabe K, Sakai R, Koike R, Sakai F, Sugiyama H, et al (2013) Clinical characteristics and risk factors for Pneumocystis jirovecii pneumonia in patients with rheumatoid arthritis receiving adalimumab: a retrospective review and case-control study of 17 patients. Mod RheumatolJpn Rheum Assoc 23:1085-1093.
- Baddley JW, Winthrop KL, Chen L, Liu L, Grijalva CG, et al (2014) Non-viral opportunistic infections in new users of tumour necrosis factor inhibitor therapy: results of the SAfety Assessment of Biologic ThERapy (SABER) study. Ann Rheum Dis 73:1942-1948.
- Dixon WG, Watson K, Lunt M, Hyrich KL, Silman AJ, et al (2006) Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 54:2368-2376.
- Greenberg JD, Reed G, Kremer JM, Tindall E, Kavanaugh A, et al. (2010) Association of methotrexate and tumour necrosis factor antagonists with risk of infectious outcomes including opportunistic infections in the CORRONA registry. Ann Rheum Dis 69: 380-386.
- Takeuchi T, Tatsuki Y, Nogami Y, Ishiguro N, Tanaka Y, et al. (2008) Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis 67: 189-194.
- Harigai M, Koike R, Miyasaka N; Pneumocystis Pneumonia under Anti-Tumor Necrosis Factor Therapy (PAT) Study Group (2007) Pneumocystis pneumonia associated with infliximab in Japan. N Engl J Med 357: 1874-1876.
- Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, et al. (2012) Serious infection and mortality in patients with Crohn's disease: more than 5 years of follow-up in the TREATâ„¢ registry. Am J Gastroenterol 107: 1409-1422.
- Salmon-Ceron D, Tubach F, Lortholary O, Chosidow O, Bretagne S, et al (2011) Drug-specific risk of non-tuberculosis opportunistic infections in patients receiving anti-TNF therapy reported to the 3-year prospective French RATIO registry. Ann Rheum Dis 70:616-623.
- Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, et al. (2013) Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 369: 699-710.
- Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, et al. (2013) Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med 369: 711-721.
- Loftus EV Jr, Colombel JF, Siegel C, Lewis J, Abhyankar B, et al (2014) Safety of Vedolizumab Alone or With Concomitant Corticosteroids and/or Immunosuppressants in Patients with Ulcerative Colitis or Crohn’s Disease. Am J Gastroenterol 109:1617.
- Okafor PN, Wasan SK, Farraye FA (2013) Pneumocystis jiroveci pneumonia in patients with inflammatory bowel disease: a survey of prophylaxis patterns among gastroenterology providers. Inflamm Bowel Dis 19:812-817.
- Wissmann G, Varela JM, Calderón EJ (2008) Prevention of Pneumocystis pneumonia in patients with inflammatory bowel disease based on the detection of Pneumocystis colonization. Inflamm Bowel Dis 14: 1751-1752.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15375
- [From(publication date):
December-2015 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 14405
- PDF downloads : 970