Plant-Originated Kaempferol and Luteolin as Allelopathic Algaecides Inhibit Aquatic Microcystis Growth Through Affecting Cell Damage, Photosynthetic and Antioxidant Responses
Received: 10-Feb-2018 / Accepted Date: 26-Feb-2018 / Published Date: 28-Feb-2018 DOI: 10.4172/2155-6199.1000431
Abstract
Harmful algal blooms dominated by cyanobacterium Microcystis aeruginosa increasingly occur in freshwaters worldwide, and adversely threat ecosystem functioning. Plant allelopathic effects can be applied as an emerging biological option to control and remediate HABs pollution. This study aimed to explore the growth-inhibition effects of plant-originated kaempferol and luteolin on bloom-forming Microcystis aeruginosa (FACHB-915 strain) and elucidate their anti-algal mechanisms from the views of photosynthesis, antioxidant responses and cell oxidative damage. Results showed that kaempferol and luteolin stress on M. aeruginosa growth were dose-and time-dependent. In contrast to 0.5~4 mg/L dose, 16~32 m/L kaempferol and luteolin significantly inhibited growth after 6 days-exposure and achieved 92.05% ~95.20% and 74.40%~85.35% inhibition, respectively, by day 14, partially caused by inhibited chlorophyll-a content at late phase. On day 4 and 8, stimulated photosynthetic responses (except phycocyanin content on day 4) at 32 mg/L kaempferol and stimulated superoxide dismutase activity at 16~32 mg/L kaempferol and 32 mg/L luteolin acted as adaptive and antioxidant defense against oxidative stress. Despite these, enhanced oxidative damage at 16~32 mg/L kaempferol and 32 mg/L luteolin and inhibited phycobiliproteins (e.g., phycocyanin, allophycocyanin) synthesis at 16~32 luteolin throughout the test and/or during mid-late phase still caused inhibited growth. Innovatively, this study for the first time to reveal that plant-originated kaempferol and luteolin at 16~32 mg/L could inhibit M. aeruginosa growth due to enhanced cell oxidative damage and/or inhibited photosynthesis despite activated antioxidant responses and could be potentially developed as algaecides for efficient M. aeruginosa bloomremoval and bioremediation.
Keywords: Antioxidant response; Kaempferol; Luteolin; Microcystis aeruginosa ; Oxidative damage; Photosynthesis
Introduction
Harmful algal blooms (HABs) have a cosmopolitan distribution, and adversely affect aquatic organisms and ecosystem functioning [1]. Much attempt has been taken to remove aquatic HABs, but the physicchemical methods including coagulation, sedimentation, filtration, and copper- and chlorine-based algaecides cannot be applied in large scale due to low efficiency, high cost and/or secondary pollution [2,3]. The HAB-remediation strategies based on biological regulation and control is always eco-friendly and environmentally-benign. Noteworthy, many plants produce secondary metabolites that known as allelochemicals with high target-selectivity and specificity, low spectrum toxicity and easily-degradability [4]. Therefore, plant allelopathy acts as a bioremediation strategy and can be applied as an emerging biological option to control HABs.
Flavonoids are a family of compounds mainly synthesized by plant roots, stems and leaves [5]. Such flavonoid compounds as rutin, quercetin 3-β-D-glucoside, salcolin and dihydroxyflavone were recently found to pose inhibitory effect on harmful algal growth [6-8]. However, few researches have explored the anti-algal mechanisms of flavonoids. To reveal flavonoid-caused anti-algal mechanisms may be of greater ecological interest and environmental implication.
Besides, kaempferol and luteolin are also common flavonoid compounds which are more extensively distributed in many botanical types such as fruits, vegetables and medicinal herbs [9]. Previous studies showed that kaempferol and luteolin had multi-beneficial effects on biological health-care, including anti-bacteria, antiinflammation, anti-allergy and anti-tumor [10,11]. To date, the effects of kaempferol and luteolin on harmful algae, especially Microcystis aeruginosa that exists as the dominant algae during HABs, still remains unidentified, and the influence mechanisms of them on M. aeruginosa growth is largely unknown.
To address knowledge gaps, this study employed M. aeruginosa FACHB-915 as model harmful algae, aiming to i) reveal the dosedependent effects of kaempferol and luteolin on its growth and ii) elucidate the influence mechanisms from the views of photosynthetic, antioxidant responses and oxidative damage during a exposure period of 14 days. To achieve these, this study measured cell density, contents of chlorophyll-a (CLA), phycobiliproteins (PBPs) (e.g., phycocyanin (PC), allophycocyanin (APC), phycoerythrin (PE)), total protein (TP) and malondialdehyde (MDA), and activities of antioxidases such as superoxide dismutase (SOD) and catalase (CAT) of M. aeruginosa exposed to kaempferol and luteolin at various doses. Understanding kaempferol and luteolin stress on M. aeruginosa growth and underlying mechanisms may provide further insights into the ecological roles of these two common flavonoids, and also be instructive to HABs-removal.
Materials and Methods
M. aeruginosa cultivation and chemicals
M. aeruginosa FACHB-915 was pre-cultured in sterile BG11 medium at 25 ± 1 and a 12 h diurnal-nocturnal alternation with cool white fluorescent lights at 25 μmol photons /m2·s. After 20 days precultivation, M. aeruginosa cells reaching exponential growth phase were collected by centrifugation (4000 g, 4, 10 min), and used as inoculums for downstream experiments.
Kaempferol and luteolin were purchased from Energy Chemical, Inc. (Shanghai, China). Individual stock solution were prepared in dimethyl sulfoxide (DMSO) and stored at 4 before use. Other chemicals used in this study were analytical grade except as specified by kit.
Experimental setup
All Erlenmeyer flasks were autoclaved at 121 for 20 min before experimental use. For each exposure test group, individual 250 mL flask containing 100 mL sterile BG11 medium was spiked with certain dose of kaempferol or luteolin at test beginning without any replenishment along test period. The final concentrations of kaempferol or luteolin were 0.5, 1, 4, 16 or 32 mg/L in each 100 mL medium, with DMSO amount below 0.1% (v/v). Initially-inoculated M. aeruginosa density was 4 × 105 cell/mL. Non-stress control group containing M. aeruginosa containing M. aeruginosa of the same density and < 0.1% (v/v) DMSO was established in parallel. Each group performed in triplicate was capped and cultivated for 14 days under the identical conditions as pre-cultivation.
Cell density analysis
Before each sampling, the flask was shaken to thoroughly mix the culture, and 10 mL of M. aeruginosa culture was aseptically sampled from each flask every two days for cell counting. The cell number was recorded by hemocytometer method using microscopy (BX53F Olympus, Japan) at 200-folds magnification. The inhibitory ratio (IR) of M. aeruginosa was calculated by following equation:
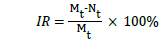 (1)
(1)
where Mt and Nt denote the cell density in control (i) and test group at day t, respectively.
CLA and PBPs synthesis analyses
Eight milliliter of culture was sampled on day 4, 8 and 14, respectively, during test, and divided into two equal aliquots. To quantify CLA in M. aeruginosa , the cells in one aliquot were collected by centrifugation at 4000 g for 10 min, and re-suspended in 90% ethanol to extract CLA under assisted ultrasonic-process at 150 W for 30 min. One milliliter of extraction supernatant after centrifugation was taken to monitor absorbance by spectrophotometry, with 90% ethanol as blank control. CLA was assayed following Yao [12] and expressed in pg/cell.
The other aliquot was used to analyze PBPs in M. aeruginosa . The cells in aliquot were collected after centrifugation at 4°C and resuspended in 4 mL of phosphate buffered solution (PBS) (50 mM, pH 7.28). Such re-suspension was freezed at -80°C for 8 h, and thawed in dark at room temperature. The freezing-and-thawing cycle was then repeated thrice. After centrifugation, the supernatant was taken to monitor absorbance at 650, 620 and 565 nm wavelengths, respectively. The PC, APC and PE contents (CPE, CPC and CAPC, expressed in pg/ cell) were calculated by following equations [13]:
CPC= (A620‐ 0.72 × A620)/629 (2)
CAPC= (A620‐ 0.19 × A620)/5.97 (3)
CPE=[A565‐ (2.41 × CPC‐1.41 × CAPC)]/13.0 (4)
where A620, A620 and A5\65 denote the absorbance at 650, 620 and 565 nm, respectively.
TP and antioxidant response analyses
To analyze TP and antioxidant response, 5 mL of M. aeruginosa culture was sampled on day 4, 8 and 14, respectively, of test period. The cells were collected by centrifugation at 8000 r/min for 15 min and resuspended in 5 mL of PBS (50 mM, pH 7.4). The re-suspension was homogenized on ice by ultrasonic cell pulverizer working at 150 W for 60 min, during which every working time of 5 s was alternated with an interval of 5 s. The homogenate was then centrifuged at 4000 g for 10 min to collect the supernatant.
TP content was determined according to the method of Braford [14] and expressed in mg/106 cells. SOD and CAT antioxidases activities, together with MDA content, were detected using specific kit provided by Nanjing Jiancheng Bioengineering Institute, China, according to manufacturer’s instruction. The antioxidases activities and MDA content in M. aeruginosa were expressed in Units (U)/106 cells and ng/106 cells, respectively.
Statistical analysis
Statistical analysis was performed using SPSS Statistics (Version 20.0). Parametric one-way analysis of variance was applied to identify the statistically significant difference (defined as those with p<0.05) between different groups on the same day.
Results and Discussion
Growth response to kaempferol or luteolin exposure
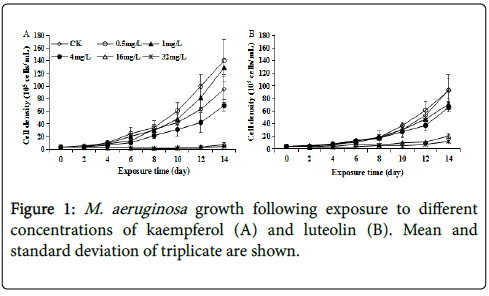
M. aeruginosa growth was not significantly (p>0.05) affected at kaempferol or luteolin exposure in early phase (until day 6) (Figure 1). Afterward, low dose kaempferol and luteolin as 0.5~4 mg/L posed no significant or even slightly stimulated effect on M. aeruginosa growth as compared to control, while 16~32 mg/L kaempferol and luteolin posed significantly inhibitory effect (p<0.05), with the IR of 92.05% ~95.20% and 74.40%~85.35%, respectively, by day 14 (Figure 1). Thus, for both kaempferol and luteolin, M. aeruginosa growth was increasingly inhibited with rising exposure concentration from 16 to 32 mg/L, and kaempferol was more effective to M. aeruginosa than luteolin at the same concentration, implying higher susceptibility of M. aeruginosa to kaempferol than luteolin. Above results showed that both kaempferol and luteolin slightly promoted or did not affect M. aeruginosa growth at low doses but caused growth inhibition at high concentrations. Interestingly, the toxicity of other environmental chemicals, including pesticides, antibiotics and industrial contaminants, to M. aeruginosa also followed this pattern, and their effects on M. aeruginosa growth changed from no obvious effect or stimulation to inhibition as exposure concentration elevated [15-17]. The slight growth stimulation at low doses of kaempferol and luteolin could be regarded as a hormesis effect on M. aeruginosa at low toxicity level.
Photosynthetic response to kaempferol or luteolin exposure
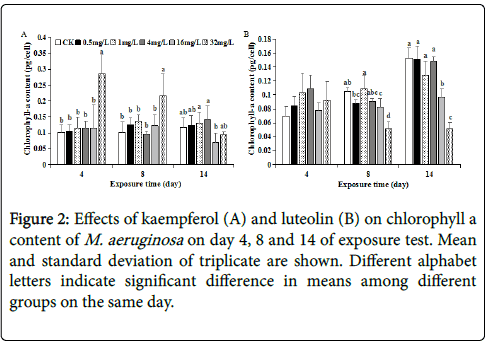
PBPs including PC, APC and PE are major light-harvesting photosynthetic accessory pigments in cyanobacteria and maintain the basic mode of energy metabolism via photosynthesis. PBPs, together with CLA, are proposed to absorb majority of incoming solar, and play imperative roles in electron/energy transfer and energy production during photosynthesis process [18,19]. Coupled to inhibited growth, the CLA content at 16~32 mg/L kaempferol and luteolin was inhibited by 19.71%~40.77% and 36.99%~66.50%, respectively, on day 14 as compared to control, implying that decreased photosynthetic activity was involved in M. aeruginosa growth-inhibition caused by 16~32 mg/L kaempferol and luteolin. Accordingly, no significant (p>0.05) difference in CLA content at all kaempferol or luteolin levels on day 4 was coupled with no significant different growth at the same time, except the case for 32 mg/L kaempferol (Figures 1 and 2). Such coupling between CLA and growth was reported in amoxicillinexposure study [17]. Exceptionally, CLA was significantly (p<0.05) stimulated to 0.28 pg/cell earlier on day 4 at 32 mg/L kaempferol, hence CLA synthesis was more sensitive than cell division for growth at 32 mg/L kaempferol (Figures 1A and 2A). Such stimulated CLA synthesis in early phase of test was likely to be an adaptive response that reflects a cellular requirement for protection against damage via producing excessive energy [20]. From these results, the inhibitory effects of 16~32 mg/L kaempferol and luteolin on both M. aeruginosa growth and CLA synthesis could appear with the prolongation of exposure time.
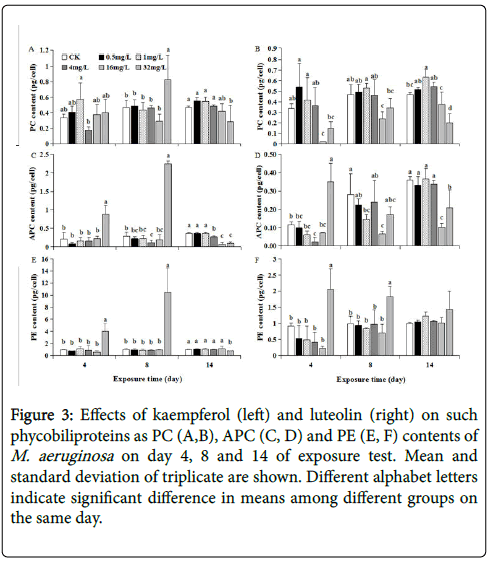
As time elapsed, PC, APC and PE contents of M. aeruginosa at each kaempferol level varied in the manner generally similar to that of CLA ( Figures 2A, 3A, 3C and 3E), implying that these PBPs synthesis was synchronously regulated in conjunction with CLA under kaempferol exposure. One exception was that the PC content exposed to 32 mg/L kaempferol cannot be significantly stimulated as CLA, APC and PE contents on day 4 (Figures 2A, 3A, 3C and 3E), probably because PC did not participate in such protective response as elevating photosynthesis activity and energy production during early phase.
Figure 3: Effects of kaempferol (left) and luteolin (right) on such phycobiliproteins as PC (A,B), APC (C, D) and PE (E, F) contents of M. aeruginosa on day 4, 8 and 14 of exposure test. Mean and standard deviation of triplicate are shown. Different alphabet letters indicate significant difference in means among different groups on the same day.
At each of 0~4 mg/L luteolin, PC, APC and PE contents of M. aeruginosa generally increased as time elapsed, coupled with synchronous cell growth at each of 0~4 mg/L luteolin (Figures 1B, 3B, 3D and 3F). This manifested the contribution of rising photosynthetic activity to cell growth at lower luteolin doses. Such time-dependent increase were also observed for PBPs contents at 16 mg/L luteolin exposure, with PC, APC and PE contents increasing from 0.02, 0.07 and 0.23 pg/cell on day 4 to 0.38, 0.10 and 1.01 pg/cell on day 14, respectively. Despite this, the PC, APC or PE content at 16 mg/L luteolin exposure were almost lower than that at 0~4 mg/L luteolin on each day, suggesting that the inhibited synthesis for light-harvesting pigments from day 4 to 14 was one reason for the inhibited growth at 16 mg/L luteolin in later phase of test (Figure 3B, 3D and 3F). By contrast, at 32 mg/L luteolin, the time-dependent trends of PC, APC and PE contents differed between each other. Specifically, the significantly inhibited PC (down to 0.15 pg/cell) and significantly stimulated APC and PE contents (rose to 0.35 and 2.05 pg/cell, respectively) simultaneously appeared on day 4, in contrast to no significant change on CLA on the same day (Figures 2B, 3B, 3D and 3F). These results shed that the response of PBPs synthesis to 32 mg/L luteolin was more sensitive than CLA synthesis in early phase. Despite the stimulated APC on day 4 and enhanced PE content along the test probably as adaptive strategy to 32 mg/L luteolin stress, the inhibited PC throughout the test and the inhibited APC and CLA contents from day 8 to 14 jointly resulted in an inhibited growth during later phase at 32 mg/L luteolin. Among three PBPs, PC was negatively affected most severely by 32 mg/L luteolin (Figure 3B), agreeing with Bi et al. [21] who also revealed that PC was the most vulnerable PBP under 20 mg/L berberine stress, and decreased photosynthetic pigments contents was involved in berberine-induced anti-algal mechanism.
TP synthesis at kaempferol and luteolin exposure
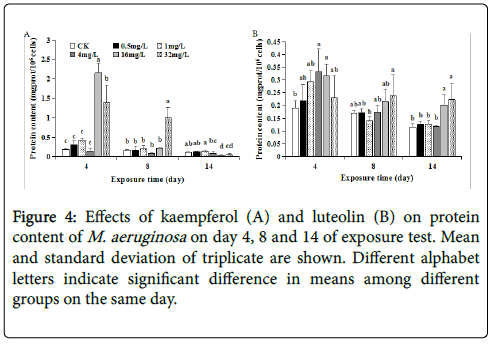
The TP content of M. aeruginosa at 0~4 mg/L kaempferol exposure remained relatively low and almost the same levels (without significant difference between each other) on each day, generally coinciding with the case at 0~4 mg/L luteolin (Figure 4). This corresponded to the similar growth trends at 0~4 mg/L kaempferol or luteolin exposure. In contrast, the TP content showed a continuously decreasing trend as time elapsed at 16~32 mg/L kaempferol or luteolin exposure (Figure 4). Moreover, compared to control, the TP at 16~32 mg/L luteolin was stimulated to 0.32~0.23, 0.21~0.24 and 0.20~0.22 mg/106 cells on day 4, 8 and 14 (Figure 4B), while TP at 16~32 mg/L kaempferol was significantly stimulated to 1.40~2.15 mg/106 cells on day 4 but significantly inhibited to 0.03~0.05 mg/106 cells on day 14 (p<0.05), exhibiting a transition from stimulation at early phase to inhibition at later phase (Figure 4A). Stimulated TP synthesis was also observed in M. aeruginosa exposed to antibiotic amoxicillin and herbicide diclofop [ 17,22]. Previous studies proposed the stimulated TP synthesis also as an adaptive response to alleviate exogenous stress, and a potential result of stimulated antioxidase responses [17,23].
Antioxidant responses and cell damage at kaempferol or luteolin exposure
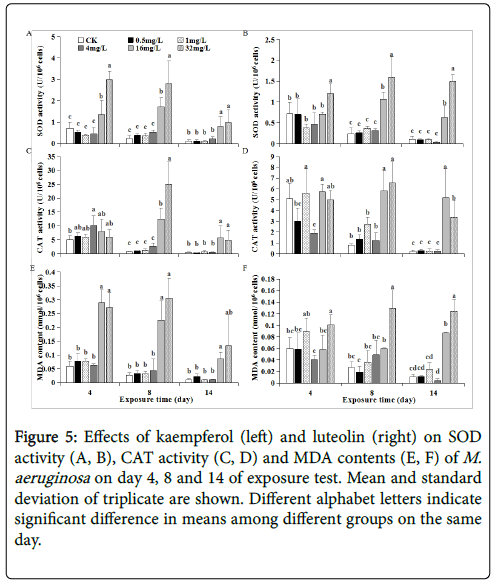
Exogenous stress exposure can induce excess reactive oxygen species (e.g., free radicals, H2O2) via aerobic metabolism and cause oxidative damage to cyanobacteria. Synthesis of antioxidants SOD and CAT with a protein or pepetide structures exerts pivotal roles in antioxidant defense by scavenging free radicals to ameliorate oxidative stress [24]. SOD is the first defense against oxidative stress and converts superoxide radical into O2 and H2O2 (i.e., 2O2 •−+2H +→H2O2+O2), while CAT further converts H2O2 into O2 [25]. Hence, to determine antioxidant responses and oxidative damages qualifies as a promising scheme to elucidate the mechanisms of exogenous stress on M. aeruginosa .
In this study, both SOD and CAT activities were not stimulated and/or remained relatively low levels at 0~4 mg/L kaempferol or luteolin exposure, corresponding to slight or no effects of such exposure on M. aeruginosa growth along test period (Figures 5A-5D). In comparison, SOD activity exposed to 16~32 mg/L kaempferol was significantly stimulated to 1.36~2.98, 1.72~ 2.80 and 0.80~0.98 U/106 cells, respectively, on day 4, 8 and 14. Likewise, SOD activity exposed to 32 mg/L luteolin was stimulated to 1.20, 1.59 and 1.49 U/106 cells, respectively, on day 4, 8 and 14 (Figures 5A and 5B). The stimulated SOD activity meant that M. aeruginosa cells suffered oxidative stress at higher kaempferol or luteolin levels. However, at 16~32 mg/L kaempferol and luteolin, apparent CAT activity stimulation was absent on day 4 and only emerged until day 8 and 14 (Figures 5C and 5D). The reason might be that H2O2 amount produced by SOD activity on day 4 was insufficient to induce CAT activity, as CAT can only be activated by exogenous stress up to certain extent [26]. To restrict H2O2 amount within an acceptable range is important for algal growth [ 27]. Thus, low CAT activity on day 4 might result in H2O2 accumulation in M. aeruginosa , which allowed MDA content (as indicator for cell damage) to increase to 0.29~0.27 and 0.1 nmol/106 cells at 16~32 mg/L kaempferol and at 32 mg/L luteolin exposure since the early phase as day 4 (Figures 5E and 5F).
Figure 5: Effects of kaempferol (left) and luteolin (right) on SOD activity (A, B), CAT activity (C, D) and MDA contents (E, F) of M. aeruginosa on day 4, 8 and 14 of exposure test. Mean and standard deviation of triplicate are shown. Different alphabet letters indicate significant difference in means among different groups on the same day.
As an index of lipid peroxidation, increased MDA content is a widely-used indicator for oxidative damage to cell [28]. Intracellular redox environment is sustained by the balance between production rate of oxidants and their removal by antioxidant system, but when such balance transforms to more oxidized state and/or the oxidants cannot be sufficiently removed by antioxidants, oxidative damage could occur [29,30]. The significantly increased MDA content indicated M. aeruginosa cell damage via lipid peroxidation (Figures 5E and 5F). Similar to SOD/CAT activities, MDA formation was also exposure concentration- and time-dependent, and MDA content trend was generally correlated to SOD/CAT activities at each kaempferol or luteolin level: i) Exposed to 16~32 mg/L kaempferol and 32 mg/L luteolin, significantly-increased MDA content on each day indicated M. aeruginosa cell damage, causing inhibited M. aeruginosa growth. Noteworthy, most significant SOD and CAT activity stimulation on day 8 could subsequently alleviate oxidative damage on day 14, according to decreasing MDA content from day 8 to 14 (down to 0.09, 0.13 and 0.12 nmol/106 cells, respectively) (Figure 5). To some extent, such relatively-alleviated oxidative stress on day 14 further inactivated SOD/CAT, leading to lower SOD/CAT activities on day 14 (SOD down to 0.80, 0.98 and 1.49 U/106 cells, CAT down to 5.67, 4.88, 3.38 U/106 cells) (Figures 5A-5D).
ii) At 16 mg/L luteolin, significantly-stimulated SOD and CAT activities were absent on day 4 but both stimulation presented on day 8, as compared to control, exhibiting that SOD activity linked to CAT activity as discussed above. Together considering no stimulation in MDA content on day 4, it is conferred that 16 mg/L luteolin did not induce oxidative stress and antioxidant responses at early phase as day 4. Until day 8 and 14, oxidative stress occurred as indicated by increased MDA content, accordingly SOD and CAT antioxidant responses was activated (Figures 5B-5F). This showed that the interactive scenario between oxidative stress-antioxidant responses at 16 mg/L luteolin differed with that at 16~32 mg/L kamepferol and at 32 mg/L luteolin. Thus, growth-inhibition at 16 mg/L luteolin could be partly due to enhanced cell oxidative damage during mid-late phase (from day 8 to 14), differing with the case at 16~32 mg/L kamepferol and at 32 mg/L luteolin where cell damage occurred throughout test period.
Above data revealed that 16~32 mg/L kaempferol induced not only antioxidant responses but also oxidative damages in M. aeruginosa during 14 day-exposure, largely due to the upset in cellular redoxbalance. In contrast, no stimulation or relatively low MDA content coupling to non-stimulated SOD/CAT activity responses at 0~4 mg/L kaempferol and luteolin could ensure a smooth growth of M. aeruginosa (Figure 5).
Conclusion
Applying the allelophathic effects of plant-originated compounds to inhibit the growth of bloom-forming algae is a promising environmentally-benign and eco-friendly option to control and remediate HABs. Flavonoids are a family of compounds mainly synthesized by plant roots, stems and leaves. Innovatively, plantoriginated kaempferol and luteolin were tested as algaecides against excessive growth of M. aeruginosa here. In conclusion, this study for the first time to reveal growth-inhibition effects of kaempferol and luteolin on M. aeruginosa and their algicidal mechanisms during a longer exposure than most previous studies. Results showed that kaempferol and luteolin stress on M. aeruginosa growth were dose-and time-dependent. The time-dependent data clearly clarified the dynamic relationship between physiological responses and cell growth of M. aeruginosa under various kaempferol or luteolin doses. In contrast to 0.5~4 mg/L doses, 16~32 m/L kaempferol and luteolin significantly inhibited growth after 6 days-exposure and achieved 92.05% ~95.20% and 74.40%~85.35% inhibition, respectively, by day 14, partly caused by inhibited CLA content at late phase. On day 4 and 8, stimulated photosynthetic responses (except PC content on day 4) at 32 mg/L kaempferol and stimulated SOD activity at 16~32 mg/L kaempferol and 32 mg/L luteolin acted as adaptive and antioxidant defense against oxidative stress. Despite these, enhanced oxidative damage at 16~32 mg/L kaempferol or luteolin and inhibited PBPs (e.g., PC, APC) synthesis at 16~32 luteolin throughout test and/or during mid-late phase, in conjunction to inhibited CLA contents at late phase, still caused inhibited M. aeruginosa growth. This study shed that two of the widely-distributed flavonoids kaempferol and luteolin could be potentially developed as anti-algal agents for efficient M. aeruginosa bloom-removal and bioremediation.
Acknowledgements
This work was supported by the Research Fund for the Doctoral Program of Higher Education of China (grant number: 20130008120026).
References
- Hu X, Liu Y, Zeng G, Hu X, Wang Y, et al. (2014) Effects of limonene stress on the growth of and microcystin release by the freshwater cyanobacterium Microcystis aeruginosa FACHB-905. Ecotoxicol Environ Saf 105: 121-127.
- JanÄula D, Maršálek B (2011) Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms. Chemosphere 85: 1415-1422.
- Xiao X, Chen Y, Liang X, Lou L, Tang X (2010) Effects of Tibetan hulless barley on bloom-forming cyanobacterium (Microcystis aeruginosa) measured by different physiological and morphologic parameters. Chemosphere 81: 1118-1123.
- Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55: 481-504.
- Jiang D, Huang L, Lin Y, Nie L, Lv S, et al. (2012) Inhibitory effect of Salicornia europaea on the marine alga Skeletonema costatum. Sci China Life Sci 55: 419-426.
- Xiao X, Huang H, Ge Z, Rounge TB, Shi J, et al. (2014) A pair of chiral flavonolignans as novel anti-cyanobacterial allelochemicals derived from barley straw (Hordeum vulgare): characterization and comparison of their anti-cyanobacterial activities. Environ Microbiol 16: 1238-1251.
- Huang H, Xiao X, Lin F, Grossart HP, Nie Z, et al. (2016) Continuous-release beads of natural allelochemicals for the long-term control of cyanobacterial growth: preparation, release dynamics and inhibitory effects. Water Res 95: 113-123.
- Miean KH, Mohamed S (2001) Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agri Food Chem 49: 3106-3112.
- Calderón-Montaño JM, Burgosmorón E, Pérezguerrero C, Lópezlázaro M (2011) A review on the dietary flavonoid kaempferol. Mini-Rev Med Chem 11: 298-344.
- Lin Y, Shi R, Wang X, Shen H (2008) Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Tar 8: 634-646.
- Yao N (1987) Algological Physiology. Dalian Institute of Technology Press, China.
- Chapman DJ, Kremer BP (1988) Experimental phycology: a laboratory manual. Cambridge University Press, Cambridge, United Kingdom.
- Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Qiu H, Geng J, Ren H, Xia X, Wang X (2013) Physiological and biochemical responses of Microcystis aeruginosa to glyphosate and its Roundup® formulation. J Hazard Mater 248: 172-176.
- De Morais P, Stoichev T, Basto MCP, Ramos V, Vasconcelos VM, et al. (2014) Cyanobacterium Microcystis aeruginosa response to pentachlorophenol and comparison with that of the microalga Chlorella vulgaris. Water Res 52: 63-72.
- Liu Y, Chen X, Zhang J, Gao B (2015) Hormesis effects of amoxicillin on growth and cellular biosynthesis of Microcystis aeruginosa at different nitrogen levels. Microb Ecol 69: 608-617.
- Blankenship RE (2014) Molecular Mechanisms of Photosynthesis. John Wiley and Sons, United Kingdom.
- Rastogi RP, Sonani RR, Madamwar D (2015) Effects of PAR and UV radiation on the structural and functional integrity of phycocyanin, phycoerythrin and allophycocyanin isolated from the marine cyanobacterium Lyngbya sp. A09DM. Photochem Photobiol 91: 837-844.
- Riethman H, Bullerjahn G, Reddy KJ, Sherman LA (1988) Minireview: regulation of cyanobacterial pigment-protein composition and organization by environmental factors. Photosynth Res 18: 133-161.
- Bi X, Zhang S, Xing K (2012) Effects of berberine on the photosynthetic pigments compositions and ultrastructure of cyanobacterium Microcystis aeruginosa. Adv Mater Res 343: 1117-1125.
- Ye J, Zhang Y, Chen S, Liu C, Zhu Y, et al. (2014) Enantioselective changes in oxidative stress and toxin release in Microcystis aeruginosa exposed to chiral herbicide diclofop acid. Aquat Toxicol 146: 12-19.
- Liu Z, Cui F, Ma H, Fan Z, Zhao Z, et al. (2014) The transformation mechanism of nitrobenzene in the present of a species of cyanobacteria Microcystis aeruginosa. Chemosphere 95: 234-240.
- Belchik SM, Xun L (2011) S-glutathionyl-(chloro) hydroquinone reductases: a new class of glutathione transferases functioning as oxidoreductases. Drug Metab Rev 43: 307-316.
- Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutase (SODs) in controlling oxidative stress in plants. J Exp Bot 53: 1331-1341.
- Li J, Ou D, Song L (2008) Decline of Microcystis aeruginosa FACHB-905 under four stress conditions. J Lake Sci 20: 549-555.
- Bhargava P, Atri N, Srivastava AK, Rai LC (2007) Cadmium mitigates ultraviolet-B stress in Anabaena doliolum: enzymatic and non-enzymatic antioxidants. Biol Plant 51: 546-550.
- Wang P, Wong M, Tam NFY (2013) Antioxidant responses of two microalgae, Selenastrum capricornutum and Chlorella sp., to estradiol and ethinylestradiol. J Appl Phycol 25: 891-903.
- Kumar S, Habib K, Fatma T (2008) Endosulfan induced biochemical changes in nitrogen-fixing cyanobacteria. Sci Tot Environ 403: 130-138.
- Cano-Europa E, Ortiz-ButrÓn R, Gallardo-Casas CA, Blas-Valdivia V, Pineda-Reynoso M, et al. (2010) Phycobiliprotein from Pseudanabaena tenuis rich in c-phycoerythrin protect against HgCl2-caused oxidative stress and cellular damage in the kidney. J Appl Phycol 22: 495-501.
Citation: Cao LH, Li J (2018) Plant-Originated Kaempferol and Luteolin as Allelopathic Algaecides Inhibit Aquatic Microcystis Growth Through Affecting Cell Damage, Photosynthetic and Antioxidant Responses. J Bioremediat Biodegrad 9: 431. DOI: 10.4172/2155-6199.1000431
Copyright: © 2018 Cao LH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5311
- [From(publication date): 0-2018 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 4396
- PDF downloads: 915