Research Article Open Access
Plasma Treatment-Promising Tool for Preparation of Disposable Monolithic Columns
Ita Junkar1*, Gregor Primc1, Tanja Mivsek2, Matic Resnik1,3, Janez Kovac1, Andrijana Sever Skapin4, Ales Podgornik2 and Miran Mozetic1
1Jozef Stefan Institute, Jamova cesta 39, 1000 Ljubljana, Slovenia
2 University of Ljubljana, Faculty of Chemistry and Chemical Technology, Vecna pot 113, 1000 Ljubljana, Slovenia
3 Jozef Stefan International Postgraduate School, Jamova cesta 39, 1000 Ljubljana, Slovenia
4 Slovenian National Building and Civil Engineering Institute, Dimiceva ulica 12, 1000 Ljubljana, Slovenia
- *Corresponding Author:
- Ita Junkar
Jamova cesta 39
1000 Ljubljana, Slovenia
Tel: +38614773398
Fax: +38614773440
E-mail: ita.junkar@ijs.si
Received date: May 29, 2015; Accepted date: June 25, 2015; Published date: July 02, 2015
Citation: Junkar I, Primc G, Mivsek T, Resnik M, Kovac J, et al. (2015) Plasma Treatment-Promising Tool for Preparation of Disposable Monolithic Columns. J Anal Bioanal Tech 6:253 doi: 10.4172/2155-9872.1000253
Copyright: © 2015 Junkar I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Atmospheric pressure plasma jet was employed to improve adhesion between polypropylene (PP) column wall and monolith used in chromatography. Different treatment conditions for modification of PP tube were used and the effects of treatment were analysed with X-ray photoelectron spectroscopy (XPS) and atomic force microscopy (AFM). Modified surfaces had higher oxygen content and surface was covered with small grain like structures. To explore effects of modification on adhesion between PP tube and monolith tensile strength measurements were conducted. It was shown that appropriate treatment conditions significantly increased bonding strength. The improvement of adhesion was attributed to increased oxygen functional groups obtained from plasma.
Keywords
Atmospheric pressure plasma jet; Polypropylene; Adhesion; Functionalization; Polymer; Chromatography column
Introduction
Chromatography is currently one of the most important techniques for analytics and for purification of different compounds. The separation mechanism is based on interaction between stationary phase, containing chemical moieties, being able to interact with the molecules dissolved in a liquid phase. Due to movement of mobile phase through the stationary phase, various molecules interact differently with the stationary phase resulting in different retention of various molecular species and consequently their separation. To exhibit high binding capacity, stationary phase commonly consists of porous particles to provide very high accessible surface area. Since pores of the particles are closed on one side, the only transport mechanism of molecules within the pores is molecular diffusion. The diffusivity of large biomolecules is very low and their purification time-which allows molecules to access most of available surface area-is very long. Both features are rate limiting steps for production of large biological molecules and consequently decrease process productivity.
To overcome this problem novel type of stationary phase called monolith was recently introduced [1]. In contrast to particulate stationary phases, monolith is a single piece of highly porous material with pore voids being formed of highly interconnected channels. Because of such structure, entire mobile phase is forced to move through these channels and molecules are transported to the stationary phase surface by convection, which in this way accelerate entire separation process several orders of magnitude [2]. They exist in variety of different microscopic structures as well as skeleton chemistries, either inorganic, where most extensively studied are silica monoliths having bicontinuous structure, or organic based on methacrylate, acrylamide, cellulose and many other which exhibit particulate, polyHIPE, or sponge like structure (cryogels) [3]. Due to their monolithic structure preparation monolithic chromatographic columns require to overcome several technical challenges. Among many of them have already been successfully solved, such as scaling-up of monolithic columns [4,5] or their non-invasive characterization [6], while bypassing of the mobile phase between a monolith and column wall, especially for columns operating in axial mode, is still challenging [7]. This can to some extent be overcome by changing axial operating mode to a radial one [8] or designing complex housing [7], while one straightforward approach is covalent attachment of the monolith to the column wall. This approach was elaborated in details for covalent bonding to silica [9] but there are almost no reports about covalent bonding of monoliths to plastic materials [10]. Since disposable technology is being increasingly accepted by pharmaceutical and biotech industry it would be very attractive to find simple method for covalent attachment of monoliths to inert FDA compliant plastic material such as polypropylene (PP). While this can be to some extent achieved using oxidizing chemicals, such as ozone, potassium persulfate or ammonium persulfate [11], an alternative approach might be to treat PP with highly reactive gaseous plasma [11-13].
Many studies showed that plasma treatment of polymers enables modification of polymer surface in terms of surface roughness, chemical composition and surface energy [12-14] by which improved adhesion of various coatings can be achieved [15]. Cold plasma consist of high energy electrons, low energy ions, excited species and long lived metastable particles which enable surface modification. In comparison to low pressure plasma the surface treated by atmospheric pressure exhibits lower oxidation, which can be due to different plasma chemistry. In case of low vacuum oxygen plasma the reactive species are mainly produced by collisions with high energy electrons while in case of atmospheric pressure plasma the oxygen species are produced by interaction of Ar metastable (in case of argon gas used for plasma) with air molecules [16,17]. Polymeric surface is modified by interaction with high energy ions, functionalization of the surface with reactive species and crosslinking [18,19]. There are growing interest in atmospheric pressure plasma jets due to its low cost and versatility: modification of 3D objects [20] are enabled at low temperature [21,22]; in addition, since there is no need for vacuum, it could be easily integrated into existing production lines. However modification of surfaces, especially long narrow tubes with plasma jets are not quite well understood, mostly due to electromagnetic interaction, flow dynamics, thermal instabilities and effects of tube diameter on plasma modification [23].
The main purpose of our work was to achieve not only increased surface roughness but mainly to incorporate novel functional groups on the surface which would enable covalent adhesion of monolith with PP tube. In present work we describe effects of plasma treatment on surface properties of PP tubes. We studied changes in chemical composition by X-ray photoelectron spectroscopy (XPS), morphological changes by atomic force microscopy and differences in adhesion of methacrylate monoliths on untreated and plasma treated PP tubes, which were evaluated by tensile strength measurements.
Materials and Methods
Plasma treatment
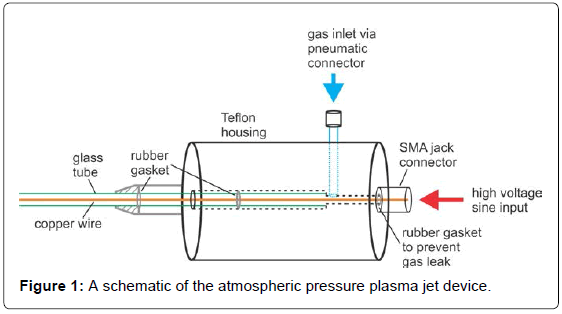
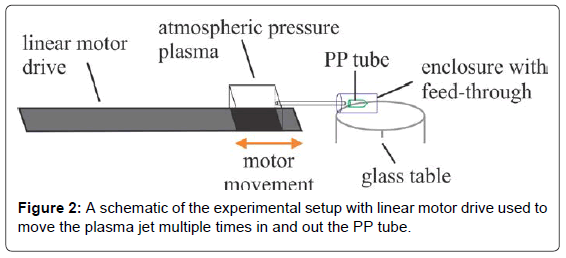
Experimental system consisted of an atmospheric pressure plasma (free plasma jet), a linear motor drive and a plastic enclosure to create argon rich atmosphere (Figure 1) [24].
The atmospheric pressure plasma system used belongs to the atmospheric pressure plasma jet (APPJ) group, specifically singleelectrode (SE) configuration was used. Plasma was excited using a sinusoidal power supply. A timer was used to generate excitation frequency of 23 kHz. The output voltage at the electrode was about 2.5 kV and plasma current consumption was about 1 mA [25]. Teflon housing served as a vessel for the copper round electrode (diameter 0.5 mm) that was center into a quartz tube (outer and inner diameter 4 mm and ~2.4 mm, respectively). Flow controller (Bronkhorst) was used for leaking the gas through the Teflon housing and the quartz tube. Plasma was created in Argon gas with constant flow of 2.2 L/min.
APP device was mounted onto a linear motor drive, 500 mm long, controlled by a computer. Such configuration enabled for precise movement and speed control of the APP device and thus linear movement of the APP jet. A PP tube, 39 mm long with the largest diameter of 10.7 mm and the smallest diameter of 5.3 mm, was mounted into the plastic housing. A small hole was made in the center of the plastic housing, allowing free movement of an APP quartz tube through it. Plastic housing was fixed so that the height of the PP tube inside the housing was the same as the height of the APP jet (or APP device quartz tube). The linear motor drive enabled a repeated backand- forth linear movement of the APP jet inside the plastic housing into and out of the PP tube. Closing one end of the housing enabled the establishment of argon rich atmosphere. The lowest speed of motor movement was 12 mm/s, which is quite fast; therefore we made several repeated motor movements of the APP jet into and out of the PP tube. Typical time needed to move the jet into and outside of the PP tube was about 5 s. We made repeated motor movements; starting at the end of PP tube and going to the beginning and then back again (one treatment). In order to study the effects of treatment on surface modification and adhesion we made repeated movements, ranging from a single movement to 30 consecutive movements. Samples treated more than once are annotated in the text as PP 1x, PP 2x, PP 5x, and so on (Figure 2).
Water contact angle measurements
The surface wettability was measured immediately after plasma treatment by determination of water contact angle with a demineralized water droplet of a volume of 2 μl. PP tubes were cut into half and a water contact angle was measured. A water contact angle was measured by Advex Instruments See System E equipped with a CCD camera and a PC computer, which enabled us to make high resolution pictures of a water drop on the sample surface. For each sample 10 measurements were perform in order to minimize the statistical error. The relative humidity was kept at 45% and the temperature at 25°C. The contact angles were determined by software See System 6.3 which enables fitting of the water drop on the surface in order to allow a relatively precise determination of the contact angle. An estimated error for each WCA value is less than 3.0°. In order to evaluate ageing of PP tube after plasma treatment water contact angle measurements were done on tubes stored for 4 days in air at room temperature and constant humidity.
AFM analysis
Surface morphology was observed by atomic force microscopy (AFM). Topographic changes of PP tube surface before and after plasma treatment were monitored with AFM (Solver PRO, NT-MDT) in the tapping mode in air. Samples were scanned with standard Si cantilever with a force constant of 10 N/m at a resonance frequency of 170 kHz. All measurements were done in air, one day after plasma treatment, on an area size of 5 × 5 μm and 2 × 2 μm. The average surface roughness (Ra) was calculated from images taken on area size of 2 × 2 μm (corresponds to the average value of the surface height). To obtain representative results average surface roughness was measured on the same sample on 5 different areas.
XPS analysis
The surface of the sample was analysed with an XPS instrument (TFA XPS Physical Electronics). The base pressure in the XPS analysis chamber was about 6 × 10-8 Pa. Samples were excited with X-rays over a 400 μm spot area with a monochromatic Al Kα radiation at 1486.6 eV. The photoelectrons were detected with a hemispherical analyzer positioned at an angle of 45° with respect to the normal vector of the sample’s surface. The energy resolution was about 0.65 eV. Survey scan spectra were recorded at a pass energy of 187 eV. Also individual high resolution spectra at pass energy of 29 eV and 0.1 eV energy step were recorded for C 1s and O 1s peaks. Since the investigated samples are insulators, an additional electron gun has been used to provide surface neutralization during measurements. All spectra were referenced against the main C 1s peak of the carbon atoms, which was given a value of 285.0 eV. The XPS spectra were measured for the untreated and plasma treated samples. The concentration of different chemical states of carbon in the C 1s peak has been determined by fitting the curves with symmetrical Gauss Lorentz functions. The spectra were fitted using software MultiPak ver. 8.1 (Physical Electronics), supplied with the spectrometer. A Shirley type background subtraction was used. It should be noted that the analysis depth of the XPS method is about 8 nm.
Polymerization
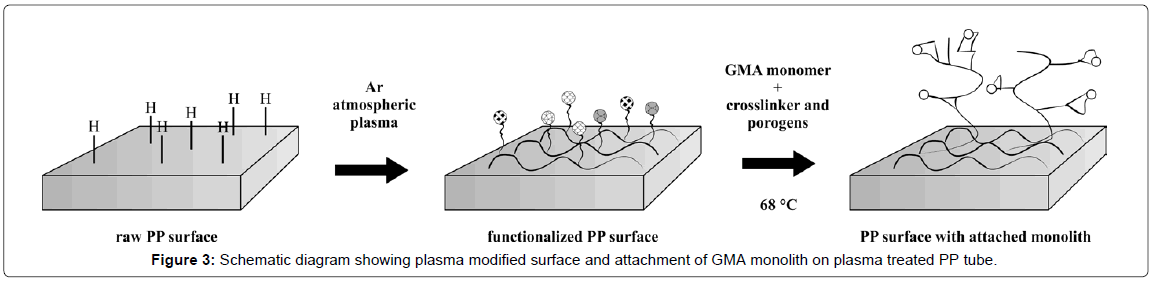
Polymerization mixture consisted of 16 wt% of glycidyl methacrylate (GMA) as a monomer, 24 wt% of ethylene glycol dimethacrylate as a crosslinker, porogens cyclohexanol and dodecanol (48 wt% and 12 wt% respectively) as pore forming agents, and initiator benzoyl peroxide [26]. Each of plasma treated and untreated PP tubes were filled with 1.5 mL of polymerization mixture. In the PP tube center, a hook bolt (diameter of 4.5 mm and length of 35 mm) was immersed into the polymerization mixture. Bolt dimensions allowed a PP tube to be closed before polymerization. Bolt length was properly chosen to be firmly secured inside the PP tube. The bolt’s head was inside a PP tube but out of the polymerization mixture. To provide fast polymerization reaction the polymerization temperature was set to 68°C [27] in order to benefit from the presence of active moieties on inner PP tube surface which were formed by plasma treatment. Schematic diagram on attachment of GMA monolith with plasma modified surface is presented in Figure 3. The polymerization process was allowed to proceed overnight in water bath and resulted in solid porous monolith skeleton containing non reacted porogens in pores [28] PP tubes were cooled to room temperature inside the water bath and a cover was opened afterwards.
Tensile strength measurements
The testing of mechanical properties was performed by measuring the tensile stress (tensile strength and strain at break). A calibrated mechanical testing equipment was used (ZWICK/ROELL Z100). The PP tube was mounted onto a holder that enabled pulling the monolith (with firmly secured hook bolt that served as anchorage) out of the tube. The preload was achieved with the speed of 0.5 mm/min and was 0.5N. The testing procedure was set at the speed of 1 mm/min and ended when the pulling force decreased to 80% of the maximum achieved force. The tests were performed on 3 parallels and the mean values were reported. The temperature and relative humidity during measurements was 23°C and 50%, respectively.
Results and Discussion
Water contact angle measurements
Water contact angle measurements showed that untreated surface has the highest contact angle, about 80.0° ± 1.3°, while the water contact angle on PP 5x is about 50.4° ± 1.8° and on PP 20x to about 46.1° ± 1.3°. Therefore slight differences in wettability are observed. Changes in wettability after plasma treatment are due to formation of new polar oxygen functionalities, electric charges, free radicals and roughness. In order to gain better insights on differences in wettability, the stability of functional groups on plasma treated PP tubes was measured after 4 days of ageing in controlled environment. Both plasma treated surfaces exhibited ageing effects, which were more prominent for the case of PP 5x plasma treated surface. The water contact angle increased to about 57.9° ± 1.1° and to about 47.8° ± 1.3° for PP 5x and PP 20x, respectively. The calculated difference in water contact angle before and after ageing for PP 5x was 7.5° and only about 1.7° for PP 20x. The longer treated surface seems to age much slower, which could be explained by higher crosslinking of polymer surface [29] due to formation of free radicals from plasma. Slight differences in surface morphology were observed by AFM between PP 5x and PP 20x, but the morphology of aged surface remained unchanged. Therefore changes in wettability after ageing can be explained only by changes in surface chemistry.
XPS analysis
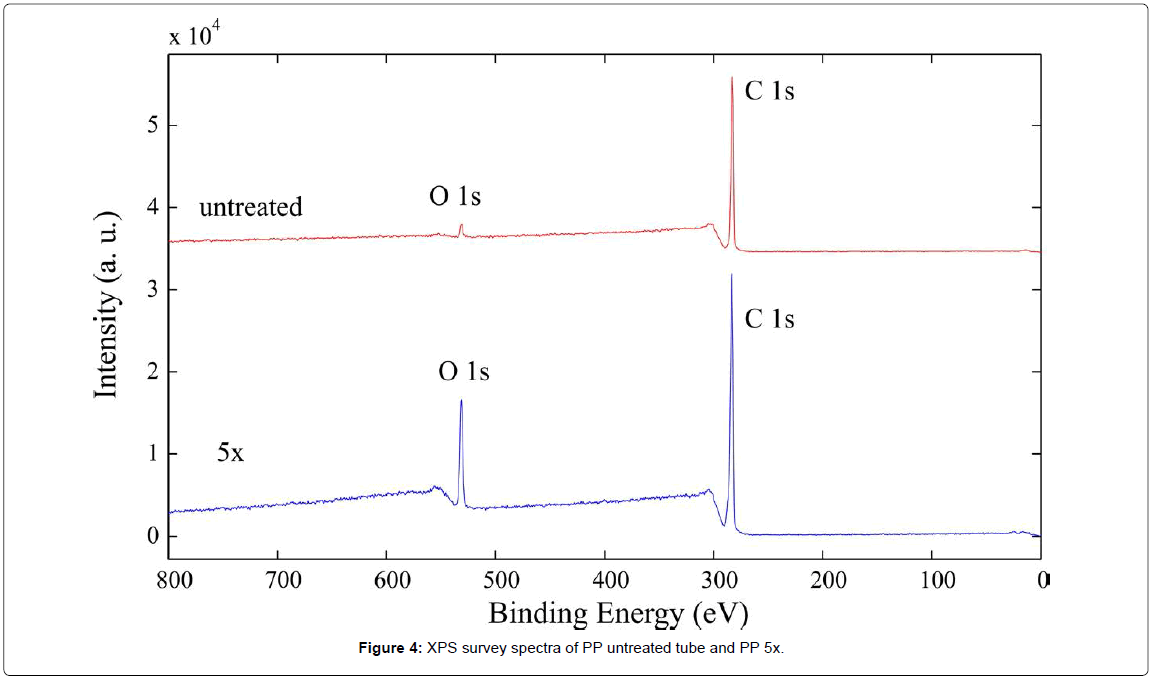
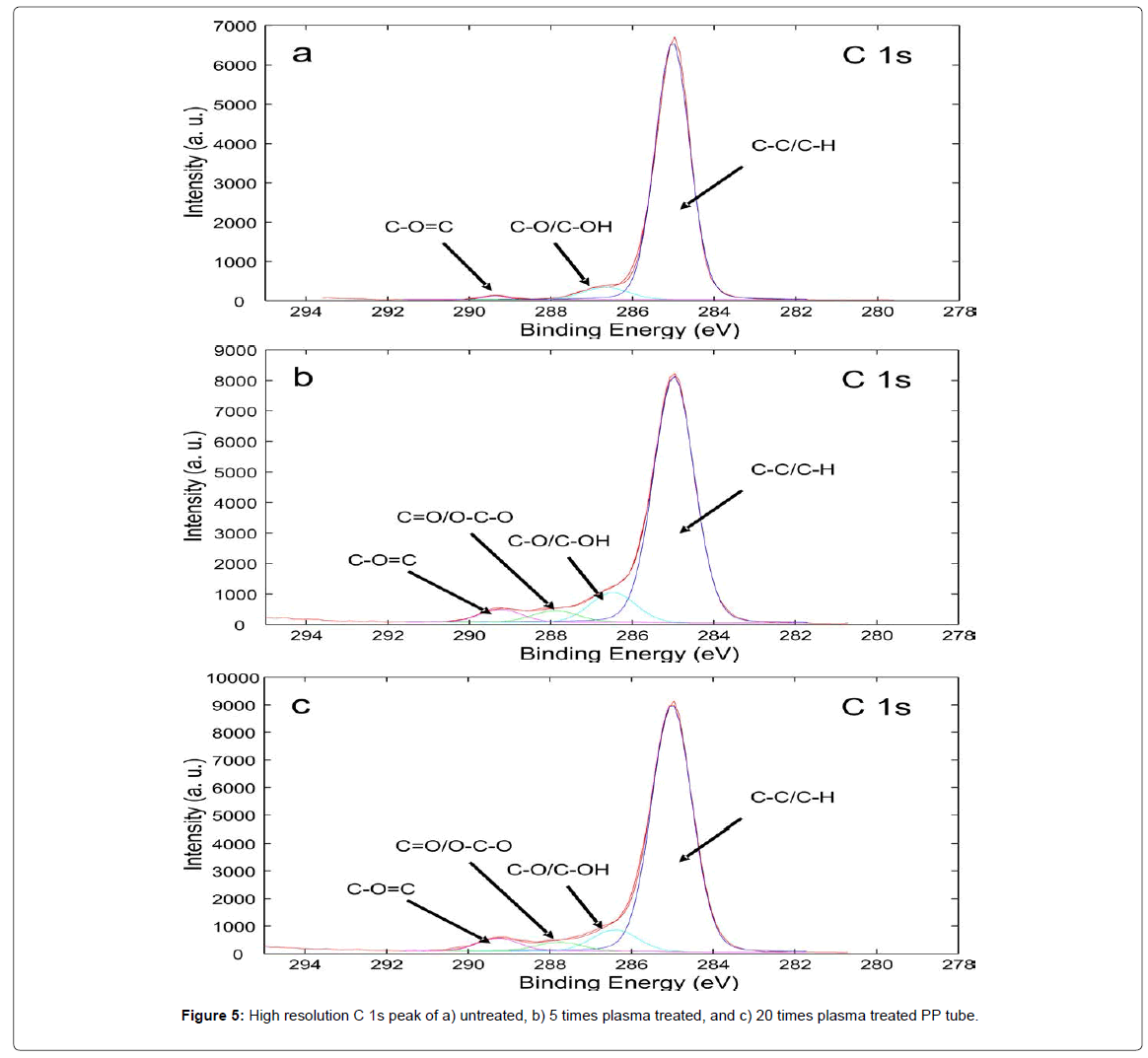
From XPS analysis it can be observed that surface treated with plasma has higher oxygen content and lower carbon content than untreated surface. As seen from survey spectra (Figure 4 and Table 1), untreated PP tube has about 95 at% of carbon and 5 at% of oxygen, which is due to a manufacturing process. The plasma treated surface has 84.2 at% of carbon and 15.6 at% of oxygen. This indicates that novel oxygen functional groups were formed on the surface of a PP tube after plasma treatment. In order to determine if functionalization is uniform along the PP tube’s surface, analysis was performed on different areas on the same PP tube. It has been found out that there were no significant discrepancies between different areas, so the modification was rather uniform. The ratio of O 1s/C 1s increases from 0.05 to 0.19 for untreated and plasma treated surface, respectively. XPS analysis has been performed also on the PP tube treated for 20 times in order to observe changes in a chemical composition. Interestingly, chemical composition of the PP 20x did not significantly differ from the PP 5x, as the concentration of oxygen species was very similar. On the surface of PP 20x nitrogen functional groups were also present, due to incorporation of nitrogen from air.
| Sample | C (at%) | O (at%) | N (at%) | C-C,C-H (%) | C-O, C-OH (%) | C=O,O-C-O (%) | O-C=O (%) |
|---|---|---|---|---|---|---|---|
| 285.0 eV | 286.5 eV | 287.8 eV | 289.2 eV | ||||
| Untreated | 95.0 | 5.0 | 0 | 93.3 | 5.7 | 0.1 | 0.9 |
| PP 5x | 84.2 | 15.7 | 0.13 | 81.5 | 10.4 | 4.0 | 4.1 |
| PP 20x | 82.9 | 15.4 | 1.76 | 84.6 | 7.9 | 3.3 | 4.3 |
Table 1: Atomic concentration obtained from XPS spectra and relative concentration of functional groups obtained from high-resolution C 1s spectra for untreated and plasma treated PP 5x and PP 20x tube.
In order to investigate novel oxygen functional groups on the PP tube’s surface high resolution XPS analysis of C 1s peak was performed. The relative concentration of each chemical component in C 1s peak has been determined by deconvolution of experimental spectra using Gaussian-Lorentzian curves. As seen in Figure 5 the C 1s spectrum of the untreated PP tube has first peak at 285.0 eV, which corresponds to C-C and C-H bonds from PP, a small peak at 285.7 eV corresponding to C-O groups, minor peaks at 286.8 eV corresponding to C=O and O-C-O and at 289.1 eV corresponding to O-C=O. On the other hand the C 1s spectra of plasma treated PP 5x and PP 20x tube consist of a broad shoulder at a high binding energy side which is due to incorporation of novel oxygen functional groups (Figures 5b and 5c), such as alcohols, carbonyls and carboxylic acid. It can be clearly seen that the PP 5x exhibits more pronounced peaks corresponding to C O and a small increase in C=O and O C O functional groups. The percentage of each functional group determined from high resolution C 1s spectra is presented in Table 1. Slight differences in oxygen functional groups between PP 5x and PP 20x are observed and could influence on adhesion with monolith. Immediately after plasma treatment the surface is covered with radicals which provide reactive sites for adhesion with epoxy or vinyl groups from GMA monolith. Moreover oxygen radicals on the surface can also form peroxides, which cannot be detected by XPS, but could covalently attach with monolith.
AFM analysis
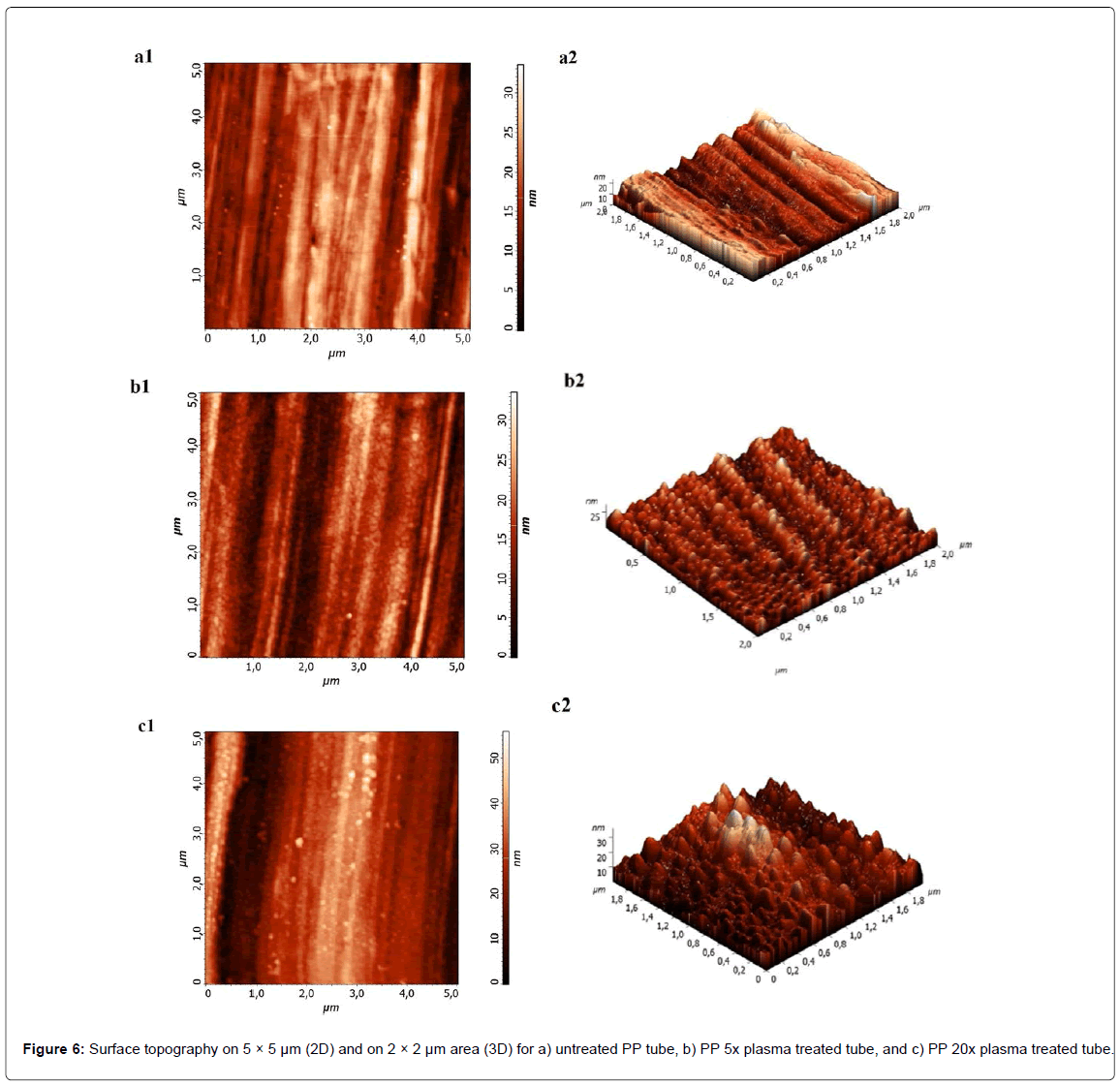
The PP tubes’ morphology was analyzed with AFM. The untreated PP surface has no special morphology. On the surface only razes have been observed, which are probably present due to manufacturing process. The average roughness measured on 5 × 5 μm area is about 5.2 nm. On the other hand, plasma treated surfaces exhibits small grain like structures. The average roughness of the plasma treated surface on 5 × 5 μm area is 4.5 nm. Besides the presence of the manufacturing razes, the surface is covered with small grains which are about 6 nm high. These structures are better observed at higher magnification, as seen from Figures 6. Structures formed on the surface are probably the result of a preferential etching of amorphous parts of polymer by oxygen plasma. It seems that roughness is not significantly altered by plasma treatment. Even after longer treatment times the change in roughness is negligible and is in the range of experimental error. However, it has been noticed that the PP 20x surface exhibits more pronounced grain like structures with a bit wider and higher dimensions (the average height was about 8 nm).
Tensile strength measurement
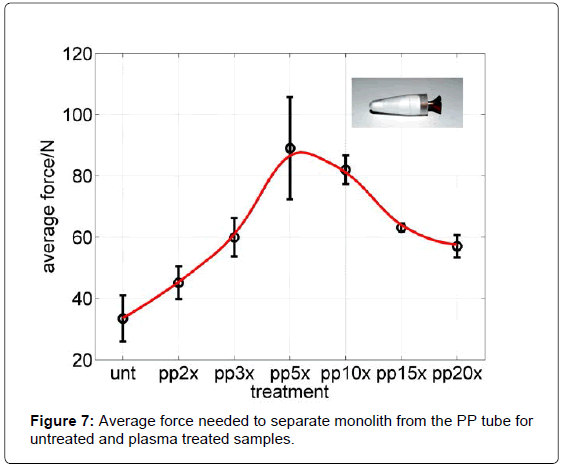
According to results obtained on PP tube after different plasma treatment conditions (repetition of treatments inside and out of PP tube) a different force was needed to remove polymer from the PP tube. In Figure 7 different treatments and measured maximum forces are presented. The highest force needed for removal of polymer from the PP tube was for the PP 5x tube. Longer treatments actually caused a decrease in adhesion force.
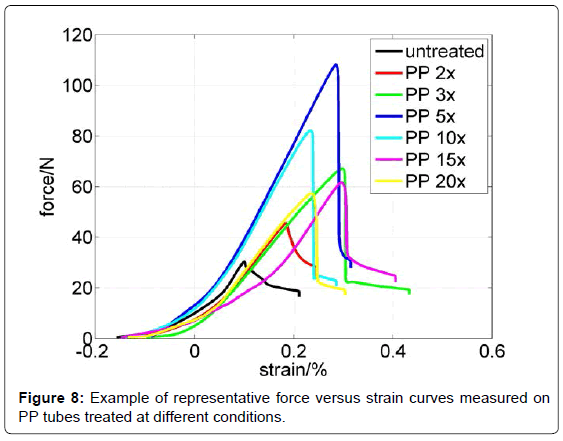
In Figure 8 representative force versus strain curves are presented. It can be clearly seen that the highest force is needed for the PP 5x tube. In this case the adhesion is increased by a factor of 2. Interestingly longer treatment times lower adhesion force. As observed from the XPS analysis surface seems to be saturated with oxygen functional groups already after 5 time plasma treatment. Moreover longer treatment even decreases the number of C-O functional groups, which may have an important role in adhesion with monolith. Another reason for decreased adhesion after longer treatment could be due to formation of low molecular weight fragments (LMWF) or crosslinking of polymer matrix. The LMWF are unstable on the surface and could be easily removed from the surface or can be quickly reoriented inside the polymer matrix. Since the XPS analyzing depth is about 8 nm, it was not possible to distinguish between reoriented functional groups and groups that are oriented outside of polymer matrix, nor was it possible to detect the crosslinking. However it can be observed from water contact angle measurements that PP 20x is ageing much slower, which could be explained by crosslinking. In this case adhesion with monolith could be lower as the reactive species from plasma crosslink and thus lower the number of possible active sites for adhesion with monolith. Increased adhesion with monolith could be due to covalent bonding between PP tube’s surface and monolith, but we could not fully exclude the possible morphological features of the surface which could to some extent also influence on adhesion. Although surface roughness is not much altered the grain like structures formed on PP 5x and PP 20x have slight variation in size (about 2 nm in height) as well as different distribution on the surface (Figures 6b and 6c). The grain like structures formed on PP 5x are not as far apart as in case of PP 20x, which could provide higher friction force and thus better adhesion with monolith.
Conclusion
Results from our study show that 5 time atmospheric pressure plasma treatment was the optimal treatment procedure which significantly increased adhesion force between PP tube and monolith. As revealed by XPS novel oxygen functional groups were formed on the surface. According to AFM analysis surface exhibited small grain like structures, which were uniformly distributed along the PP tube. Tensile strength measurements show that the optimal plasma treatment more than doubled the force necessary to remove monolith from the PP surface (wall). APP treatment is simple and robust technique which could be used for preparation of GMP compliant cost effective disposable monolithic columns applicable in pharmaceutical and biotech industry.
References
- Jungbauer A, Hahn R (2008) Polymethacrylate monoliths for preparative and industrial separation of biomolecular assemblies. J Chromatogr A 1184: 62-79.
- Podgornik A, Krajnc NL (2012) Application of monoliths for bioparticle isolation. J Sep Sci 35: 3059-3072.
- Wang PG (2010) Monolithic Chromatography and Its Modern Applications. ILM Publications.
- Podgornik A, Jancar J, Merhar M, Kozamernik S, Glover D, et al. (2004) Large-scale methacrylate monolithic columns: design and properties. J Biochem Biophys Methods 60: 179-189.
- Podgornik A, Barut M, Strancar A, Josic D, Koloini T (2000) Construction of large-volume monolithic columns. Anal Chem 72: 5693-5699.
- Podgornik A, Vidic J, Jancar J, Lendero N, Frankovic V, et al. (2005) Noninvasive methods for characterization of large-volume monolithic chromatographic columns. Chemical engineering & technology 28: 1435-1441.
- Schulte M, Dingenen J (2001) Monolithic silica sorbents for the separation of diastereomers by means of simulated moving bed chromatography. J Chromatogr A 923: 17-25.
- Strancar A, Barut M, Podgornik A, Koselj P, Schwinn H, et al. (1997) Application of compact porous tubes for preparative isolation of clotting factor VIII from human plasma. J Chromatogr A 760: 117-123.
- Unger KK, Tanaka N, Machtejevas E (2010) Monolithic silicas in separation science: concepts, syntheses, characterization, modeling and applications. John Wiley & Sons.
- Stachowiak TB, Rohr T, Hilder EF, Peterson DS, Yi M, et al. (2003) Fabrication of porous polymer monoliths covalently attached to the walls of channels in plastic microdevices. Electrophoresis 24: 3689-3693.
- Alexander JV, Neely JW, Grulke EA (2014) Effect of chemical functionalization on the mechanical properties of polypropylene hollow fiber membranes. Polym Sci B Polym Phys 52: 1366-1373.
- Lehocky M, Drnovska H, LapciKova B, Barros-Timmons AM, Trindade T, et al. (2003) Plasma surface modification of polyethylene. Colloids Surf A Physicochem Eng Asp 222: 125-131.
- Vesel A, Mozetic M (2012) Surface modification and ageing of PMMA polymer by oxygen plasma treatment. Vacuum 86: 634-637.
- Cvelbar U, Mozetic M, Junkar I, Vesel A, Kovac J, et al. (2007) Oxygen plasma functionalization of poly (p-phenilene sulphide). Appl Surf Sci 253: 8669-8673.
- Lehocky M, Mracek A (2006) Improvement of dye adsorption on synthetic polyester fibers by low temperature plasma pre-treatment. Czechoslovak Journal of Physics 56: B1277-B1282.
- Van Gaens W, Bogaerts A (2013) Kinetic modelling for an atmospheric pressure argon plasma jet in humid air. J Phys D Appl Phys 46: 275201.
- Zaplotnik R, Biscan M, Kregar Z, Cvelbar U, Mozetic M, et al. (2015) Influence of a sample surface on single electrode atmospheric plasma jet parameters. Spectrochim Acta Part B At Spectrosc 103: 124-130.
- Wertheimer MR (2014) Plasma Processing and Polymers: A Personal Perspective. Plasma Chemistry and Plasma Processing 34: 363-376.
- Mozetic M, Ostrikov K, Ruzic DN, Curreli D, Cvelbar U, et al. (2014) Recent advances in vacuum sciences and applications. J Phys D Appl Phys 47: 153001.
- Lommatzsch U, Pasedag D, Baalmann A, Ellinghorst G, Wagner HE (2007) Atmospheric pressure plasma jet treatment of polyethylene surfaces for adhesion improvement. Plasma Process Polym 4: S1041-S1045.
- Hofmann S, Van Gessel A, Verreycken T and Bruggeman P (2011) Power dissipation, gas temperatures and electron densities of cold atmospheric pressure helium and argon RF plasma jets. Plasma Sources Sci Technol 20: 065010.
- Hong Y, Lu N, Pan J, Li J, Wu Y, et al. (2013) Characteristic study of cold atmospheric argon plasma jets with rod-tube/tube high voltage electrode. J Electrostat 71: 93-101.
- Kostov K, Nishime T, Castro A, Toth A, Hein L (2014) Surface modification of polymeric materials by cold atmospheric plasma jet. Appl Surf Sci 314: 367-375.
- Mozetic M, Primc G, Vesel A, Modic M, Junkar I, et al. (2015) Application of extremely non-equilibrium plasmas in the processing of nano and biomedical materials. Plasma Sources Sci Technol 24: 015026.
- Zaplotnik R, Kregar Z, Biscan M, Vesel A, Cvelbar U, et al. (2014) Multiple vs. single harmonics AC-driven atmospheric plasma jet. Europhys Lett 106: 25001.
- Mihelic I, Krajnc M, Koloini T, Podgornik A (2001) Kinetic model of a methacrylate-based monolith polymerization. Ind Eng Chem Res 40: 3495-3501.
- Mihelic I, Koloini T, Podgornik A (2003) Temperature distribution effects during polymerization of methacrylate-based monoliths. J Appl Polym Sci 87: 2326-2334.
- Svec F, Frechet J M (1995) Kinetic control of pore formation in macroporous polymers. Formation of "molded" porous materials with high flow characteristics for separations or catalysis. Chemistry of materials 7: 707-715.
- Punga I-L (2014) Surface modification and stability of polymers treated by atmospheric-pressure helium plasma. Journal of Advanced Research in Physics 4: 1-8.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15559
- [From(publication date):
August-2015 - Dec 19, 2024] - Breakdown by view type
- HTML page views : 11054
- PDF downloads : 4505