Research Article Open Access
Plasma Concentration of Oxycodone and Pain during Hemodialysis in a Patient with Cancer
Satoshi Murakami1, Mizuki Herai2, Satoshi Suzuki 3, Takayuki Fujii3, Hiroaki Tanaka3, Mizuki Shinozaki3, Hideya Kokubun4, Toru Akagi5, Yasuhito Uezono6,7, Yuko Murakami-Ando8, Seiji Shiraishi6,9, Motohiro Matoba10*1Department of Palliative Medicine, Seirei Sakura Citizen Hospital, Chiba, Japan
2Department of Nursing, Seirei Sakura Citizen Hospital, Chiba, Japan
3Department of Internal Medicine, Seirei Sakura Citizen Hospital, Chiba, Japan
4Department of Pharmacy, Kitasato University Hospital, Kanagawa, Japan
5Department of Pharmacy, National Cancer Center Hospital, Tokyo, Japan
6Cancer Pathophysiology Division, National Cancer Center Research Institute, Tokyo, Japan
7Division of Supportive Care Research, Exploratory Oncology Research & Clinical Trial Center, Tokyo, Japan
8Department of Anesthesia, Iwai Orthopaedic Medical Hospital, Tokyo, Japan
9Department of Anesthesia and Intensive Care, National Cancer Center Hospital, Tokyo, Japan
10Department of Palliative Care, Japanese Red Cross Medical Center, Tokyo, Japan
- *Corresponding Author:
- Motohiro Matoba, M.D., PhD
Department of Palliative Care
Japanese Red Cross Medical Center
4-1-22 Hiroo, Shibuya-ku
Tokyo 150-8935, Japan
Tel: +81-3-3400-1311
Fax: +81-3-3409-1604
E-mail: teamkanwa@gmail.com
Received date: Feb 15, 2016; Accepted date: Mar 09, 2016; Published date: Mar 12, 2016
Citation: Murakami S, Herai M, Suzuki S, Fujii T, Tanaka H, et al. (2016) Plasma Concentration of Oxycodone and Pain during Hemodialysis in a Patient with Cancer. J Palliat Care Med 6:252. doi:10.4172/2165-7386.1000252
Copyright: © 2016 Murakami S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Palliative Care & Medicine
Abstract
Pain is one of the most common problems in palliative medicine, and opioid therapy should be used adequately. Clinically, many cancer patients with severe renal dysfunction receive opioids for pain control. However, the pharmacokinetics of opioids during hemodialysis are not completely understood. We investigated the time course of the plasma concentration of oxycodone and pain during hemodialysis in a 55-year-old man using oxycodone for cancer pain. The patient’s plasma concentration of oxycodone decreased during hemodialysis, and increased after it ended. Conversely, his pain increased after the beginning of hemodialysis, and improved after it was finished. Additionally, our results did not show a relationship between the plasma concentration of oxycodone and his pain. Breakthrough pain occurred several times during hemodialysis irrespective of the plasma concentration of oxycodone. The decrease in plasma oxycodone during hemodialysis appeared to be caused by the removal of oxycodone by hemodialysis, and the later increase after hemodialysis appeared to be related to the redistribution of oxycodone. On the other hand, breakthrough pain during hemodialysis may be caused by hemodialysis itself, rather than due to the decrease of the plasma concentration of oxycodone, given that no relationship between the plasma concentration of oxycodone and pain could be identified. When building a strategy of pain management during hemodialysis, both the possibilities of a decrease in the plasma concentration of oxycodone by hemodialysis and the increase of pain by hemodialysis itself should be considered.
Keywords
Oxycodone; Hemodialysis; Cancer pain
Introduction
Pain is one of the most common problems in palliative medicine. According to a systematic literature review, prevalence of pain was 33% in patients after curative treatment, 59% in patients being treated for cancer, and 64% in patients with metastatic, advanced, or terminal phase cancer [1]. Reported response rates of adequate analgesia when using The World Health Organization analgesic ladder guidelines [2] have varied between 71 and 100% [3]. Opioid therapy should be used as the main palliative in all cancer patients with at least moderate pain.
Clinically, many cancer patients receive opioids for pain control in spite of concomitant renal dysfunction. This treatment carries significant risks, including metabolite accumulation, increased elimination half-lives, and toxicity (e.g., accidental overdose) in such patients [4-8]. Morphine, for example, has active metabolites such as morphine-3-glucuronide and morphine-6-glucuronide [4-6]. Oxycodone is relatively safe because it produces fewer active metabolites [7,9], and fentanyl's metabolite, norfentanyl, has no biological activity. However, the pharmacokinetics of opioids during hemodialysis are not completely understood [10]. We investigated the time course of the plasma concentration of oxycodone and pain during hemodialysis in a 55-year-old male using oxycodone for cancer pain.
The patient provided written permission for publishing this report.
Case Report
A 55-year-old man with a history of lung cancer, liver metastasis, a liver abscess, and glomerulonephritis (RPGN) associated with methicillin-resistant Staphylococcus aureus (MRSA) infection presented at our department. He underwent a right upper lobectomy, with resection of the parietal pleura, for right lung cancer. He had a recurrence of the cancer, liver metastasis, and a liver abscess postoperatively, and was treated via a left hepatic lobectomy for the metastastic liver tumor. Oxycodone (Oxycontin®; 80 mg/day) was used to treat severe pain in his right chest. He didn’t receive drugs which influence the metabolism of oxycodone such as CYP3A4 and CYP2D6 inhibitors and inducers. Elevated serum creatinine, proteinuria, and hematuria indicated glomerulonephritis-induced renal failure. He then presented at our department for pain control during hemodialysis.
He presented to us with pain in his right chest, right back, and right arm. The right chest and back pain were intermittent sharp pains, and localized in his right precordia and around his scapula. The maximum, minimum, and average pain scores based on the 0–10 numeric rating scale (NRS) were 4/10, 2/10 and 2/10 respectively. Neither neurological disturbances, hypaesthesia, nor listlessness was identified, and the douleur neuropathique 4 questions (DN4) score was a 0/10. We therefore diagnosed these right chest and back pains as somatalgia. However, the right arm pain was continuous and intermittent, relatively sharp, and localized in the area supplied by the ulnar nerve. No shooting pain was identified. The maximum, minimum, and average pain scores on the 0–10 NRS were 4/10, 2/10, 2/10 respectively. The arm pain was accompanied by allodynia and hypoesthesia as identified by cold test (6-7/10), pinprick test (6-7/10), and touch test (6-8/10). Muscle weakness, scored 3/5 on the manual muscle test (MMT), was also identified in the areas innervated by the ulnar, radial, and musculotaneous nerves. The DN4 score was 5/10. We therefore diagnosed his right arm pain as neuropathic pain, mainly caused by disturbance of the right brachial plexus related to his right lung cancer.
His blood urea nitrogen (BUN) and serum creatinine were 111–169 mg/dL and 11.6-14.6 mg/dL, respectively, and estimated glomerular filtration rate (GFR) was 3.5-4.2 mL/min/1.73 m2. His liver function was classified into Child-Pugh class B, according to a platelet level of 14.9×104/μL, a prothorombin time of 56.6% of normal, and a total bilirubin level of 1.1 mg/dL. His pain was successfully controlled with 80 mg/day of oxycodone sustained action tablets (Oxycontin®), and several uses of 20 mg of immediate release oxycodone hydrochloride (Oxinorm®) as rescue.
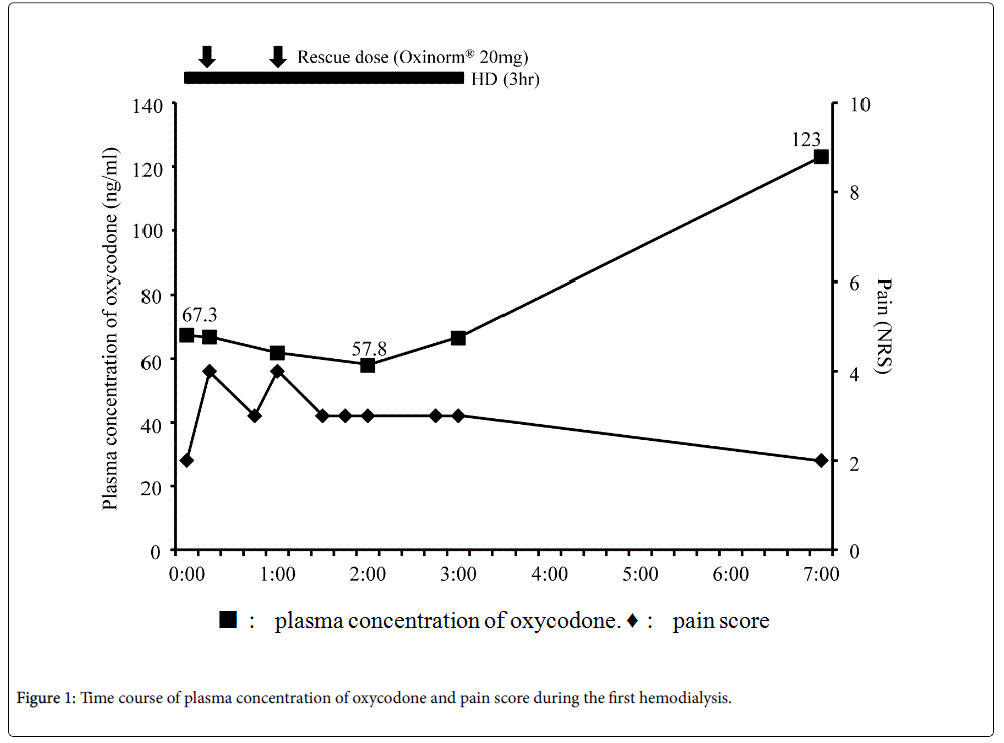
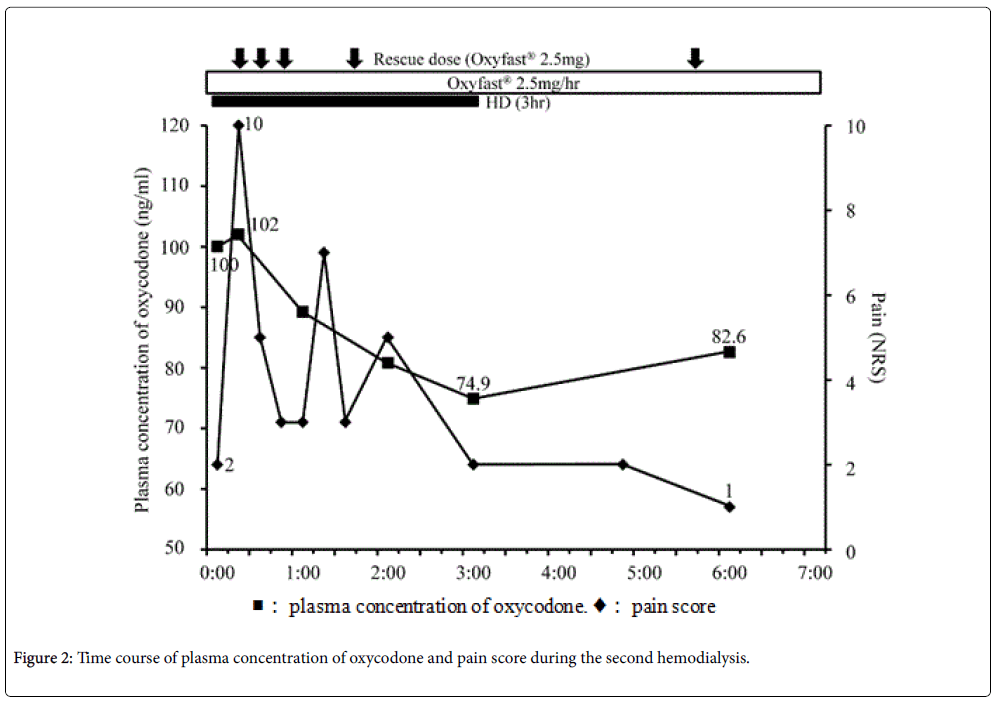
Blood samples were collected to determine the plasma concentration of oxycodone at the following time points: Before hemodialysis; 15 min, 60 min, 120 min, and 180 min after the beginning of hemodialysis; 3 h (second hemodialysis) and 4 h (first hemodialysis) after the end of hemodialysis (Figure 1 and 2). Vital signs including heart rate, blood pressure, respiratory rate, Ramsay Sedation Score, and 0–10 NRS were recorded at the same time points. The listed vital signs were additionally recorded at 45 min after the beginning of hemodialysis, and at any time at which the patient complained of symptoms such as increasing pain or nausea (Figure 1 and 2). When the patient's pain increased during the study period, he was given 20 mg of Oxinorm® orally on the first day of hemodialysis, or 2.5 mg of Oxyfast® administered subcutaneously using a patientcontrolled analgesia (PCA) pump on the second day of hemodialysis, as rescue medicine.
The first hemodialysis treatment used a dialysis system (DCS-27; Nikkiso, Tokyo, Japan) with a dialyser (KF-15C; Asahi Kasei medical, Tokyo, Japan) set to a flow rate of 120 mL/min, and began 140 min after he took the oxycodone sustained action tablets. We decided the flow rate relatively low at 120 ml/min following the advice of Board Certified Nephrologist of the Japanese Society of Nephrology considering the risk of the prevention of the disequilibrium syndrome, and this setting is common in Japan. His right chest and back pain increased at 15 and 60 min after the beginning of hemodialysis (Figure 1), so he took rescue medicine each time. The location and quality of the chest and back pain were not changed. The plasma concentration of oxycodone decreased over the course of hemodialysis treatment, and then increased after the end of hemodialysis (Figure 1). After hemodialysis was finished, his right chest and back pain immediately improved, and no side effects were observed that would indicate an overdose of oxycodone.
On the next day, oxycodone was administrated continuously via subcutaneous injection instead of orally, in order to better control the plasma concentration of oxycodone. He was administered 2.5 mg/h of Oxyfast® subcutaneously, and a bolus of 2.5 mg of Oxyfast® using the PCA system as rescue medicine. This system successfully controlled his pain.
He underwent a second hemodialysis 2 days later, using the same dialysis system and flow rate as above. His right chest and back pain increased at 15 and 69 min after the beginning of hemodialysis, so rescue medicine was administered via the PCA pump (Figure 2). As before, the plasma concentration of oxycodone decreased sequentially over the course of hemodialysis, and then increased after the end of hemodialysis (Figure 2). After hemodialysis ended, his right chest and back pain immediately improved, and no overdose-related side effects were observed.
Discussion
In this study, a patient with cancer who used oxycodone for pain control underwent hemodialysis for renal failure. The plasma concentration of oxycodone decreased over the course of hemodialysis, and increased after it ended. In contrast, the patient's pain increased during hemodialysis, requiring rescue medicine each time it increased, and then improved after hemodialysis ended. No side effects indicating an overdose of oxycodone were identified throughout the study periods.
Evidence from several studies indicates that good renal function is necessary for eliminating oxycodone and its metabolites from the body. For example, Pöyhiä et al. reported that 8–14% of the dose of oxycodone is not metabolized before being excreted in the urine [11]. Kirvela et al. reported that the elimination half-life of oxycodone is lengthened in uremic patients, and that excretion of metabolites is severely impaired [12]. Heiskanen et al. and Kaiko et al. reported that oxymorphone has no significant pharmacodynamic effect in subjects with normal renal function [9,13], but its accumulation could be expected in subjects with renal failure.
A significant amount of oxycodone is removed by hemodialysis, because its molecular weight and the degree of plasma protein binding are relatively small [13]. Conversely, plasma oxycodone levels should rebound (increase) after hemodialysis is stopped because of the drug's high volume of distribution.
In this study, the plasma concentration of oxycodone decreased during hemodialysis and increased after it ended. On the other hand, his pain increased after the beginning of hemodialysis, and improved after hemodialysis was finished. Lee et al. reported that, similarly to our results, the plasma concentration of oxycodone, noroxycodone, and oxymorphone decreased during hemodialysis in a patient undergoing nephrectomy for renal tumors [7]. However, the pain scores in their study did not change during hemodialysis [7]. Our results in this regard were different from theirs, despite the patients having similar plasma concentrations of oxycodone.
Additionally, our results did not show a relationship between the plasma concentration of oxycodone and the patient’s pain. Breakthrough pain occurred several times during hemodialysis, irrespective of the plasma concentration of oxycodone. Although the rescue medicine was effective to some extent, the plasma concentration of oxycodone did not increase with its use. Davison reported that 50% of hemodialysis patients reported pain, and 83% of those patients rated it moderate to severe [14]. Douglas reported that pain is not only a common symptom in patients on hemodialysis, it is in fact the most common reason for withdrawing from hemodialysis [15]. Mercadante et al. reported that 25% of patients in 95 patients with end-stage renal disease who were receiving hemodialysis experienced breakthrough pain during hemodialysis [16]. In this study, a decrease of the plasma concentration of oxycodone during hemodialysis was believed to be caused by the removal of oxycodone by hemodialysis, and the increased oxycodone concentration after hemodialysis stopped was apparently due to the redistribution of oxycodone. On the other hand, breakthrough pain during hemodialysis may be caused by hemodialysis itself, rather than by the reduced plasma concentration of oxycodone, given that no relationship between the plasma concentration of oxycodone and pain could be identified.
Our results suggest that other strategies are needed to control pain during hemodialysis in patients using oxycodone. When we build a strategy of pain management during hemodialysis, we should consider both the decrease of the plasma concentration of oxycodone by hemodialysis, and the increase of pain by hemodialysis itself, as putative causes of pain requiring mitigation. When we consider the plasma concentration of oxycodone, increasing the basal dose of oxycodone during hemodialysis may be effective, because the plasma concentration of oxycodone decreased during hemodialysis. Increasing the rescue dose may also be effective. Insofar as the route of rescue medicine is concerned, intravenous injection may be more effective than subcutaneous or oral administration, because it increases the plasma concentration of oxycodone more quickly. Finally, changing the opioid from oxycodone to fentanyl prior to hemodialysis could be the most efficacious strategy, since fentanyl is not removed by hemodialysis [17]. When we consider the management of increasing pain caused by hemodialysis itself, the use of acetaminophen or adjuvant analgesics with opioids may be effective, because various mechanisms of pain induction, like neuropathic pain, might be the source of the pain.
As limitation of this study, it is difficult to make recommendations based on pharmacodynamics / kinetics of Oxycodone relying solely on its plasma concentration only in this case, but we think that our results could be useful for the making clinical decision. Additionally we did not measure oxycodone metabolites such as oxymorphone and noroxycodone. The activity of oxymorphone is negligible clinically in subjects with normal renal function but it could influence the analgesic effect in End-stage Renal Disease (ESRD) population. On the other hand, noroxycodone has no analgesic effect. Concerning data points, we should have set more measuring points of plasma concentration of oxycodone and pain score for further information.
In summary, clinicians should consider increased pain during hemodialysis, and the possibility of opioid-related side effects that may appear after hemodialysis when oxycodone levels rebound in patients. Further investigation is required to determine the best way to control pain during hemodialysis in such patients requiring opioid use.
Acknowledgements
This work was supported in part by the National Cancer Center Research and Development Fund (23-A-29).
References
- Everdingen MHJVB, De Rijke JM, Kessels AG, Schouten HC, van Kleef M, et al. (2007) Prevalence of pain in patients with cancer: a systematic review of the past 40years. Ann Oncol 18: 1437-1449.
- World Health Organization (1996) Cancer Pain Relief (2nd edn.), World Health Organization, Genova.
- Hanks GW, de Conno F, Cherny N, Hanna M, Kalso E, et al. (2001) Morphine and alternative opioids in cancer pain: the EAPC recommendations. Br J Cancer 84: 587-593.
- Davies G, Kingswood C, Street M (1996) Pharmacokinetics of opioids in renal dysfunction. ClinPharmacokinet 31:410-422.
- Izumi N, Okuyama S, Abe K, Ishikawa D, Suzuki S (2012) Prolonged disturbance of consciousness and respiratory depression induced by controlled-release morphine, requiring long-term naloxone administration in a hemodialysis patient with cancer--a case report. Gan To Kagaku Ryoho 39:1295-1259.
- Sear JW, Hand CW, Moore RA, McQuay HJ (1989) Studies on morphine disposition: influence of renal failure on the kinetics of morphine and its metabolites.Br J Anaesth62:28-32.
- Lee MA, Leng ME, Cooper RM (2005) Measurements of plasma oxycodone, noroxycodone and oxymorphone levels in a patient with bilateral nephrectomy who is undergoing haemodialysis. Palliat Med 19: 259-260.
- Tran BW, Kohan LR, Vorenkamp KE (2015) Postoperative oxycodone toxicity in a patient with chronic pain and end-stage renal disease.Anesthesia and Analgesia Case Reports 4: 44-46.
- Heiskanen T, Olkkola KT, Kalso E (1998) Effects of blocking CYP2D6 on the pharmacokinetics and pharmacodynamics of oxycodone. ClinPharmacolTher 64: 603-611.
- Mervyn Dean, MB, ChB, CCFP (2004) Opioids in Renal Failure and Dialysis Patients. Journal of Pain and Symptom Management28: 497-604.
- Pöyhiä R, Seppälä T, Olkkola KT, Kalso E (1992) The pharmacokinetics and metabolism of oxycodone after intramuscular and oral administration to healthy subjects. Br J ClinPharmac 33:617-621.
- Kirvela M, Lindgren L, Seppala T, Olkkola KT (1996) The pharmacokinetics of oxycodone in uremic patients undergoing renal transplantation. J Clin Anaesthesia 8: 13-18.
- Kaiko RF, Benziger DP, Fitzmartin RD, Burke BE, Reder RF, et al. (1996) Pharmacokinetic-pharmacodynamic relationships of controlled-release oxycodone. ClinPharmacolTher 59:52-61.
- Davison SN, Koncicki H, Brennan F (2014) Pain in chronic kidney disease: a scoping review.Semin Dial27:188-204.
- Douglas CA (2014) Palliative care for patients with advance chronic kidney disease. J R Coll Physicians Edinb44: 224-231.
- Mercadante S, Ferrantelli A, Tortorici C, Lo Cascio A, Lo Cicero M, et al. (2005) Incidence of chronic pain in patients with end-stage renal disease on dialysis. J Pain Symptom Manage30: 302-304.
- Bastani B, Jamal JA (1997) Removal of morphine but not fentanyl during haemodialysis. Nephrol Dial Trasplant12: 2802-2804.
Relevant Topics
- Caregiver Support Programs
- End of Life Care
- End-of-Life Communication
- Ethics in Palliative
- Euthanasia
- Family Caregiver
- Geriatric Care
- Holistic Care
- Home Care
- Hospice Care
- Hospice Palliative Care
- Old Age Care
- Palliative Care
- Palliative Care and Euthanasia
- Palliative Care Drugs
- Palliative Care in Oncology
- Palliative Care Medications
- Palliative Care Nursing
- Palliative Medicare
- Palliative Neurology
- Palliative Oncology
- Palliative Psychology
- Palliative Sedation
- Palliative Surgery
- Palliative Treatment
- Pediatric Palliative Care
- Volunteer Palliative Care
Recommended Journals
- Journal of Cardiac and Pulmonary Rehabilitation
- Journal of Community & Public Health Nursing
- Journal of Community & Public Health Nursing
- Journal of Health Care and Prevention
- Journal of Health Care and Prevention
- Journal of Paediatric Medicine & Surgery
- Journal of Paediatric Medicine & Surgery
- Journal of Pain & Relief
- Palliative Care & Medicine
- Journal of Pain & Relief
- Journal of Pediatric Neurological Disorders
- Neonatal and Pediatric Medicine
- Neonatal and Pediatric Medicine
- Neuroscience and Psychiatry: Open Access
- OMICS Journal of Radiology
- The Psychiatrist: Clinical and Therapeutic Journal
Article Tools
Article Usage
- Total views: 11632
- [From(publication date):
February-2016 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 10756
- PDF downloads : 876