PI3K/AKT/mTOR Pathway in ATLL: from Basic Biology to Preclinical Study
Received: 26-Nov-2018 / Accepted Date: 12-Feb-2019 / Published Date: 18-Feb-2019
Abstract
Adult T-cell leukemia/ lymphoma (ATLL) is high resistance fatal malignancy which has a poor prognosis and exhibits resistance to conventional chemotherapy. The development of novel therapies for ATLL relies on a comprehensive understanding of the occurrences that result in cellular survival and proliferation regulating pathways that control growth signals is an emerging and complementary approach to ATL treatment. The PI3K/AKT/mTOR is a pivotal gatekeeper for cell growth, viability, migration, proliferation, and development drug resistance. Activation of PI3K, AKT, mTOR regulates important genes and proteins like mTOR, p53, NF-κB, P27, P21, S6K, FKHR and BAD. So this rout has a central role in handling cell cycle regulators, transcription factors and anti-apoptotic proteins. This review focuses on the role of PI3K/AKT/mTOR in ATL progression and development drug resistance.
Keywords: PI3K; AKT; mTOR; HTLV-1; ATLL
Introduction
Human T lymphotropic virus type 1 (HTLV-1) is the earliest human retrovirus discovered from the deltaretrovirus family and is estimated to infect approximately 10–20 million of all people, however only 3-5% will eventually progress to adult T-cell leukemia/ lymphoma (ATLL) or tropical spastic paraparesis/HTLV-associated myelopathy (TSP/HAM) [1-3]. Survival of patients with ATLL is considerably low. The average survival of these patients is only 13 months [6-8]. So it is an emergency to provide effective and novel therapy for this patient. One of the pathways that have a central role in cell survival and proliferation is PI3K/Akt/mTOR pathway that has especially role in tumorigenesis [9-14]. There are many studies that proved the role of this pathway in malignancies such multiple myeloma [15-17]. This review especially focuses on the role of PI3K/Akt/mTOR in ATL.
HTLV-1
HTLV-1 is one of the members of the retrovirus family. Like other retroviruses, a proviral genome of HTLV-1 has structural genes, pol, gag and env, along with long terminal repeat (LTR) at both ends [18-20]. The diagnostic feature of the HTLV-1 proviral genome is the existence of pX region between env and 3’ LTR and encoded several adornment genes, which comprise tax, rex, p12, p21, p30, p13, and HTLV-1 bZIP factor [21-23]. Between these viral proteins, HBZ and TAX play significant roles in the cellular transformation and the activation in T-cell [24,25].
From diverse proteins that code by HTLV-1 genome, HBZ is the only protein expressed in all ATL cases without mutation in other hands, wild-type expressed [26,27]. There are two types of HBZ in ATL, spliced sHBZ and unspliced HBZ, which differs in 7 amino acids [28- 30]. Both HBZ isoforms consist of three domains: activation domain (AD), central domain (CD), and basic leucine zipper domain (bZIP). HBZ encompass a functional nuclear export signal (NES) sequence within its N-terminal region that disrupts the cellular autophagic response in the cytoplasm. In addition, three nuclear signals (NLSs) exist that responsible for nuclear localization of HBZ protein. There is the sp1 region in 3’ LTR of HTLV-1 have been shown to be necessary for HBZ promoter activity [31-33].
Diver’s function of HBZ in ATL was recognized, these functions occurred by interaction with several proteins. HBZ suppresses the p53 expression induced by ATF3 via binds to ATF3/p53 complexes [34,35]. Both BCL2 and Flip could be expressed via induction by HBZ. HBZ also has a role in decreased activation of P53 through binding to p300/ CBP, inhibits p53 acetylation and deregulates the p53 activity [36,37].
Several studies were showed the pleiotropic function of TAX in tumor genesis. CREB/ATF, SRF, and NF-kappa B-associated pathways are regulated by TAX, so TAX is able to modulate expression of many viral and cellular genes [38-40]. Tax also downregulate the function of various regulatory proteins via direct protein-protein interaction. Tax forces the infected T-cells into unstoppable replication and interfering with the function of telomerase and Topoisomerase-I via inhibiting DNA repair [41-43].
PI3K/Akt/mTOR Pathway
In several cancers especially hematologic cancers role of PI3K/Akt/ mTOR in tumorigenesis were highlighted [44-49]. This pathway from diver’s mechanisms helps to cancers to be more aggressive through increase proliferation and promote viability [50-52]. These functions earn from interacting with important cellular proteins like cell cycle regulators, anti-apoptotic proteins and transcriptional factors. PI3K is an extracellular sensor of this rout [53]. When growth factors like PDGF or FGF bind to its surface receptor PI3K activated and stimulate AKT [54,55]. AKT is an effector of this pathway, which has a diverse function. In terms of anti-apoptotic effect, anti-apoptotic effects of FLICE-like inhibitory protein (FLIP) and cIAP2 and XIAP is improved by AKT [56,57]. AKT also suppresses pro-apoptotic proteins like FAS, BIM and BAD [58,59]. Phosphorylation of BAD by AKT leads to stabilization of BCL-xL [60,61]. AKT activates NF-kB indirectly via phosphorylation of IKK. AKT also controls cell cycle through interaction with main cell cycle regulators like P27, P21, and P53, which can induce cell cycle arrest [62-64]. AKT stimulates suppression of P53 through activation of murine double murine 2 (MDM2) [65,66]. AKT induces activation of mTOR via 3 ways, phosphorylate TSC2, inhibition PRAS40 and enhance the nutrition level by stabilizing GLUT1 in cell surface [67,68].
mTOR is the last component of this pathway. There are two types of mTOR complex that is different in ingredients and functions. The main mTORC1 components include mTOR, Raptor, and mLST8/GbL and the mTORC2 ingredients are mTOR, Rictor, and mLST8/GbL [69,70]. First different between mTORC1,2 is sensitivity to rapamycin (mTORC1 is sensitive and mTORC2 is resistance). The substrates of mTORC1 are p70S6K and 4E-BP1. But substrates of mTORC2 are protein kinase C-a (PKC-a), serum- and glucocorticoid-inducible kinase (SGK), and AKT [71,72]. mTORC1 as a sensor of nutrition level in a cell, which responsible for protein synthesis and proliferation [73- 75]. Phosphorylation of S6K (ribosomal S6 kinase) activates ribosome biogenesis, and phosphorylation of 4E-BP1 (eukaryotic translation initiation factor 4E [eIF-4E] binding protein 1) inhibits its binding to eIF-4E [71-77]. Promoting cap-dependent translation is occurred through Liberation of eIF-4E and then participate in a translation initiation complex. mTORC1 enhances cell cycle proteins like myc and cyclins [78,79]. mTORC1 has an anti-apoptotic effect by enhancing expression anti-apoptotic protein MCL-1. Furthermore, mTORC1 indirectly inhibits apoptosis signal-regulating kinase 1 (ASK1) [80,81].
Activation of PI3K/AKT/mTORC1 in ATLL
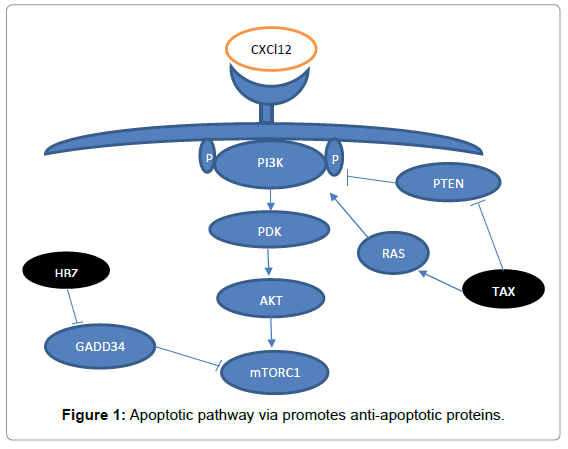
Scientists were proved a special role of PI3K/Akt/mTORC1 in ATLL [82,83]. This pathway could be activated via a diver’s mechanism like activation of HBZ or TAX [84]. Mutation of the chemokine receptor CCR4 also lead to activation of PI3K and stimulate activation of PI3K/ AKT/mTORC1 pathway in addition, TAX can activate this pathway from two different ways 1: Through stimulating of RAS signalling. 2: By inhibition of PTEN (a PI3K inhibitor) [85-87]. HBZ also stimulate activation of downstream of mTOR through interaction with GADD34 [88]. In HTLV-1 Loss of N-myc downstream regulated gene 2 (NDRG2), a negative regulator of PI3K, enhanced activation of the NF-κB pathway by PTEN and NIK phosphorylation for ATL [89].
A diverse study showed a particular role of PI3K/AKT/mTOR in ATLL. When they inhibit these route, the proliferation of HTLV-1 cell line was suppressed [90]. In HTLV-1, PI3K/Akt/mTOR activity result in stimulation activity of NF-kB and triggers transcriptional factor like activator protein 1 (AP1) and also reduces cell cycle inhibitors like P53, P27, P21. In addition, AKT regulates apoptotic pathway via promotes anti-apoptotic proteins such as BCL-xL and deregulate pro-apoptotic proteins like BAD and BAX (Figure 1) [91].
Preclinical Trial
Because PI3K/Akt/mTOR is an important route in tumorigenesis and has a special role in hematologic malignancy, several studies were done to investigate the efficacy of PI3K/Akt/mTORC1 inhibitors in HTLV-1 cell lines. Very recently Hiroo Katsuya et al. [92] demonstrated idelalisib, an inhibitor of PI3k-δ, induce apoptosis and cell toxicity in ATL cells in vitro and overcome stimulation via CCL22. Chie Ishikawa et al. [93] Reported NVP-BEZ235, a dual PI3K, and mTOR inhibitor induces cell cycle arrest and also induce apoptosis in HTLV-1 infected T-cell. Recently Chie Ishikawa et al. [82] showed Butein, an inhibitor of Akt, simultaneously induces cell apoptosis and cell cycle arrest and also induce caspase activation. Chie Ishikawa et al. [83] demonstrated Peridinin inhibits IκBα, RelA, Akt and p70 S6 kinase and induces cell cycle arrest and also induce apoptosis in MT-2 cell line (Table 1).
| Drug | Drug target | Cell line | Result | Reference |
|---|---|---|---|---|
| Idelalisib | PI3k-δ | patient derived cell line(PDC) | Reduce viability of ATL cells | 92 |
| NVP-BEZ235 | PI3K, mTOR | MT-2, MT-4, HUT-102 | Reduce proliferation of infected cells | 93 |
| RAD001 | mTOR | MT-2, MT-4, HUT-102 | Reduce proliferation of infected cells | 93 |
| Butein | AKT | MT-4, HUT-102, PDC | Induce apoptosis and cell cycle arrest | 82 |
| Curcumin | PDK | MT-2, C5/M SLB-1, HUT-102 | Suppress activation of AKT | 2 |
Table 1: PI3K/AKT/mTOR inhibitors.
Conclusion
The PI3K/ AKT/mTOR signalling pathway is implicated in multiple aspects of HTLV-1 infected T-cell. The Basic studies performed on the PI3K/ AKT/mTOR pathway in HTLV-1 infected T-cell have shown that it plays an integral role in ATLL disease biology. PI3K/Akt/ mTOR signaling has been reported to promote HTLV-1 infected T-cell survival and thereby establishes disease progression and acquired drug resistance. Targeting the PI3K/AKT/mTOR pathway could, therefore, be an interesting new avenue to treat ATLL. It would increase apoptosis of HTLV-1 infected cells and as such decrease tumor growth and survival.
References
- Futsch N, Mahieux R, Dutartre H (2017) HTLV-1, the Other Pathogenic Yet Neglected Human Retrovirus: From Transmission to Therapeutic Treatment. Viruses 10: E1.
- Gallo RC (2005) The discovery of the first human retrovirus: HTLV-1 and HTLV-2. Retrovirology 2:17.
- Coffin JM (2015) The discovery of HTLV-1, the first pathogenic human retrovirus. Proc Natl Acad Sci USA 112: 15525–15529.
- Gallo RC, Willems L, Hasegawa H (2016) Screening transplant donors for HTLV-1 and -2. Blood 128: 3029–3031.
- Matutes E (2007) Adult T-cell leukemia/lymphoma. J Clin Pathol 60: 1373–1377.
- Fukushima T, Miyazaki Y, Honda S, Kawano F, Moriuchi Y, et al. (2005) Allogeneic hematopoietic stem cell transplantation provides sustained long-term survival for patients with adult T-cell leukemia/lymphoma. Leukemia 19: 829–834.
- Takasaki Y, Iwanaga M, Tsukasaki K, Kusano M, Sugahara K, et al. (2007) Impact of visceral involvements and blood cell count abnormalities on survival in adult T-cell leukemia/lymphoma (ATLL). Leuk Res 31: 751–757.
- Montoto S (2011) Use of Zidovudine and Interferon Alfa With Chemotherapy Improves Survival in Both Acute and Lymphoma Subtypes of Adult T-Cell Leukemia/Lymphoma. Arctic J Clin Oncol 29: 4696-4701.
- Kawauchi K, Ogasawara T, Yasuyama M, Otsuka K, Yamada O (2009) Regulation and Importance of the PI3K/Akt/mTOR Signaling Pathway in Hematologic Malignancies. Anticancer Agents Med Chem 9:1024–1038.
- Altman JK, Platanias LC (2009) Prospects for mTOR targeting in adult T cell leukemia. Leuk Lymphoma 50: 525–526.
- Hirase C, Maeda Y, Yamaguchi T, Miyatake JI, Kanamaru A (2009) mTOR inhibition, and adult T-cell leukemia. Leuk Lymphoma 50: 645–647.
- Mori N (2009) Cell signaling modifiers for molecular targeted therapy in ATLL. Front Biosci 14: 1479-1489.
- Nakahata S, Ichikawa T, Maneesaay P, Saito Y, Nagai K, et al. (2014) Loss of NDRG2 expression activates PI3K-AKT signaling via PTEN phosphorylation in ATLL and other cancers. Nat Commun 5: 3393.
- Mozhgani SH, Zarei-Ghobadi M, Teymoori-Rad M, Mokhtari-Azad T, Mirzaie M, et al. (2018) Human T-lymphotropic virus 1 (HTLV-1) pathogenesis: A systems virology study. J Cell Biochem 119: 3968–3979.
- Hoang B, Frost P, Shi Y, Belanger E, Benavides A, et al. (2010) Targeting TORC2 in multiple myeloma with a new mTOR kinase inhibitor. Blood 116: 4560-4568.
- Ramakrishnan V, Kumar S (2018) PI3K/AKT/mTOR pathway in multiple myeloma: from basic biology to clinical promise. Leuk Lymphoma 59: 2524-2534.
- Li J, Zhu J, Cao B, Mao X (2014) The mTOR signaling pathway is an emerging therapeutic target in multiple myeloma. Curr Pharm Des 20: 125–135.
- Saiga A, Orita S, Minoura-Tada N, Maeda M, Aono Y, et al. (1997) cis-Acting Inhibitory Elements within the pol-env Region of Human T-Cell Leukemia Virus Type 1 Possibly Involved in Viral Persistence. J Virol 71: 4485-4494.
- Trentin B, Rebeyrotte N, Mamoun RZ (1998) Human T-cell leukemia virus type 1 reverse transcriptase (RT) originates from the pro and pol open reading frames and requires the presence of RT-RNase H (RH) and RT-RH-integrase proteins for its activity. J Virol 72: 6504–6510.
- Younis I, Khair L, Dundr M, Lairmore MD, Franchini G, et al. (2004) Repression of human T-cell leukemia virus type 1 and type 2 replication by a viral mRNA-encoded posttranscriptional regulator. J Virol 78: 11077–11083.
- Robek MD, Wong FH, Ratner L (1998) Human T-cell leukemia virus type 1 pX-I and pX-II open reading frames are dispensable for the immortalization of primary lymphocytes. J Virol 72: 4458–4462.
- Caputo A, Haseltine WA (1992) Reexamination of the coding potential of the HTLV-1 pX region. Virology 188: 618–627.
- Mahieux R, Gessain A (2003) HTLV-1 and associated adult T-cell leukemia/lymphoma. Rev Clin Exp Hematol 7: 336–361.
- Akbarin MM, Shirdel A, Bari A, Mohaddes ST, Rafatpanah H, et al. (2017) Evaluation of the role of TAX, HBZ, and HTLV-1 proviral load on the survival of ATLL patients. Blood Res 52: 106–111.
- Belrose G, Gross A, Olindo S, Lézin A, Dueymes M, et al. (2011) Effects of valproate on Tax and HBZ expression in HTLV-1 and HAM/TSP T lymphocytes. Blood 118: 2483–2491.
- Arnold J, Yamamoto B, Li M, Phipps AJ, Younis I, et al. (2006) Enhancement of infectivity and persistence in vivo by HBZ, a natural antisense coded protein of HTLV-1. Blood 107: 3976–3982.
- Matsuoka M, Green PL (2009) The HBZ gene, a key player in HTLV-1 pathogenesis. Retrovirology 6: 71.
- Shiohama Y, Naito T, Matsuzaki T, Tanaka R, Tomoyose T, et al. (2016) Absolute quantification of HTLV-1 basic leucine zipper factor (HBZ) protein and its plasma antibody in HTLV-1 infected individuals with different clinical status. Retrovirology 13: 29.
- Sugata K, Yasunaga JI, Kinosada H, Mitobe Y, Furuta R, et al. (2016) HTLV-1 Viral Factor HBZ Induces CCR4 to Promote T-cell Migration and Proliferation. Cancer Res 76: 5068–5079.
- Mozhgani SH, Jaberi N, Rezaee SA, Bustani R, Jazayeri SM, et al. (2017) Evaluation of HTLV-1 HBZ and proviral load, together with host IFN λ3 , in pathogenesis of HAM/TSP. J Med Virol 89: 1102–1107.
- Bangham CRM, Matsuoka M (2017) Human T-cell leukaemia virus type 1: parasitism and pathogenesis. Philos Trans R Soc B Biol Sci 372: 20160272.
- Al-Saleem J, Kvaratskhelia M, Green PL (2017) Methods for Identifying and Examining HTLV-1 HBZ Post-translational Modifications. Methods Mol Biol 1582: 111–126.
- Kinosada H, Yasunaga J, Shimura K, Miyazato P, Onishi C, et al. (2017) HTLV-1 bZIP Factor Enhances T-Cell Proliferation by Impeding the Suppressive Signaling of Co-inhibitory Receptors. PLOS Pathogens 13: e1006120.
- Wright DG, Marchal C, Hoang K, Ankney JA, Nguyen ST, et al. (2016) Human T-cell leukemia virus type-1-encoded protein HBZ represses p53 function by inhibiting the acetyltransferase activity of p300/CBP and HBO1. Oncotarget 7: 1687–1706.
- Zhi H, Yang L, Kuo YL, Ho YK, Shih HM, et al. (2011) NF-κB Hyper-Activation by HTLV-1 Tax Induces Cellular Senescence, but Can Be Alleviated by the Viral Anti-Sense Protein HBZ. PLoS Pathogens 7: e1002025.
- Hagiya K, Yasunaga J, Satou Y, Ohshima K, Matsuoka M (2011) ATF3, an HTLV-1 bZip factor binding protein, promotes proliferation of adult T-cell leukemia cells. Retrovirology 8: 19.
- Basbous J, Arpin C, Gaudray G, Piechaczyk M, Devaux C, et al. (2003) The HBZ Factor of Human T-cell Leukemia Virus Type I Dimerizes with Transcription Factors JunB and c-Jun and Modulates Their Transcriptional Activity. J Biol Chem 278: 43620–43627.
- Kusano S, Yoshimitsu M, Hachiman M, Ikeda M (2015) I-mfa domain proteins specifically interact with HTLV-1 Tax and repress its transactivating functions. Virology 486: 219–227.
- Bergamo E, Diani E, Bertazzoni U, Romanelli MG (2017) A Luciferase Functional Quantitative Assay for Measuring NF-ĸB Promoter Transactivation Mediated by HTLV-1 and HTLV-2 Tax Proteins. Methods Mol Biol 1582: 79–87.
- Gross C, Thoma-Kress A (2016) Molecular Mechanisms of HTLV-1 Cell-to-Cell Transmission. Viruses 8: 74.
- Rafatpanah H, Torkamani M, Valizadeh N, Vakili R, Meshkani B, et al. (2016) Prevalence and phylogenetic analysis of HTLV-1 in a segregated population in Iran. J Med Virol 88: 1247–1253.
- Groussaud D, Khair M, Tollenaere AI, Waast L, Kuo MS, et al. (2017) Hijacking of the O-GlcNAcZYME complex by the HTLV-1 Tax oncoprotein facilitates viral transcription. PLOS Pathogens 13: e1006518.
- Jabareen A, Abu-Jaafar A, Abou-Kandil A, Huleihel M (2017) Effect of TPA and HTLV-1 Tax on BRCA1 and ERE controlled genes expression. Cell Cycle 16: 1336–1344.
- Wang Z, Valera JC, Zhao X, Chen Q, Silvio Gutkind J (2017) mTOR co-targeting strategies for head and neck cancer therapy. Cancer Metastasis Rev 36: 491–502.
- Zeng H (2017) mTOR signaling in immune cells and its implications for cancer immunotherapy. Cancer Lett 408: 182–189.
- Guerrero-Zotano A, Mayer IA, Arteaga CL (2016) PI3K/AKT/mTOR: role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev 35: 515–524.
- Hsieh A, Edlind M (2014) PI3K-AKT-mTOR signaling in prostate cancer progression and androgen deprivation therapy resistance. Asian J Androl 16: 378.
- Yim CY, Bikorimana E, Khan E, Warzecha JM, Shin L, et al. (2017) G0S2 represses PI3K/mTOR signaling and increases sensitivity to PI3K/mTOR pathway inhibitors in breast cancer. Cell Cycle 16: 2146–2155.
- Cao G, Chen K, Chen B, Xiong M (2017) Positive prognostic value of HER2-HER3 co-expression and p-mTOR in gastric cancer patients. BMC Cancer 17: 841.
- Yoon SO, Shin S, Karreth FA, Buel GR, Jedrychowski MP, et al. (2017) Focal Adhesion- and IGF1R-Dependent Survival and Migratory Pathways Mediate Tumor Resistance to mTORC1/2 Inhibition. Mol Cell 67: 512–527.e4.
- Gordon MA, D’Amato NC, Gu H, Babbs B, Wulfkuhle J, et al. (2017) Synergy between Androgen Receptor Antagonism and Inhibition of mTOR and HER2 in Breast Cancer. Mol Cancer Ther 16: 1389–1400.
- Gautam P, Karhinen L, Szwajda A, Jha SK, Yadav B, et al. (2016) Identification of selective cytotoxic and synthetic lethal drug responses in triple negative breast cancer cells. Mol Cancer 15: 34.
- Zingg JM, Azzi A, Meydani M (2015) Induction of VEGF Expression by Alpha-Tocopherol and Alpha-Tocopheryl Phosphate via PI3Kγ/PKB and hTAP1/SEC14L2-Mediated Lipid Exchange. J Cell Biochem 116: 398–407.
- Bertacchini J, Heidari N, Mediani L, Capitani S, Shahjahani M, et al. (2015) Targeting PI3K/AKT/mTOR network for treatment of leukemia. Cell Mol Life Sci 72: 2337–2347.
- Ersahin T, Tuncbag N, Cetin-Atalay R (2015) The PI3K/AKT/mTOR interactive pathway. Mol Biosyst 11: 1946–1954.
- Gagnon V, St-Germain ME, Parent S, Asselin E (2003) Akt activity in endometrial cancer cells: regulation of cell survival through cIAP-1. Int J Oncol 23: 803–810.
- Prasad S, Tyagi AK (2015) Ginger and Its Constituents: Role in Prevention and Treatment of Gastrointestinal Cancer. Gastroenterol Res Pract 2015: 1–11.
- Bin G, Bo Z, Jing W, Jin J, Xiaoyi T, et al. (2016) Fluid shear stress suppresses TNF-α-induced apoptosis in MC3T3-E1 cells: Involvement of ERK5-AKT-FoxO3a-Bim/FasL signaling pathways. Exp Cell Res 343: 208–217.
- Farhan M, Wang H, Gaur U, Little PJ, Xu J, et al. (2017) FOXO Signaling Pathways as Therapeutic Targets in Cancer. Int J Biol Sci 13: 815–827.
- Phatak NR, Stankowska DL, Krishnamoorthy RR (2016) Bcl-2, Bcl-xL, and p-AKT are involved in neuroprotective effects of transcription factor Brn3b in an ocular hypertension rat model of glaucoma. Mol Vis 22: 1048–1061.
- Choudhary GS, Al-harbi S, Mazumder S, Hill BT, Smith MR, et al. (2015) MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies. Cell Death Dis 6: e1593–e1593.
- Ezhilarasan D, Evraerts J, Sid B, Calderon PB, Karthikeyan S, et al. (2017) Silibinin induces hepatic stellate cell cycle arrest via enhancing p53/p27 and inhibiting Akt downstream signaling protein expression. Hepatobiliary Pancreat Dis Int 16: 80–87.
- Hsu YH, Chang CC, Yang NJ, Lee YH, Juan SH (2014) RhoA-Mediated Inhibition of Vascular Endothelial Cell Mobility: Positive Feedback Through Reduced Cytosolic p21 and p27. J Cell Physiol 229: 1455–1465.
- Jain MV, Jangamreddy JR, Grabarek J, Schweizer F, Klonisch T, et al. (2015) Nuclear localized Akt enhances breast cancer stem-like cells through counter-regulation of p21 Waf1/Cip1 and p27 kip1. Cell Cycle 14: 2109–2120.
- Kim YY, Jee HJ, Um JH, Kim YM, Bae SS, et al. (2017) Cooperation between p21 and Akt is required for p53-dependent cellular senescence. Aging Cell 16: 1094–1103.
- Leszczynska KB, Foskolou IP, Abraham AG, Anbalagan S, Tellier C, et al. (2015) Hypoxia-induced p53 modulates both apoptosis and radiosensitivity via AKT. J Clin Invest 125: 2385–2398.
- Covarrubias AJ, Aksoylar HI, Horng T (2015) Control of macrophage metabolism and activation by mTOR and Akt signaling. Semin Immunol 27: 286–296.
- Pierobon M, Ramos C, Wong S, Hodge KA, Aldrich J, et al. (2017) Enrichment of PI3K-AKT–mTOR Pathway Activation in Hepatic Metastases from Breast Cancer. Clin Cancer Res 23: 4919–4928.
- Yoon MS (2017) The Role of Mammalian Target of Rapamycin (mTOR) in Insulin Signaling. Nutrients 9: 1176.
- Yoon MS (2017) mTOR as a Key Regulator in Maintaining Skeletal Muscle Mass. Front Physiol 8: 788.
- Linke M, Fritsch SD, Sukhbaatar N, Hengstschläger M, Weichhart T (2017) mTORC1 and mTORC2 as regulators of cell metabolism in immunity. FEBS Lett 591: 3089–3103.
- Kim LC, Cook RS, Chen J (2017) mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene 36: 2191–2201.
- Perez-Schindler J, Hamilton DL, Moore DR, Baar K, Philp A (2015) Nutritional strategies to support concurrent training. Eur J Sport Sci 41–52.
- Adegoke OAJ, Abdullahi A, Tavajohi-Fini P (2012) mTORC1 and the regulation of skeletal muscle anabolism and mass. Appl Physiol Nutr Metab 37: 395–406.
- Gonzalez AM, Hoffman JR, Stout JR, Fukuda DH, Willoughby DS (2016) Intramuscular Anabolic Signaling and Endocrine Response Following Resistance Exercise: Implications for Muscle Hypertrophy. Sport Med 46: 671–685.
- Qin X, Jiang B, Zhang Y (2016) 4E-BP1, a multifactor regulated multifunctional protein. Cell Cycle 15: 781–786.
- Ben-Sahra I, Manning BD (2017) mTORC1 signaling and the metabolic control of cell growth. Curr Opin Cell Biol 45: 72–82.
- Liu P, Ge M, Hu J, Li X, Che L, et al. (2017) A functional mammalian target of rapamycin complex 1 signaling is indispensable for c-Myc-driven hepatocarcinogenesis. Hepatol 66: 167–181.
- Shi Y, Yang Y, Hoang B, Bardeleben C, Holmes B, et al. (2016) Therapeutic potential of targeting IRES-dependent c-myc translation in multiple myeloma cells during ER stress. Oncogene 35: 1015–1024.
- Müller A, Zang C, Chumduri C, Dörken B, Daniel PT, et al. (2013) Concurrent inhibition of PI3K and mTORC1/mTORC2 overcomes resistance to rapamycin induced apoptosis by down-regulation of Mcl-1 in mantle cell lymphoma. Int J Cancer 133: 1813–1824.
- Zhang Y, Li X, Tan S, Liu X, Zhao X, et al. (2017) Mcl-1 expression and JNK activation induces a threshold for apoptosis in Bcl-xL-overexpressing hematopoietic cells. Oncotarget 8: 11042–11052.
- Ishikawa C, Senba M, Mori N (2017) Butein inhibits NF-κB, AP-1 and Akt activation in adult T-cell leukemia/lymphoma. Int J Oncol 51: 633–643.
- Ishikawa C, Jomori T, Tanaka J, Senba M, Mori N (2016) Peridinin, a carotenoid, inhibits proliferation and survival of HTLV-1-infected T-cell lines. Int J Oncol 49: 1713–1721.
- Jaworski E, Narayanan A, Van Duyne R, Shabbeer-Meyering S, Iordanskiy S, et al. (2014) Human T-lymphotropic Virus Type 1-infected Cells Secrete Exosomes That Contain Tax Protein. J Biol Chem 289: 22284–22305.
- Vajente N, Trevisan R, Saggioro D (2009) HTLV-1 Tax protein cooperates with Ras in protecting cells from apoptosis. Apoptosis 14: 153–163.
- Stoppa G, Rumiato E, Saggioro D (2012) Ras signaling contributes to survival of human T-cell leukemia/lymphoma virus type 1 (HTLV-1) Tax-positive T-cells. Apoptosis 17: 219–228.
- Lin HC, Hickey M, Hsu L, Medina D, Rabson AB (2005) Activation of human T cell leukemia virus type 1 LTR promoter and cellular promoter elements by T cell receptor signaling and HTLV-1 Tax expression. Virology 339: 1–11.
- Mukai R, Ohshima T (2014) HTLV-1 HBZ positively regulates the mTOR signaling pathway via inhibition of GADD34 activity in the cytoplasm. Oncogene 33: 2317–2328.
- Ichikawa T, Nakahata S, Fujii M, Iha H, Morishita K (2015) Loss of NDRG2 enhanced activation of the NF-κB pathway by PTEN and NIK phosphorylation for ATL and other cancer development. Sci Rep 5: 12841.
- Tomita M, Matsuda T, Kawakami H, Uchihara JN, Okudaira T, et al. (2006) Curcumin targets Akt cell survival signaling pathway in HTLV-I-infected T-cell lines. Cancer Sci 97: 322–327.
- Kawata T, Tada K, Kobayashi M, Sakamoto T, Takiuchi Y, et al. (2018) Dual inhibition of the mTORC1 and mTORC2 signaling pathways is a promising therapeutic target for adult T-cell leukemia. Cancer Sci 109: 103–111.
- Katsuya H, Cook LBM, Rowan AG, Satou Y, Taylor GP, et al. (2018) Phosphatidylinositol 3-kinase- δ (PI3K- δ) is a potential therapeutic target in adult T-cell leukemia-lymphoma. Biomarker Research 6: 24.
- Ishikawa C, Senba M, Mori N (2018) Effects of NVP-BEZ235, a dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, on HTLV-1-infected T-cell lines. Oncol Lett 15: 5311–5317.
Citation: Naghinezhad J (2019) PI3K/AKT/mTOR Pathway in ATLL: from Basic Biology to Preclinical Study. J Cell Mol Pharmacol 3: 104.
Copyright: © 2019 Naghinezhad J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 8353
- [From(publication date): 0-2019 - Nov 22, 2025]
- Breakdown by view type
- HTML page views: 7298
- PDF downloads: 1055