Plasma Catecholamines: Blood Molecules Implicated in Alzheimers disease?
Received: 04-May-2021 / Accepted Date: 18-May-2021 / Published Date: 25-May-2021 DOI: 10.4172/2161-0460.1000522
Abstract
Current research highlighted a degeneration of the dopaminergic and noradrenergic systems in the brain, i.e. the ventral tegmental area (VTA) and the locus coeruleus (LC) at an early stage of Alzheimer’s disease (AD), and alterations of catecholamines concentrations in different body fluids (CSF, plasma and urine) of AD patients and animal models. These findings imply a potential utility of catecholamines in the molecular and mechanistic AD comprehension. Following our previous work on plasma noradrenaline in the context of AD, this retrospective study includes a cohort of 105 patients (43 AD, 29 with other dementia and 32 without dementia) from the cognitive neurology center of Lariboisière (Paris) who consulted for memory complaints. We show for the first-time different relations between plasma catecholamines and AD biomarkers at cognitive (MMSE score) and molecular (CSF biomarkers concentrations) levels. Our ROC analyses illustrate the good potential of plasma catecholamines to discriminate AD from non-AD patients with a relatively low or high MMSE score. Taken together, our results support the idea that plasma catecholamines could be blood molecules implicated in AD physiopathology, opening new frontiers in the development of a blood-based AD diagnosis.
Keywords: Alzheimer's disease; Catecholamines; MMSE score; Cerebrospinal fluid biomarkers
Introduction
Challenges in the diagnosis: The need to identify new AD biomarkers
Alzheimer’s disease (AD) is the most common cause of dementia [1]. Despite considering progress of AD research, the vast majority of clinical trials for therapies have failed to affect disease progression [2,3]. AD diagnosis is complex, the only gold standard being the direct observation of amyloid plaques and neurofibrillary tangles in postmortem brain tissue, which are specific features of AD. Due to the difficult accessibility of these observations, in vivo diagnosis requires a cluster of information describing the pathophysiological characters of the disease at a cognitive, morphological, and molecular levels. National Institute on Aging and Alzheimer’s Association (NIA-AA) guidelines [4] recommend validated AD diagnostic criteria, which include imaging techniques and cerebrospinal fluid (CSF) biomarkers. These guidelines aim to capture neurofibrillary tangles formation and cortical amyloid plaques deposition, together with their physiological consequences. However, these recommended techniques have the disadvantage of being costly, as for brain imaging, or invasive and risky, as for lumbar puncture. In this context, researchers are seeking alternative methods of investigation such as i) quantification of known biomarkers of AD in more available body fluids (blood [5], saliva [6]) or sampling (eye observation [7], skin [8], EEG [9]), ii) identification of yet unknown and noninvasively accessible biomarkers [10] of AD early stage [11], which could take place decades before the apparition of the cognitive symptoms [12]. However, the utility in clinical practice of these diagnostic alternatives were not yet convincingly demonstrated as their results are often not reproductible due to technical and/or physiological reasons [13].
Identification of early pathophysiological mechanisms in AD
Until recently, the most credited idea was that the onset and the evolution of AD probably involve two interplaying phenomena [14], i.e. progressive cortical accumulation of amyloid plaques through amyloid biochemical cascade [15] associated with cholinergic synaptic dysfunction [16]. These hypotheses have been called into question since therapeutic molecules targeting amyloid metabolism or cholinergic pathway could not slow down disease evolution [2,3]. This suggests that those signaling events may be too advanced in AD pathophysiology. The observation of early alterations in subcortical brain nuclei, i.e. the locus coeruleus (LC) and the ventral tegmental area (VTA), at a prodromal stage of the disease [17-19] opened new perspectives in the pathophysiological description of AD. LC and VTA are among the main brain sources of noradrenergic and dopaminergic neurons, respectively [20,21]. With the improvement of imaging techniques focusing on LC [22], a decreased contrast and volume of the LC in MCI and AD patients [23-27] has been described, which is consistent with LC neuronal loss and deregulated NA levels observed in post-mortem brain tissue of AD patients [27-29]. Moreover, LC alterations seem to correlate with Braak stages [30] which describe the presence of neurofibrillary tangles in different brain regions during AD development [31]. Concerning the dopaminergic system, in MCI and advanced AD patients, VTA size and its connectivity with the hippocampus were associated with hippocampal size and memory competence [32,33]. In murine model of AD, VTA [34-36] and LC [37] damage and altered NA levels were observed [37]. Moreover, VTA neuronal death correlates with reduced D outflow in the hippocampus, synaptic plasticity in the CA1, memory performance and food-reward processing. In line with these observations, it has been observed that the VTA-hippocampus-NAc circuit, essential for spatial, novelty and persistent memory formation, is early impaired in AD mice. Similarly, it was also demonstrated that provoked lesions of the LC in mice impact working memory [38,39]. Further, pharmacological LC activation was able to rescue the main cognitive and behavioral deficits [37,40]. Finally, it has been shown that neurons from the LC can co-secrete dopamine (D) with noradrenaline (NA) in the hippocampus [41,42], indicating that NA and D might cooperate in learning and memory processes [43]. Taken together, those results support the idea that brain catecholamines circuits are impaired at an early phase of AD, with a loss of LC and VTA neurons correlating with memory, but also behavioral deficits (depression, anxiety, apathy, sleep-wake cycle disruption, etc.) usually observed in AD patients long before amyloid plaques and neurofibrillary tangles formation [44].
A renewed interest for plasma catecholamines in AD
Early studies on alterations in LC structure and in catecholamines levels within brain, CSF and plasma from AD patients, were followed by a long disinterest for this topic. A recent renewed attention for the role of catecholamines in the context of AD was motivated by the recent observations in MCI patients of noradrenergic and dopaminergic systems alterations before amyloid plaques deposition. NA, D and adrenaline (A) are the three main catecholamines. These molecules are linked through several enzymatic steps. In the brain, they act as neurotransmitters and locally as a hormone by diffusion. At the peripheral level, they are synthesized by adrenal medulla and sympathetic noradrenergic neurons, and act as hormones. The need to identify AD biomarkers in alternative more accessible body fluids motivated a large number of studies on catecholamines fluctuations in urine [45,46] and plasma [47-49] of AD patients and mouse model of AD. Despite conflicting, this field of research showed interesting results. Our retrospective study examined the relationship between plasma catecholamines concentrations and concomitant diagnostic criteria such as Mini Mental Sate Examination (MMSE) score and CSF biomarker profile (Aβ1-42, Tau and p-Tau). As described in our previous report for plasma NA [50], we wanted to determine i) whether other plasma catecholamines concentrations could be correlated with clinical parameters which reflect disease stages at cognitive (MMSE score) and molecular (Aβ1-42, Tau and p-Tau CSF biomarkers) levels, and ii) whether the combination of these putative biomarkers could be exploited for the early diagnosis of AD pathology.
Materials and Methods
Study population
All patients presented to the Cognitive Neurology Center of Lariboisière (Paris) for their first consultation between 2017 and 2019. Patients involved in this study were between 53 and 72-years old at the time of blood sampling. MMSE score and lumbar puncture were performed the day of blood sampling or less than one month later. More details on MMSE cutoffs are given in our previous article [50]. Sample size was calculated with the same method of our previous article [50]. In this retrospective study, 104 patients were included: 43 AD patients (diagnoses were performed according to NIA-AA guidelines [4]), 29 patients with other dementia (OD; frontotemporal dementia, vascular dementia or dementia with Lewy bodies), and 32 neurological control (NC) patients. NC patients were defined as those with memory complaints, mental depression, or anxiety but for whom no dementia was diagnosed. Two NC patients were removed from the study because of their extremely low MMSE score (3 and 8). As we previously described [50], cutoff values for Aβ1-42 (<550 pg/mL), total- Tau (>400 pg/mL), and p-Tau (>50 pg/mL) were used to identify AD dementia. Table 1 synthetizes all information concerning demographic, cognitive, physiological, and co-medications data.
| Total number of patients | NC | OD | AD | p-value | |
|---|---|---|---|---|---|
| 32 | 29 | 43 | - | ||
| Sex | % of female patients | 40.63 | 41.38 | 58.14 | 0.2257 |
| Age | Age median (IQR) in year | 62.5 (59.25-69) | 67 (61.50-69) | 68 (63-70) | 0.0619 |
| MMSE | MMSE score median (IQR) | 27 (26-28) | 24 (19-26) | 20 (15-26) | <0.0001 |
| CSF Aβ1-40 concentration$ |
CSF Aβ concentration median (IQR) |
12449 (8961-14868) | 10865 (8924-14561) | 11878 (8707-15338) | 0.8656 |
| CSF Aβ1-42 concentration | CSF Aβ concentration median (IQR) | 1133 (938-1333) | 1112 (976-1390) | 575 (463-667) | <0.0001 |
| CSF Tau concentration | CSF Tau concentration median (IQR) | 195.5 (158.5-227) | 227 (190.5-301) | 492 (373-672) | <0.0001 |
| CSF p-Tau concentration | CSF p-Tau concentration median (IQR) | 34 (19.26-47.75) | 39.5 (26.43-50.40) | 77.7 (59-105) | <0.0001 |
| Plasma NA concentration | Plasma NA concentration median (IQR) | 2064 (1556-3117) | 2295 (1855-3034) | 2499 (1826-3086) | 0.3809 |
| Plasma A concentration | Plasma A concentration median (IQR) | 276 (146-442) | 222 (172-428) | 318 (216-478) | 0.2493 |
| Plasma D concentration | Plasma D concentration median (IQR) |
311 (110.5-494.8) | 239 (206.5-370) | 201 (100-363) | 0.1216 |
| % of patients with co- medication# | Anti-Alzheimer, neuroleptics, antidepressants | 15.625 | 20.69 | 16.667 | 0.8595 |
| Lipid-lowering agents, oral antidiabetics |
18.75 | 27.586 | 19.048 | 0.6273 | |
| Anti-hypertensive agents | 21.875 | 31.034 | 35.714 | 0.4338 | |
#co-medication information are missing for 1 AD patient; $Aß1-40concentration is missing for 1 AD patient
Quantification of NA, A and D in plasma
Blood sampling was performed on 12h-fasted patients in supine position, as described in our previous article [50]. Samples purification and analysis were performed with Chromsystems kit (order #5000) for plasma catecholamines high-performance liquid chromatography (HPLC) analysis. Briefly, after blood-stabilization with glutathione and direct centrifugation (less than 60 min after sampling) to isolate the plasma, samples were frozen and stored at-80°C. 1 mL of thawed plasma was used for catecholamines dosage by HPLC coupled with electrochemical detection. Quantification of catecholamines was made without knowing the patient group of samples. For plasma D concentration ([D]plasma), values under the range of detection were considered as equal 100 pmol/L.
Quantification of Aβ1-42, total Tau, and p-Tau in the CSF
As we previously described [50], CSF samples were obtained by lumbar punctures on fasted patient. Then, they underwent 10 min centrifugation (1 g, 4°C) within 4h after collection. 500 μL-polypropene tubes were used for aliquoting and -80°C storage. Sandwich ELISA INNOTEST® kit (Fujirebio Europe NV, formerly Innogenetics NV) was used for AD biomarkers quantification in the CSF (Aβ1-42, total Tau, and p-Tau).
Data analysis and statistical tests
4.4 Data analysis and statistical tests Similarly to what performed in our previous study [50], we first tested normality (D'Agostino-Pearson normality test) to determine results illustration and statistical test choice. For normally distribution, we presented mean with standard deviation (SD) in figures and used Student’s t-test (two-tailed) to compare two groups. In the absence of normal distribution, we presented median with interquartile range (IQR: 25-75th percentiles) (95% confidence interval in figures) and used Mann-Whitney test (two-tailed) to compare two groups or Kruskal-Wallis test to compare distribution of their data. For multiple group comparisons we performed one-way ANOVA. Linear correlations were tested using Pearson’s correlation test or Spearman’s correlation test for normally or not-normally distributed data, respectively. All analyses and multiple logistic regressions were performed with GraphPad Prism 9.0.0 software. We used Medcalc software to apply the empirical nonparametric method from Delong et al. [51] to compare AUCs from ROC curves. No outliers were identified in our cohort (p-value>0.01) by performing Rosner’s Extreme Studentized Deviate test for multiple outliers (using log-normal distribution and two-side test). AUC were compared using the empirical nonparametric method by Delong et al. [51] with Medcalc software. Cutoff for p-value was 0.05 to identify statistical significance.
Results
Cohort description
We found no difference concerning age, sex or pharmacological treatments between NC, OD and AD groups that differed by MMSE score and CSF biomarkers (one-way ANOVA, p-values are mentioned in Table 1). Patients’ clinical diagnosis was established by the neurologist accordingly to NIA-AA guidelines [4].
Relations between catecholamines and AD CSF biomarkers
Consistent with our previous results in a different cohort [50], we found that AD patients with a MMSE score above 23 (the cutoff value for dementia in the old population [52]) (n=19) had a higher[NA]plasma than non-AD patients with similar MMSE score (n=49, 31 NC and 18 OD patients) (Mann Whitney test, p-value=0.0485) (Figure 1A). On the other hand, AD patient with a MMSE score under 23 (n=24) had a comparable[NA]plasma than non-AD patient (n=12, 11 OD and 1 NC patients) (Mann Whitney test, p-value=0.4969) (Figure 1B). Knowing that NA is the precursor of the other catecholamine adrenaline, we wanted to know if there was a correlation between [NA]plasma and[A]plasma in order to determine if A could potentially be associated with specific AD features, just as NA. Interestingly, we found a significant positive linear correlation between[NA]plasma and |A]plasma of AD patients (n=43) (Spearman’s correlation, r=0.5130 (95% CI: 0.2428 to 0.7093), p-value=0.0004, equation: Y=0.1119*X +72.33) (Figure 1C), which was inexistent in OD (n=29) (Spearman’s correlation, r=0.3153 (95% IC:-0.06925 to 0.6182, equation: Y=0.04465*X +217.6), p-value=0.0957) and NC (n=32) (Spearman’s correlation, r=0.1881 (95% IC:-0.1823 to 0.5117), p-value=0.3026, equation: Y=0.01520*X +297.1) patients (Figure 1C). As we previously described for NA50, we examined whether there was a correlation between [A]plasma distance from the NC patient median-defined as the absolute value of [A]plasma-<[A]plasma/NC>, with <[A]plasma/NC> the median value of [A]plasma from NC patients (276 pmol/L)-and the CSF biomarkers profile. Indeed, we observed that |[A]plasma-<[A]plasma/NC>| for extreme [A]plasma values-meaning below 1st tercile value (33% percentile: 162.5 pmol/L) and above 3rd tercile value (67% percentile: 421 pmol/L) of [A]plasma from NC patients was significantly higher in AD patients (n=19) than in control patients (n=21) (Mann Whitney test, p-value=0.0096) (Figure 1D), which was not the case when comparing NC and OD patients (n=12) (Mann Whitney test, p-value=0.6118) (data not shown). Parallelly, concerning patients with an [A]plasma closer to the median control (meaning between 1st tercile value and 3rd tercile value of [A]plasma from NC patients), we found no significant difference between NC (n=11) and AD (n=24) (Mann Whitney test, p-value=0.9788) (Figure 1D) or NC and OD (n=17) (Mann Whitney test, p-value=0.6516) patients for |[A]plasma-<[A]plasma/NC>| (data not shown). Taken together, this suggests that, unlike for OD patients, AD cohort presents more extreme high or low [A]plasma values than NC patients. We found a significant negative linear correlation between|[A]plasma-<[A]plasma/NC>| and [Aβ1-42]CSF in AD patients (n=43) (Spearman’s correlation, r=-0.3895 (95% IC:-0.6232 to-0.09190), p-value=0.0098, equation: Y=-0.3186*X +655.4) (Figure 2A), which was not the case in OD (n=29) (Spearman’s correlation, r=-0.08594 (95% IC:-0.4478 to 0.3001), p-value=0.6576, equation: Y=0.05645*X +1151) and NC patients (n=32) (Spearman’s correlation, r=0.01148 (95% IC:-0.3481 to 0.3681), p-value=0.9503, equation: Y=0.03872*X + 1170) (Figure 2A). To understand the relation between raw [A]plasma values and CSF biomarkers concentrations, we looked at negative andpositive relative distance from <[A]plasma/NC>, i.e. when [A]plasma is respectively lower or higher than <[A]plasma/NC>. We found opposite correlations between [A]plasma and [Aβ1-42] CSF when comparing AD cohort with negative and positive distance from <[A]plasma/NC>. Indeed, in AD cohort with negative relative distance from <[A]plasma/NC>, we observed a non-significant positive linear correlation for negative distance (n=19) (Spearman’s correlation, r=0.2544 (95% IC:-0.2396 to 0.6438), p-value=0.2933, equation: Y=0.7615*X+464.4) and a significant negative correlation in AD for positive distance (Spearman’s correlation, r=-0.4478 (95% IC:-0.7270 to-0.04161), p-value=0.0282, equation: Y=-0.3419*X +769.6) (Figure 2B). We found no significant linear correlation between |[A]plasma-<[A]plasma/NC>| and [p-Tau]CSF and [Tau]CSF (data not shown). However, we found a significant positive linear correlation between [A]plasma and the ratio (p-Tau/Tau)CSF in AD patients (n=32) (Spearman’s correlation, r=0.3293 (95% IC: 0.02300 to 0.5791), p-value=0.0311, equation: Y=(4.863*10-5)*X +0.1386)) (Figure 2C), a significant negative linear correlation in NC patients (n=32) (Spearman’s correlation, r=-0.3984 (95% IC:-0.6621 to-0.04702), p-value=0.0239, equation: Y=(-2.439*10-5)*X +0.1781)), and no significant correlation in OD patients (n=29) (Spearman’s correlation, r=0.09681 (95% IC:-0.2901 to 0.4565), p-value=0.6174, equation: Y=(2.716*10-5)*X + 0.1556)) (Figure 2C). We found no correlation between negative and positive distance with (pTau/Tau)CSF in AD patients (data not shown). However, (pTau/Tau)CSF tended to be lower in 2nd tercile (n=15) of [A]plasma from AD patients ([A]plasma 33% percentile: 235.1 pmol/L and 67% percentile: 432 pmol/L) in comparison with 1st tercile (n=14), without reaching significance (Student’s t-test, p-value=0.1894), and was significantly lower in comparison with 3rd tercile (n=14) (Student’s t-test, p-value=0.0129), without difference between 1st and 3rd terciles (Student’s t-test, p-value=0.2470) (Figure 2D). Taken together, those results suggest that, like[NA]plasma , [A]plasma could be related to CSF biomarkers profile in AD patients. The recent studies emphasizing the implication of VTA and dopaminergic neurons in an early stage of AD evolution prompted us to determine whether [D]plasma could be related as well to CSF AD biomarkers. Interestingly, we found a significant positive linear correlation between [D]plasma and |[A]plasma-<[A]plasma/NC>| in AD patients (Spearman’s correlation, r=0.3057 (95% IC:-0.003251 to 0.5614), p-value=0.0462, equation: Y=0.3751*X + 189.6), which was not the case for NC (Spearman’s correlation, r=-0.1198 (95% IC:-0.4582 to 0.2490), p-value=0.5137, equation: Y=0.2295*X + 309.5) or OD (Spearman’s correlation, r=0.2691 (95% IC:-0.1193 to 0.5860), p-value=0.1581, equation: Y=0.1432*X+ 285.8) patients (Figure 3A). Moreover, we could identify that AD patients present a lower [D]plasma than non-AD (NC and OD) patients (Mann Whitney test, p-value=0.0435) (Figure 3B) with a significant different cumulative distribution (Kolmogorov-Smirnov test, p-value=0.0389) (Figure 3C). We found no significant correlation between [D]plasma and CSF biomarkers (data not shown). However, we found a significant difference of (Aβ1-42/Aβ1-40)CSF ratio between AD patients under 1st tercile value of AD [D]plasma (168 pmol/L) (n=13) and AD patients above this value, i.e. 2nd and 3rd tercile (n=29) (Student’s t-test, p-value=0.0201) (Figure 3D). In summary, we could identify in our cohort that [D]plasma seems altered in AD patients and that AD patients with the lowest values of [D]plasma differed from other AD patients with a significantly smaller (Aβ1-42/Aβ1-40)CSF ratio. Altogether, our results strongly suggest that plasma catecholamines-NA, A and D-are potential informative molecules that could mirror CSF biomarkers alterations illustrating brain AD physiopathology.
Figure 1: Plasma noradrenaline concentration in AD patients is related to MMSE score and plasma adrenaline concentration, which extreme high and low values are more distanced from median control, in AD patients. A-B: [NA]plasma in AD (orange) and non-AD (blue) patients with MMSE score above 23 (A) and below 23 (B). C: Correlation between [NA]plasma and [A]plasma in AD (orange) patients but not in NC (dark blue) and OD (light blue) patients. D: Distance |[A] plasma-[A]plasma/NC| from patients with extreme [A]plasma values (right) and [A]plasma close to median control (left) in AD (orange) and NC (blue) patients.*means p-value<0.05; ***means p-value<0.001.
Figure 2: Plasma adrenaline concentration distance from median control correlates with CSF biomarkers Aß1-42 concentration and p-Tau/Tau ratio in AD patients. A: Correlation between |[A]plasma-<[A]plasma/NC >|and [Aß1-42]CSF in AD patients (orange) but not in NC (dark blue) and OD (light blue) patients. B: Linear correlations between negative (in pink) and positive (in blue) values of|A]plasma-<[A]plasma/NC> with [Aß1–42]CSF in AD patients. C: Correlation between |[A] plasma-<[A]plasma/NC>|and (p-Tau/Tau)CSF in AD patients (orange) but not in NC (dark blue) and OD (light blue) patients. D: (p-Tau/Tau)CSF values in 1st, 2nd and 3rd [A]plasma terciles of AD patients.*means p-value<0.05 ; **means p-value<0.01.
Figure 3: Plasma dopamine concentration in AD patient, related to plasma adrenaline concentration distance from median control and to CSF Aß1-42/Aß1-40 ratio, is lower than in non-AD patient population. A: Correlation between |[A]plasma-<[A]plasma/NC >|and [D]plasma in AD patients (orange) but not in NC (dark blue) and OD (light blue) patients. B: [D]plasma in AD (orange) non-AD (blue) patients. C: Cumulative distribution of [D]plasma between AD (orange) and non-AD (blue) patients. D: (Aß1-42/Aß1-40)CSF ratio values in 1st, 2nd and 3rd [D]plasma terciles of AD patients.*means p-value<0.05; **means p-value<0.01.
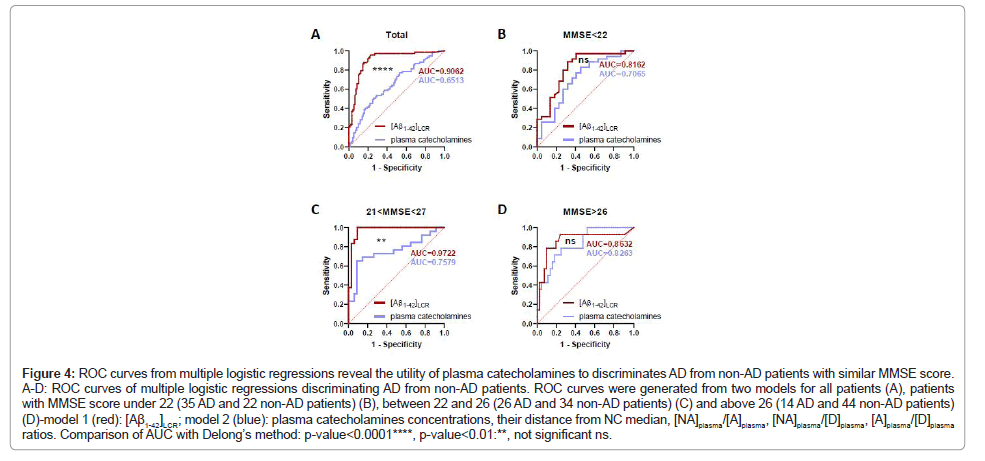
Utility of plasma catecholamines to discriminate AD from non-AD patients
We recently highlighted in our studies relations between plasma catecholamines concentrations and AD biomarkers at cognitive (MMSE score) and molecular (CSF biomarkers) levels. These results prompt us to test whether plasma catecholamines could help in AD diagnosis in the context of a first neurological consultation for memory complaints. We pooled the two cohorts of our previous [50] and present studies (75 AD, 51 OD and 49 NC patients) to perform multiple logistic regressions from two different models: a model based on one parameter [Aβ1-42]CSF and another taking into account parameters obtained from plasma catecholamines concentrations, i.e.[NA]plasma , [A]plasma, [D]plasma, |[NA]plasma-<[NA]plasma/NC>|, |[A]plasma-<[A]plasma/NC>|, |[D]plasma-<[D]plasma/NC>|, (NA/A)plasma ratio, (NA/D)plasma ratio, (A/D)plasma ratio, to discriminate AD from non-AD patients. Considering the whole cohort, we found that AUCs from the two different ROC curves were significantly different (Delong’s methodology, difference between areas=0.252, p-value<0.0001) (Figure 4A). However, knowing that[NA]plasma is related to MMSE score, we performed multiple logistic regression in three different groups based on 33% and 64% percentiles of MMSE score in the cohort (<22, between 22 and 26, >26). The different multiple logistic regressions models use the same parameters, but the model adapts to the cohort defined by the MMSE score. We found that AUCs from two different ROC curves were not different in groups with extreme MMSE scores, meaning under 22 (Delong’s methodology, difference between areas=0.110, p-value=0.2999) and above 26 (Delong’s methodology, difference between areas=0.0342, p-value=0.6741), and were significantly different in the middle group (Delong’s methodology, difference between area=0.235, p-value=0.0022) (Figures 4B-D). This implies that plasma catecholamines parameters could help to discriminate AD patients with similar performances as [Aβ1-42]LCR in an advanced or early stage of AD evolution from a cognitive view point. In other words, plasma catecholamines parameters could potentially help to distinguish advanced AD patients from other demented patients with a relatively low MMSE score and identify mild AD patients among non-demented patients with a relatively high MMSE score. Taken together, our observations open the road for the use of plasma catecholamines in the diagnosis of AD.
Figure 4: ROC curves from multiple logistic regressions reveal the utility of plasma catecholamines to discriminates AD from non-AD patients with similar MMSE score. A-D: ROC curves of multiple logistic regressions discriminating AD from non-AD patients. ROC curves were generated from two models for all patients (A), patients with MMSE score under 22 (35 AD and 22 non-AD patients) (B), between 22 and 26 (26 AD and 34 non-AD patients) (C) and above 26 (14 AD and 44 non-AD patients) (D)-model 1 (red): [Aß1-42]LCR; model 2 (blue): plasma catecholamines concentrations, their distance from NC median, [NA]plasma/[A]plasma, [NA]plasma/[D]plasma, [A]plasma/[D]plasma ratios. Comparison of AUC with Delong’s method: p-value<0.0001****, p-value<0.01:**, not significant ns.
Discussion
In this retrospective study, we showed for the first-time relations between LCR AD biomarkers and [A]plasma and [D]plasma. We also found a higher concentration of[NA]plasma in AD patients with a high MMSE score in comparison with other non-AD patients with a similar MMSE score, which is consistent with our previous independent study [50-53]. Moreover, we observed that [A]plasma correlated with[NA]plasma and was related to [D]plasma, significantly lower in AD cohort in comparison with other patients. We could then highlight the potential discrimination power of plasma catecholamines by comparing ROC analysis based on Aβ1-42 or plasma catecholamines signature to differenciate AD from non-AD patients. We observed that AUC from the two models were similar for patients with extreme MMSE scores (<22 or >26), suggesting that the catecholamines signature is informative in cognitively advanced or early stage of AD cognitive evolution.
AD early mechanisms linked to catecholaminergic system?
As mentioned in the introduction, many evidences show that subcortical catecholaminergic nuclei, i.e. LC and VTA, are among the first affected by tau protein abnormalities [53]. This, together with the fact that noradrenergic and dopaminergic neurons form a vast and complex network throughout the brain, contribute to the hypothesis of an early “prion-like” spreading of Tau pathology, from subcortical nuclei to cortex and other brain regions [54]. A reasons explaining why catecholaminergic neurons would be first impacted during the disease is their strong vulnerability in comparison with other CNS neurons [55]. Anatomically, their projections to other brain regions are long and poorly myelinated, which make them fragile. They contain neuromelanin, a heavy metal chelator resulting of catecholamine oxidation, whose accumulation becomes toxic. Moreover, they have a stronger energetic demand which could expose them to cellular and oxidative stress. Lastly, their large contact surface with blood vessels and ventricles exposes them to a high amount of toxins and pathogens. Such phenomenon could then indirectly participate in amyloid plaques formation and inflammation in AD. Moreover, NA system deregulation in the CNS has a role in amyloid pathology [56], neuronal metabolism [57], and neuroinflammation [58]. It is probable that NA system and those mechanisms interact in a synergic vicious-circle manner during AD. These features make catecholaminergic neurons the first potential actors in AD onset. Finally, it is important to mention that imaging of human brain showed an early disconnection of VTA, but not LC, with other brain structures [32]. This evidence suggests that D circuits might also participate in the onset of early non-cognitive behavioral and psychological symptoms of AD, such as irritability, or sleep disorders.
Peripheral catecholamines in AD
Adolfsson et al. first identified an altered concentration of D and NA in human postmortem brain tissue of AD patient in comparison with age-matched control, which correlated with dementia score in some brain regions [59]. This is consistent with a recent article reporting on similar results, and also including a correlation between MMSE score and cortical NA level (BA22) [60]. Other studies also show reduced cerebral NA [61,62] and D concentration in AD patients [63,64], with sometimes no change in D [65], which could depend of the observed brain regions. Parallelly, despite conflicting results showing both increased [66,67] and decreased [68] levels, NA CSF concentrations in AD patients seem to be altered. Moreover, Yohimbine-induced increase of CSF NA levels was greater in AD patients in comparison with their age-matched controls [69]. Interestingly, it was also demonstrated that cognitive performances and CSF NA concentration do correlate [70]. Finally, higher CSF concentrations of D [66] and A [71] have been measured in AD patients, and it seemed that CSF A increased with disease severity. Parallely, in other peripheral body fluids, it was previously shown that urine catecholamines concentrations in AD patients [45] and rat model of AD [46] are decreased. On the other hand, previous studies showed altered [47,48] or unchanged [49] NA and A plasma concentrations in AD patients. Conflicting results could be explained by different disease stages, as well as age and gender ratio presented in those articles. The correlations that we found between plasma catecholamines in AD patients were not observed in non-AD patients from our cohort. Correlations between [A]plasma and[NA]plasma were previously described in horses during and after intense physical exercises [72]. The coefficient of correlation between[NA]plasma and [A]plasma decreased as the intensity of exercise decreased, suggesting a different process in release, distribution and clearance of these molecules and/or a different proportion in sympathetic nervous system and surrenal medulla involvement. Interestingly, LC and VTA are involved in the regulation of sympathetic system activity [20]. Hence, it is tempting to speculate that the different correlations between plasma catecholamines concentrations measured in AD patients, but not in non-AD subjects, could be due to an alteration of the sympathetic system caused by LC and VTA dysregulation. Moreover, autonomic dysfunction seems to be implicated in AD [73].
The potential role of plasma catecholamines in the understanding of AD pathology
According to our observations, plasma catecholamines should not be considered as classical biomarkers with a defined cutoff helping in AD identification, but rather as an additional information to the clinical picture (MMSE score, memory complaints, education, age, etc.) of the patient. It is also important to precise that LC neurodegeneration is not specific to AD, as it also occurs during aging and other dementia. However, AD-related neuronal loss in the LC follows a rostro-caudal gradient, unlike for other neurodegenerative diseases where neuronal loss is scattered in the LC [23,27,30,37,55]. Knowing that LC neurons are regionalized depending on the targeted brain region [20], we could imagine that some loss of function and compensation mechanisms (such as oversecretion of catecholamines or network reorganization) in LC and VTA brain areas are specific to AD [19]. These potential compensation mechanisms due to a defined neuronal loss pattern, could explain, for example, the lower concentration of NA observed in cortical postmortem tissue of AD patients in comparison with age-matched controls without dementia or with other dementia [60,62]. Moreover, VTA deregulation seems to occur before LC degeneration [35,74], suggesting that D might participate in a specific physiological response, relative to other dementia, during AD evolution.
Study limitations
The first limitation that we identified in our work is the relatively small size of our cohort. Future studies in larger cohorts will allow to validate the observed relations between plasma catecholamines and CSF biomarkers, and the good potential of those molecules in discriminating AD from non-AD patients, as shown by multiple logistic regression analysis. Secondly, our study lacks longitudinal observations to investigate the dynamics of plasma catecholamines concentrations overtime during disease evolution. This would help understanding their potential utility as predictors of MCI to AD conversion. Finally, the relation between brain catecholamines dysregulation and plasma catecholamines alteration during AD is currently not clear. Hitherto, it is still difficult to assess whether plasma catecholamines could be a good mirror of catecholaminergic dysregulation in the brain. It is important to notice that Raskind et al. found a linear correlation between [NA]CSF and[NA]plasma in their study (AD and controls subjects), which is consistent with the correlation that we found between plasma and CSF concentration for NA and D in 10 AD patients from a cohort of our previous article [50]. This result supports the idea that catecholamines alterations in the brain and CSF could be also observed in the plasma. However, further studies are necessary to assess the relation between brain and plasma catecholamines in the context of AD. More details on our hypothesis linking[NA]plasma alterations and brain dysregulation in AD, that could be extend to A and D, are discussed in our previous article [50]. The dysregulation of plasma catecholamines concentrations during AD is a complex phenomenon as it might be the consequence of multiple interconnected physiological events (stage of the disease, compensation phenomenon, cognitive reserve effect, sympathetic system activity, etc.).
Conclusion
Based on our results and on previous literature, catecholamines seem to be good candidate to ameliorate early diagnosis. However, further investigations are needed to define a specific use of plasma catecholamines within the diagnostic pathway of AD. Our results open the possibility to explore new molecular mechanisms in order to better understand the physiopathology of this complex disease, and, by extension, to improve AD diagnosis, potentially helping the development of new drugs aimed at slowing down and/or stopping disease evolution.
Acknowledgements
We thank the patients who were involved in this retrospective study and their caregivers. We also thank Claire Paquet, MD, PhD (Memory Resources and Research Center, Cognitive Neurology Center, INSERM UMR-S 942, University Hospital of Paris Diderot Saint Louis-Lariboisière-Fernand Widal, APHP, France) for selecting and providing the biological and clinical samples as well as Jacques Callebert, PharmD, PhD (Department of Biochemistry, University Hospital of Paris Diderot Saint Louis-Lariboisière-Fernand Widal, APHP, France) for performing the blood sample analyses.
Competing Interests Statement
Alzohis is a company that has activities related to the submitted work. This study and this publication were produced and written in a responsible and ethical manner.
Ethics Approval and Consent to Participate
Patients gave their informed and written consent to have their samples stored in an officially registered and ethically approved biological collection that has been approved by the Ethics Committee of Paris University Hospitals (CEERB [Comité d’Ethique En Recherche Biomédicale], Bichat University Hospital, Paris, France).
Data Availability
The data that support the findings of this study are available from the corresponding author, Romain Verpillot, upon reasonable request.
References
- O’Connor, D (2019) World Alzheimer report 2019: Attitudes to dementia. 160.
- Barage SH, Sonawane KD (2015) Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides 52: 1-18.
- Hampel H, Mesulam MM, Cuello AC, Farlow MR, Giacobini E, et al. (2018) The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain J Neurol 141: 1917-1933.
- Jack Jr CR, Albert MS, Knopman DS, McKhann GM, Sperling RA, et al. (2011) Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 7: 257-262.
- Janelidze S, Mattsson N, Palmqvist S, Smith R, Beach TG, et al. (2020) Plasma P-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med 26: 1-8.
- Sabbagh MN, Shi J, Lee M, Arnold L, Al-Hasan Y, et al. (2018) Salivary beta amyloid protein levels are detectable and differentiate patients with Alzheimer’s disease dementia from normal controls: Preliminary findings. BMC Neurol 18: 155.
- Dehghani C, Frost S, Jayasena R, Masters CL, Kanagasingam Y (2018) Ocular biomarkers of Alzheimer’s disease: The role of anterior eye and potential future directions. Invest Ophthalmol Vis Sci 59: 3554-3563.
- RodrÃguez-Leyva I, Chi-Ahumada E, Calderón–Garcidue-as AL, Medina-Mier V, Santoyo Martha E, et al. (2015) Presence of phosphorylated tau protein in the skin of Alzheimer´s disease patients. J Mol Biomark Diagn 6: 5-10.
- Musaeus CS, Engedal K, Høgh P, Jelic V, Mørup M, et al. (2018) EEG theta power is an early marker of cognitive decline in dementia due to Alzheimer’s disease. J Alzheimers Dis 64: 1359-1371.
- Park SA, Han SM, Kim CE (2020) New fluid biomarkers tracking non-amyloid-ß and non-tau pathology in Alzheimer’s disease. Exp Mol Med 52: 556-568.
- Hudd F, Shiel A, Harris M, Bowdler P, McCann B, et al. (2019) Novel blood biomarkers that correlate with cognitive performance and hippocampal volumetry: Potential for early diagnosis of Alzheimer’s disease. J Alzheimers Dis 67: 931-947.
- Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, et al. (2016) Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement J Alzheimers Assoc 12: 292-323.
- Wood H (2016) Alzheimer disease: Biomarkers of AD risk-the end of the road for plasma amyloid-ß? Nat Rev Neurol 12: 613.
- Pákáski M, Kálmán J (2008) Interactions between the amyloid and cholinergic mechanisms in Alzheimer’s disease. Neurochem Int 53: 103-111.
- Hardy JA, Higgins GA (1992) Alzheimer’s disease: The amyloid cascade hypothesis. Science 256: 184-185.
- Contestabile A (2011) The history of the cholinergic hypothesis. Behav Brain Res 221: 334-340.
- Bozzali M, D’Amelio M, Serra L (2019) Ventral tegmental area disruption in Alzheimer’s disease. Aging 11: 1325-1326.
- D’Amelio M, Serra L, Bozzali M (2018) Ventral tegmental area in prodromal Alzheimer’s disease: Bridging the gap between mice and humans. J Alzheimers Dis 63: 181-183.
- Gannon M, Che P, Chen Y, Jiao K, Roberson ED, et al. (2015) Noradrenergic dysfunction in Alzheimer’s disease. Front Neurosci 9: 220.
- Szabadi E (2013) Functional neuroanatomy of the central noradrenergic system. J Psychopharmacol 27: 659-693.
- Morales M, Margolis EB (2017) Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci 18: 73-85.
- Betts MJ, Kirilina E, Otaduy MC, Ivanov D, Acosta-Cabronero J, et al. (2019) Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain 142: 2558-2571.
- Betts MJ, Cardenas-Blanco A, Kanowski M, Spottke A, Teipel SJ, et al. (2019) Locus coeruleus MRI contrast is reduced in Alzheimer’s disease dementia and correlates with CSF Aß levels. Alzheimers Dement Amst Neth 11: 281-285.
- Dordevic M, Müller-Fotti A, Müller P, Schmicker M, Kaufmann J, et al. (2017) Optimal cut-off value for locus coeruleus-to-pons intensity ratio as clinical biomarker for Alzheimer’s Disease: A pilot study. J Alzheimers Dis Rep 1: 159-167.
- Olivieri P, Lagarde J, Lehericy S, Valabrègue R, Michel A, Macé P, et al. (2019) Early alteration of the locus coeruleus in phenotypic variants of Alzheimer’s disease. Ann Clin Transl Neurol 6: 1345-1351.
- Takahashi J, Shibata T, Sasaki M, Kudo M, Yanezawa H, et al. (2015) Detection of changes in the locus coeruleus in patients with mild cognitive impairment and Alzheimer’s disease: High-resolution fast spin-echo T1-weighted imaging. Geriatr Gerontol Int 15: 334-340.
- Theofilas P, Ehrenberg AJ, Dunlop S, Alho AT, Nguy A, et al. (2017) Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: A stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimers Dement J Alzheimers Assoc. 13: 236-246.
- Kelly SC, He B, Perez SE, Ginsberg SD, Mufson EJ, et al. (2017) Locus coeruleus cellular and molecular pathology during the progression of Alzheimer’s disease. Acta Neuropathol Commun 5: 8.
- Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, et al. (2006) Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with Alzheimer’s disease and dementia with Lewy bodies. J Neurosci Off J Soc Neurosci 26: 467-478.
- Ehrenberg AJ, Nguy AK, Theofilas P, Dunlop S, Suemoto CK, et al. (2017) Quantifying the accretion of hyperphosphorylated tau in the locus coeruleus and dorsal raphe nucleus: The pathological building blocks of early Alzheimer’s disease. Neuropathol Appl Neurobiol 43: 393-408.
- Braak H, Braak E (1995) Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 16: 271-284.
- De Marco M, Venneri A. (2018) Volume and connectivity of the ventral tegmental area are linked to neurocognitive signatures of Alzheimer’s disease in humans. J Alzheimers Dis 63: 167-180.
- Bozzali M, Serra L, Cercignani M (2016) Quantitative MRI to understand Alzheimer's disease pathophysiology. Curr Opin Neurol 29: 437-444.
- Cordella A, Krashia P, Nobili A, Pignataro A, La Barbera L, et al. (2018) Dopamine loss alters the hippocampus-nucleus accumbens synaptic transmission in the Tg2576 mouse model of Alzheimer’s disease. Neurobiol Dis 116: 142-154.
- Nobili A, Latagliata EC, Viscomi MT, Cavallucci V, Cutuli D, et al. (2017) Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat Commun 8: 14727.
- Vorobyov V, Bakharev B, Medvinskaya N, Nesterova I, Samokhin A, et al. (2019) Loss of midbrain dopamine neurons and altered apomorphine EEG Effects in the 5xFAD mouse model of Alzheimer’s disease. J Alzheimers Dis 70: 241-256.
- Rorabaugh JM, Chalermpalanupap T, Botz-Zapp CA, Fu VM, Lembeck NA, et al. (2017) Chemogenetic locus coeruleus activation restores reversal learning in a rat model of Alzheimer’s disease. Brain 140: 3023-3038.
- Coradazzi M, Gulino R, Fieramosca F, Falzacappa LV, Riggi M, et al. (2016) Selective noradrenaline depletion impairs working memory and hippocampal neurogenesis. Neurobiol Aging 48: 93-102.
- Kelly SC, McKay EC, Beck JS, Collier TJ, Dorrance AM, et al. (2019) Locus coeruleus degeneration induces forebrain vascular pathology in a transgenic rat model of Alzheimer’s disease. J Alzheimers Dis 70: 371-388.
- Braun D, Feinstein DL (2019) The locus coeruleus neuroprotective drug vindeburnol normalizes behavior in the 5xFAD transgenic mouse model of Alzheimer’s disease. Brain Res 1702: 29-37.
- Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER (2016) Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory Proc Natl Acad Sci USA 113: 14835-14840.
- Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M, et al. (2016) Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537: 357-362.
- Ranjbar-Slamloo Y, Fazlali Z (2019) Dopamine and noradrenaline in the brain; Overlapping or dissociate functions? Front Mol Neurosci 12: 334.
- Raudino F (2013) Non-cognitive symptoms and related conditions in the Alzheimer’s disease: A literature review. Neurol Sci 34: 1275-1282.
- Liu L, Li Q, Li N, Ling J, Liu R, et al. (2011) Simultaneous determination of catecholamines and their metabolites related to Alzheimer’s disease in human urine. J Sep Sci 34: 1198-1204.
- Lv C, Li Q, Liu X, He B, Sui Z, et al. (2015) Determination of catecholamines and their metabolites in rat urine by ultra-performance liquid chromatography-tandem mass spectrometry for the study of identifying potential markers for Alzheimer’s disease. J Mass Spectrom 50: 354:363.
- Umegaki H, Ikari H, Nakahata H, Yoshimura J, Endo H, et al. (2000) Low plasma epinephrine in elderly female subjects of dementia of Alzheimer type. Brain Res 858: 67-70.
- Raskind MA, Peskind ER, Halter JB, Jimerson DC (1984) Norepinephrine and MHPG Levels in CSF and Plasma in Alzheimer’s Disease. Arch Gen Psychiatry 41: 343.
- Vitiello B, Veith RC, Molchan SE, Martinez RA, Lawlor BA, et al. (1993) Autonomic dysfunction in patients with dementia of the Alzheimer type. Biol Psychiatry 34: 428-433.
- Pillet LE, Taccola C, Cotoni J, Thiriez H, André K, et al. (2020) Correlation between cognition and plasma noradrenaline level in Alzheimer’s disease: A potential new blood marker of disease evolution. Transl Psychiatry 10: 213.
- DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 44: 837-845.
- Legdeur N, Binnekade TT, Otten RH, Badissi M, Scheltens P, et al. (2017) Cognitive functioning of individuals aged 90 years and older without dementia: A systematic review. Ageing Res Rev 36: 42-49.
- Andres-Benito P, Fernandez-Dueñas V, Carmona M, Escobar LA, Torrejon-Escribano B, et al. (2017) Locus coeruleus at asymptomatic early and middle Braak stages of neurofibrillary tangle pathology. Neuropathol Appl Neurobiol 43: 373-392.
- Iba M, McBride JD, Guo JL, Zhang B, Trojanowski JQ, et al. (2015) Tau pathology spread in PS19 tau transgenic mice following locus coeruleus (LC) injections of synthetic tau fibrils is determined by the LC’s afferent and efferent connections. Acta Neuropathol 130: 349-362.
- Satoh A, Iijima KM (2019) Roles of tau pathology in the locus coeruleus (LC) in age-associated pathophysiology and Alzheimer’s disease pathogenesis: Potential strategies to protect the LC against aging. Brain Res 1702: 17-28.
- Ross JA, Reyes BA, Van Bockstaele EJ (2019) Amyloid beta peptides, locus coeruleus-norepinephrine system and dense core vesicles. Brain Res 1702: 46-53.
- Fillenz M, Lowry JP, Boutelle MG, Fray AE (1999) The role of astrocytes and noradrenaline in neuronal glucose metabolism. Acta Physiol Scand 167: 275-284.
- Feinstein DL, Kalinin S, Braun D (2016) Causes, consequences, and cures for neuroinflammation mediated via the locus coeruleus: Noradrenergic signaling system. J Neurochem 139: 154-178.
- Adolfsson RC, Gottfries CG, Roos BE, Winblad B (1979) Changes in the brain catecholamines in patients with dementia of Alzheimer type. Br J Psychiatry 135: 216-223.
- Vermeiren Y, Janssens J, Aerts T, Martin JJ, Sieben A, et al. (2016) Brain serotonergic and noradrenergic deficiencies in behavioral variant frontotemporal dementia compared to early-onset Alzheimer’s disease. J Alzheimers Dis 53: 1079-1096.
- Matthews KL, Chen CP, Esiri MM, Keene J, Minger SL, et al. (2002) Noradrenergic changes, aggressive behavior, and cognition in patients with dementia. Biol Psychiatry 51: 407-416.
- Vermeiren Y, Van Dam D, Aerts T, Engelborghs S, De Deyn PP (2014) Monoaminergic neurotransmitter alterations in postmortem brain regions of depressed and aggressive patients with Alzheimer’s disease. Neurobiol Aging 35: 2691-2700.
- Reinikainen KJ, Soininen H, Riekkinen PJ (1990) Neurotransmitter changes in Alzheimer’s disease: Implications to diagnostics and therapy. J Neurosci Res 27: 576-586.
- Storga D, Vrecko K, Birkmayer JG, Reibnegger G (1996) Monoaminergic neurotransmitters, their precursors and metabolites in brains of Alzheimer patients. Neurosci Lett 203: 29-32.
- Francis BM, Yang J, Hajderi E, Brown ME, Michalski B, et al. (2012) Reduced tissue levels of noradrenaline are associated with behavioral phenotypes of the TgCRND8 mouse model of Alzheimer’s disease. Neuropsychopharmacology 37: 1934-1944.
- Raskind MA, Peskind ER, Holmes C, Goldstein DS (1999) Patterns of cerebrospinal fluid catechols support increased central noradrenergic responsiveness in aging and Alzheimer’s disease. Biol Psychiatry 46: 756-765.
- Elrod R, Peskind ER, DiGiacomo L, Brodkin KI, Veith RC, et al. (1997) Effects of Alzheimer’s disease severity on cerebrospinal fluid norepinephrine concentration. Am J Psychiatry 154: 25-30.
- Martignoni E, Bono G, Blandini F, Sinforiani E, Merlo P, et al. (1991) Monoamines and related metabolite levels in the cerebrospinal fluid of patients with dementia of Alzheimer type. Influence of treatment with L-deprenyl. J. Neural Transm Park Dis Dement Sect 3:15-25.
- Peskind ER, Wingerson D, Murray S, Pascualy M, Dobie DJ, et al. (1995) Effects of Alzheimer’s disease and normal aging on cerebrospinal fluid norepinephrine responses to yohimbine and clonidine. Arch Gen Psychiatry 52:774-782.
- Wang LY, Murphy RR, Hanscom B, Li G, Millard SP, et al. (2013) Cerebrospinal fluid norepinephrine and cognition in subjects across the adult age span. Neurobiol Aging 34: 2287-2292.
- Peskind ER, Elrod R, Dobie DJ, Pascualy M, Petrie E, et al. (1998) Cerebrospinal fluid epinephrine in Alzheimer’s disease and normal aging. Neuropsychopharmacol 19: 465-471.
- Baragli P, Pacchini S, Gatta D, Ducci M, Sighieri C (2010) Brief note about plasma catecholamines kinetics and submaximal exercise in untrained standardbreds. Ann Dell Istituto Supe Sanità 46: 96-100.
- Femminella GD, Rengo G, Komici K, Iacotucci P, Petraglia L, et al. (2014) Autonomic dysfunction in Alzheimer’s disease: Tools for assessment and review of the literature. J Alzheimers Dis 42: 369-377.
- Serra L, D'Amelio M, Di Domenico C, Dipasquale O, Marra C, et al. (2018) In vivo mapping of brainstem nuclei functional connectivity disruption in Alzheimer’s disease. Neurobiol Aging 72: 72-82.
Citation: Pillet L-E, Gallo A, Manivet P, Verpillot R (2021) Plasma Catecholamines: Blood Molecules Implicated in Alzheimer’s Disease? J Alzheimers Dis Parkinsonism 11: 522. DOI: 10.4172/2161-0460.1000522
Copyright: © 2021 Pillet L-E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2545
- [From(publication date): 0-2021 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 1713
- PDF downloads: 832