Research Article Open Access
Phytoplankton Abundance Constrains Planktonic Energy Subsidy to Benthic Food Web
Peiyu Zhang1,2, Huijuan Tang3, Zhijun Gong4, Ping Xie1 and Jun Xu1*1Donghu Experimental Station of Lake Ecosystems, State Key Laboratory of Freshwater Ecology and Biotechnology of China, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, P. R. China
2Graduate School of the Chinese Academy of Sciences, Beijing 100080, P. R. China
3College of Environment and Natural Resources, South China Agricultural University, Guangzhou 510642, P.R. China
4Nanjing Institute of Geography and Limnology, Chinese Academy of Science, Nanjing 210008, P. R. China
- *Corresponding Author:
- Jun Xu
Donghu Experimental Station of Lake Ecosystems
State Key Laboratory of Freshwater Ecology and Biotechnology of China
Institute of Hydrobiology, Chinese Academy of Sciences
Wuhan 430072, P. R. China
Tel: +86-27-6878 0195
E-mail: xujun@ihb.ac.cn
Received date: August 15, 2013; Accepted date: October 25, 2013; Published date: November 01, 2013
Citation: Zhang P, Tang H, Gong Z, Xie P, Xu J (2013) Phytoplankton Abundance Constrains Planktonic Energy Subsidy to Benthic Food Web. J Ecosys Ecograph 4:139. doi:10.4172/2157-7625.1000139
Copyright: © 2013 Zhang P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Ecosystem & Ecography
Abstract
Linkages across habitats between habitats are fundamental components of ecological processes. As a major characteristic of within-lake food webs, linkage of benthic and planktonic food webs has received a great deal of attention in recent aquatic studies. However, planktonic energy subsidy to benthic consumers has been infrequently tested in experiment. In a field experiment manipulating nutrients and phytoplankton abundance, we ask how changes in phytoplankton abundance affect planktonic energy subsidy to benthic primary consumers across nutrient levels. Our results suggest that the planktonic subsidy to benthic primary consumers depends on the phytoplankton abundance. Furthermore, highly taxon-specific planktonic energy subsidy to benthic primary consumers suggests that the benthic community composition partially determines the degree of the planktonic-benthic linkage. Because shallow and eutrophic lakes distribute worldwide, and many ecological functions are mediated by planktonic-benthic linkage, it will be important to consider these findings to protection and restoration of lake ecosystems.
Keywords
Chironomidae larvae; Feeding strategy; Oligochaeta; Resource; Stable carbon isotope
Introduction
Linkages across ecosystems and within-ecosystem segregated habitats are fundamental components of all ecological systems, which can affect ecological compartments ranging from individual organisms to entire ecosystems [1-3]. Therefore, elucidating the constraints on ecosystem linkages is necessary for understanding ecological processes and functions, and is also in favor of ecosystem services and management [1-3]. In lakes, linkages of distinct habitats can influence productivity, resource abundance, consumer behavior, and trophic interactions in the basic components of food webscross spatial boundaries [1-3]. Recent researches have importance of linkages of Lake Ecosystem, including benthic–planktonic and subsurface–surface within a particular aquatic ecosystem, and river–lake, wetland–lake, and terrestrial–lake among ecosystems [3-6]. These linkages are coupled by nutrient and energy flows through physical and biological activities.
As one of the major characteristics of within-lake food webs, the linkage of benthic and planktonic (or pelagic) food webs has received a great deal of attention in recent aquatic studies [2,3]. The magnitude of planktonic-benthic linkage is constrained by various factors, such as lake trophic status, morphometry, and hydrodynamics [2,3,7]. Eutrophication is one of the most common water quality problems that affect the status of lake trophic worldwide. In eutrophic ecosystems, the increased input of anthropogenic nutrients from watersheds with intense human activity [8,9] causes the imbalance in the primary producers of the food web; this results in high levels of phytoplankton biomass[10] that, in turn, alters the pathways and the magnitude of planktonic-benthic linkage in lakes. Therefore, planktonic energy subsidy to benthic consumers may increase [3,11]. However, this hypothesis has been rarely tested experimentally in planktonic-benthic linkage in eutrophic lakes.
Assessment of the responses of benthic food web to resource perturbations in plantonic food web leads to a comprehensively understanding of the energy pathways and the subsequent dynamics of food webs [2,3,12]. Therefore, in a field experiment manipulating nutrients and afterwards phytoplankton abundance, we ask how changes in phytoplankton abundance affect planktonic energy subsidy to benthic consumers across nutrient levels. We focus our analysis on quantifying planktonic energy subsidy to benthic food web that indicates the importance of direct resource perturbations of planktonicbenthic linkage in ecosystems.
Materials and Methods
Mesocom experimental design
To investigate the effect of planktonic energy subsidy on benthic zoobenthos, we manipulated nutrient supply of both water column and sediment in 8 polyethylene 8000L mesocosms in Lake Donghu near the Donghu Experimental Station of Lake Ecosystems, Chinese Ecosystem Research Network. We used a full-factorial design, with 2 levels of water nutrients (high and low) and 2 levels of sediment nutrients (high and low) for a total of 4 treatments with 2 replicates of each. Nutrient supply in both water column and sediment was manipulated by filling the mesocosms with water and sediment (thickness 5cm) from a hypereutrophic site (water TP=0.172 ± 0.022 and sediment TP=4.017 ± 0.017 mgg-1) and a mesoeutrophic site (0.025 ± 0.003 and sediment TP=0.777 ± 0.031 mgg-1) at the lake, providing local assemblages of plankton and zoobenthos and allowing us to assess their response to nutrients. In addition, fishes were removed by netting before the experiment. In the sediment, three tolerate species of zoobenthos, Branchiura sowerbyi (Oligochaeta: tubificidae), Limnodrilus hoffmeisteri (Oligochaeta: tubificidae) and Tanypus chinensis (Diptera: Chironomidae), constitutes 80%–95% of the total benthic macro invertebrate density and biomass in this eutrophic lake [13,14]. The experiment lasted for 11 weeks from July 1 to September 21, 2001, until the effect of planktonic energy subsidy on benthic food web was evaluated.
Nutrient analysis and phytoplankton monitoring
Total phosphorus concentration in the water column was measured by colorimetry after digestion of the total samples with K2S2O8+NaOH to orthophosphate [15]. Total phosphorus content in the sediment was measured by H2SO4-HClO4 digestion-molybdenum blue colorimetric method [15]. Phytoplankton samples were preserved with 1% acidified Lugol’s iodine solution and concentrated to 30 ml after undergoing sedimentation for 48 h. After mixing, 0.1 ml concentrated samples were counted and phytoplankton species were identified directly using a Leitz microscope with a magnification of 400. Phytoplankton biomass was estimated from approximate geometric volumes of each taxon, assuming that 1 mm3,/ equaled 10-6 μg fresh weights. The geometric dimensions were measured on ~30 individuals for each dominant species in each sample.
Benthic invertebrate samplings
Three abundant benthic macroinvertebrate species in enclosures, including B. sowerbyi, L. hoffmeisteri and T. chinensis were sampled at the end of the experiment for stable isotope analyses by collecting constant surface sediments using an Ekman grab to gain enough analytical biomass. Animals were sorted and identified from samples while alive, placed in filtered lake water overnight to clear their guts, then oven-dried to a constant weight at 60°C, before being finally ground to a fine homogeneous powder with a mortar and pestle for δ13C. Lipids were extracted using methanol-chloroform (2:1 by volume) because lipids are depleted in 13C [16,17]. The mortar and pestle were acid-washed and dried to prevent cross-contamination between samples. The powdered samples were kept in acid-washed glass tubes and sealed in desiccators with silica gel for future analysis.
Isotopic baseline sampling
Sedimentary organic matters and seston were collected at each mesocosms at the end of the experiment. After collection and transport to the laboratory, the samples were acidified with superfluous 1 N HCl and oven-dried to a constant weight at 60°C. Samples were then ground to a fine homogeneous powder with a mortar and pestle. The mortar and pestle were acid-washed and dried to prevent cross-contamination between samples. The powdered samples were kept in acid-washed glass tubes and sealed in desiccators with silica gel for future analysis.
Stable isotope analyses
Stable carbon isotope ratios of seston, sediment organic matter, and invertebrate were analyzed with Delta Plus (Finnigan, German) continuous-flow isotope ratio mass spectrometer (CF–IRMS) directly coupled to an NC2500 elemental analyzer (Carlo Erba, Italy). The isotopic compositions of samples were expressed as δ13C notation using the following equation:
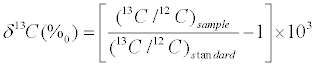
where 13C/12C is the atomic ratios of the number of atoms in the sample or standard, δ is the measure of heavy to light isotope in the sample, and higher δ values denote a greater proportion of the heavy isotope. The international standard is Vienna Pee Dee Belemnite (VPDB). Carbonatite (IAEA–NBS18) was used as the international reference material; on a daily basis, an internal working standard, urea (δ13C=- 49.44‰), was used. More than 20% of the samples were analyzed for more than three times, and the standard deviations of δ13C replicate analyses were less than 0.2‰.
Modeling the planktonic energy subsidy of zoobenthos
In the present study, the seston and sediment organic matter were used to quantify the isotopic baseline for benthic and planktonic energetic pathways. Since the diet of the zoobenthos was switched from the lake to the mesocoms, the new dietary δ13C signature was expected to manifest itself rapidly in the body tissues of the small invertebrates after several days as previous study suggested [18]. The δ13C values of the three dominant benthic species in each mesocoms at the end of the experiment were converted into proportions of planktonic and benthic energetic pathways with a two end-member mixing model using the software ISOERROR 1.04 [19]. When calculating mixing models, ISOERROR takes into account the variability in the δ13C of both the sources and the zoobenthos, and provides 95% confidence intervals around the estimated proportions.
Statistical Analysis
To test the correlations between phytoplankton biomass and the percentage of planktonic energy, simple linear regression was selected which was performed using SPSS statistical software for Windows (Version 17.0, SPSS Inc.).
Results
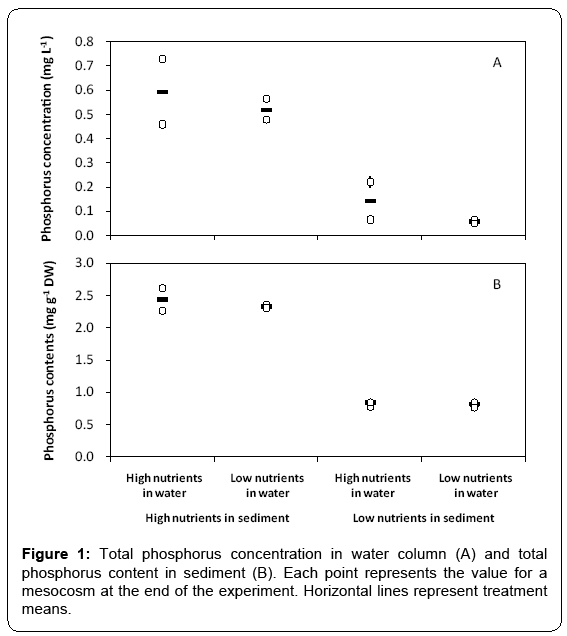
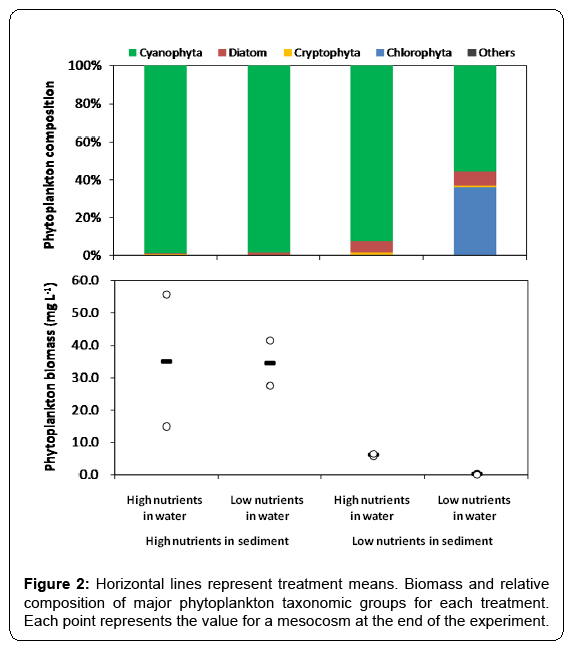
At the end of the experiment which lasted for 11 weeks, total phosphorus concentration in water column varied between 0.07 and 0.73 mg/L (Figure 1A), and total phosphorus content in sediment varied between 0.76 and 2.61 mg g-1 (Figure 1B) in the mesocosms. Total phosphorus concentration in water column was much higher in the treatments with high total phosphorus content in sediment than in those of low contents. Across all treatments, microscopic examination showed that Cyanophyta was dominant in phytoplankton (Figure 2). The total biomass of phytoplankton varied between 0.05 and 55.62 mg L-1. Biomass of phytoplankton was much higher in the treatments with high total phosphorus content in sediment than in those of low contents (Figure 2), and a significant positive relationship between phytoplankton biomass and total phosphorus concentration in water column was observed across all mesocosms (F1, 6=18.012, p < 0.01, r2=0.757).
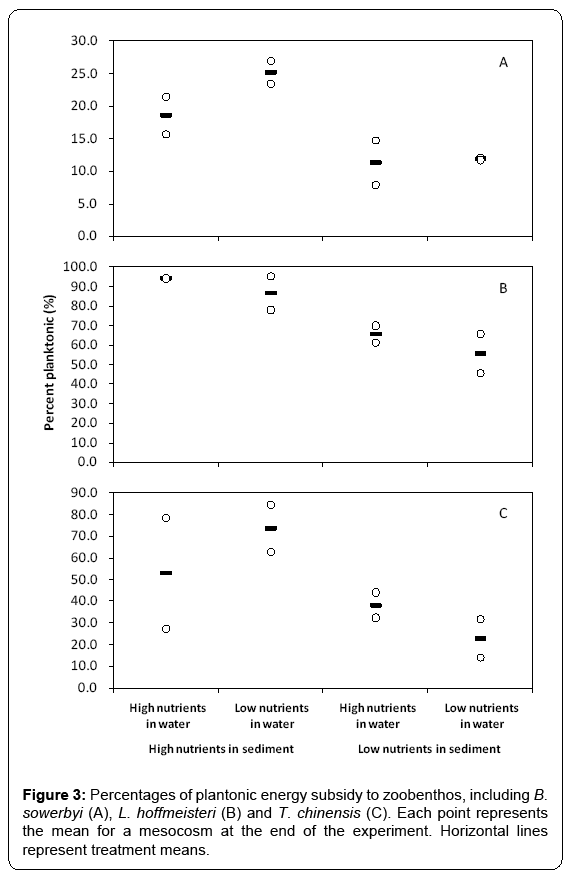
The contribution of planktonic energy subsidy to benthic animals, which was estimated using mixing models, ranged from 7.9% to 26.8% for B. sowerbyi (Figure 3A), from 45.5% to 95.0% for L. hoffmeisteri (Figure 3B), and from 13.9% to 84.4% for T. chinensis (Figure 3C), showing significant intra-species difference across all mesocosms. In the treatments with high total phosphorus content in sediment, planktonic energy subsidy to zoobenthos was higher with high phytoplankton biomass in the water column than those treatments with low phytoplankton biomass in the water column at the end of the experiment (Figure 3A-3C). We used linear regression to better understand the phytoplankton production mediating the planktonic energy subsidy to B. sowerbyi (Figure 4A), L. hoffmeisteri (Figure 4B) and T. chinensis (Figure 4C), respectively. There were significant positive relationships between percentage of planktonic carbon in zoobenthos and the phytoplankton biomass (PB) (Planktonic B. sowerbyi=11.745 + 0.261PB, F1, 6=12.993, p < 0.05, r2=0.683; Planktonic L. hoffmeisteri=63.835 + 0.612PB, F1, 6=5.683, p < 0.05, r2 =0.490; Planktonic T. chinensis=27.455 + 1.024PB, F1, 6=12.941, p < 0.05, r2=0.682).
Figure 4: Relationships between the percentages of plantonic energy subsidy to zoobenthos, including B. sowerbyi (A), L. hoffmeisteri (B) and T. chinensis (C), and the phytoplankton production. Each point represents the value for an individual mesocosm. Best fit lines, r2, and P values are given for each relationship, using linear regression.
Discussion
Benthic and planktonic food webs in shallow water bodies are thought to be linked by trophic relationships and by biologicallymediated or physically-mediated resuspension or sedimentation processes [2,3,12]. Therefore, focusing only on benthic or only on planktonic components of aquatic food webs can lead to an ambiguous comprehension of the interactions between components in different habitats [3,12,20]. In natural water bodies, seston samples comprise a variable fraction of the particulate organic carbon and are not easily separated from non-phytoplankton particulate organic carbon [20-23], which results in uncertainty in the evaluation of the importance of planktonic sources to the benthic food web using natural abundances of stable isotopes. Here, the results of our experiment provided a unique opportunity to test responses of planktonic energy subsidy to benthic invertebrates along a gradient of phytoplankton abundance, and to assess the magnitude of the responses.
In the experiment, the planktonic subsidy increases in Chironomidae and oligochaetes along the evident gradient of phytoplankton abundance, which supports the general paradigm claims that benthic secondary production is strongly linked to planktonic primary production. This view has also been supported by field observations that benthic secondary production is high in eutrophic lakes [24] and that benthic secondary production increases when lakes are experimentally fertilized [11,25,26]. With the enrichment of nutrients in eutrophic aquatic ecosystems, not only can zooplanktons benefit from the increased phytoplankton production, many benthic invertebrates can also benefit from and regulate increases in phytoplankton biomass or production [11,25,27] by direct filter feeding or by collecting newly sedimented phytoplankton. For example, sedimentation of phytoplankton served as the major carbon resource in the production of benthic amphipods (Diporeia) in Lake Michigan [28]. Our study, along with others, clearly shows that benthic secondary production is strongly linked to pelagic production in eutrophic lakes.
Planktonic-benthic linkage can be affected by abiotic factors, such as lake morphometry, hydrodynamics and trophic status, all of which influence the magnitude of phytoplankton biomass, the rate of degradation, the velocity of downward transport, and the biological activities [3,5]. Increases in phytoplankton cause significant increases in secondary production by enhancing the planktonic energy subsidy to both planktonic and benthic animals. In addition, benthic food web structure can play an important role in efficiency of converting planktonic resources into benthic invertebrate biomass [26]. In the current study, highly taxon-specific planktonic energy subsidy to the three dominant invertebrate species suggests that the benthic community composition constrains the efficiency of planktonic energy subsidy to benthic food web. For example, a community dominated by macrofaunal consumers that graze directly on algal phytodetritus could transfer the energy from deposited diatoms to higher trophic levels (fish) more efficiently than a community with large populations of meiobenthic invertebrates [29].
The relative importance of planktonic energy subsidy to benthic food web provided the understanding of the key ecological processes that functionally link these habitats in aquatic ecosystems. For example, strong evidence has been provide that benthic invertebrate feeding on phytoplankton is widespread through filter-feeding phytoplankton directly from the water column and/or collecting newly sedimented phytoplankton [20,24,30]. Thus, benthic animal can play an important role in organizing the planktonic community through their grazing or predatory activity [12,31,32]. In addition, extensive zoobenthivory can subsidize fish populations, leading to apparent competition, if not altering trophic dynamics and ecosystem processes, in the pelagic zone [33,34].
Conclusively, our results claims the generally accepted paradigm that benthic secondary production is strongly linked to pelagic primary production [2,3]. The linkage of benthic-planktonic food webs remains an important area for research considering that the majority of lakes globally are small and shallow [20,35], and that eutrophication is one of the most common water quality problems in lakes worldwide [36,37]. The planktonic energy subsidy to benthic food web documented here is probably characteristic to the majority of eutrophic lakes worldwide, and thus is important for protecting ecosystems and for restoring critical habitats of natural linkages before ecosystem structure and function return [1,3].
Acknowledgements
We thank the members of the Donghu Experimental Station of Lake Ecosystem, Chinese Ecological Research Network for their field and laboratory assistance. This research was partially supported by the National Natural Science Foundation of China (Grant Nos. 31170439 and 30870428 to JX and Grant No. 30800145 to HT) and the National Basic Research Program of China (Grant No. 2008CB418001-1).
References
- Polis GA, Anderson WB, Holt RD (1997) Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu Rev Ecol Syst 28: 289-316.
- Schindler DE, Scheuerell MD (2002) Habitat coupling in lake ecosystems. Oikos 98: 177-189.
- Lamberti GA, Chaloner DT, Hershey AE (2010) Linkages among aquatic ecosystems. J N Am Benthol Soc 29: 245-263.
- Blumenshine S, Vadeboncoeur Y, Lodge D, Cottingham K, Knight S (1997) Benthic-pelagic links: responses of benthos to water-column nutrient enrichment. J N Am Benthol Soc 16: 466-479.
- Dolson R, McCann K, Rooney N, Ridgway M (2009) Lake morphometry predicts the degree of habitat coupling by a mobile predator. Oikos 118: 1230-1238.
- Gratton C, Vander Zanden MJ (2009) Flux of aquatic insect productivity to land: comparison of lentic and lotic ecosystems. Ecology 90: 2689-2699.
- Doi H, Chang KH, Ando T, Imai H, Nakano Si (2010) Shoreline bank construction modifies benthic–pelagic coupling of food webs. Ecol Eng 36: 601-604.
- Carpenter S, Kitchell J, Hodgson J, Cochran P, Elser J, et al. (1987) Regulation of lake primary productivity by food web structure. Ecology 68: 1863-1876.
- Carpenter SR, Kitchell JF (1996) The trophic cascade in lakes. Cambridge University Press.
- Jacoby JM, Collier DC, Welch EB, Hardy FJ, Crayton M (2000) Environmental factors associated with a toxic bloom of Microcystis aeruginosa. Can J of Fish Aquat Sci 57: 231-240.
- Welch HE, Jorgenson JK, Curtis MF (1988) Emergence of Chironomidae (Diptera) in fertilized and natural lakes at Saqvaqjuac, NWT. Can J of Fish Aquat Sci 45: 731-737.
- Threlkeld ST (1994) Benthic-pelagic interactions in shallow water columns: an experimentalist's perspective. Hydrobiologia 275: 293-300.
- Gong Z, Xie P (2001) Impact of eutrophication on biodiversity of the macrozoobenthos community in a Chinese shallow lake. J Freshwater Ecol 16: 171-178.
- Xu J, Xie P (2004) Studies on the food web structure of Lake Donghu using stable carbon and nitrogen isotope ratios. J Freshwater Ecol 19: 645-650.
- Xie L, Xie P, Li S, Tang H, Liu H (2003) The low TN:TP ratio, a cause or a result of Microcystis blooms? Water Res 37: 2073-2080.
- Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annual review of ecology and systematics 18: 293-320.
- Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83: 703-718.
- Schmidt O, Scrimgeour CM, Curry JP (1999) Carbon and nitrogen stable isotope ratios in body tissue and mucus of feeding and fasting earthworms (Lumbricus festivus). Oecologia 118: 9-15.
- Phillips DL, Gregg JW (2001) Uncertainty in source partitioning using stable isotopes. Oecologia 127: 171-179.
- Vadeboncoeur Y, Vander Zanden MJ, Lodge DM (2002) Putting the Lake Back Together: Reintegrating Benthic Pathways into Lake Food Web Models: Lake ecologists tend to focus their research on pelagic energy pathways, but, from algae to fish, benthic organisms form an integral part of lake food webs. Bioscience 52: 44-54.
- Gu B, Alexander V, Schell D (1999) Seasonal and interannual variability of plankton carbon isotope ratios in a subarctic lake. Freshwater Biol 42: 417-426.
- Lehmann MF, Bernasconi SM, McKenzie JA, Barbieri A, Simona M, et al. (2004) Seasonal variation of the d13C and d15N of particulate and dissolved carbon and nitrogen in Lake Lugano: Constraints on biogeochemical cycling in a eutrophic lake. Limnol Oceanogr 49: 415-429.
- Xu J, Li S, Xie P (2005) Differences in delta13C and delta15N of particulate organic matter from the deep oligotrophic Lake Fuxian connected with the shallow eutrophic Lake Xingyun, People's Republic of China. Bull Environ Contam Toxicol 74: 281-285.
- Jónasson PM (1972) Ecology and production of the profundal benthos in relation to phytoplankton in Lake Esrom. Munksgaard.
- Davies I (1980) Relationships between dipteran emergence and phytoplankton production in the Experimental Lakes Area, northwestern Ontario. Can J of Fish Aquat Sci 37: 523-533.
- Hershey A, Beaty S, Fortino K, Kelly S, Keyse M, et al. (2006) Stable isotope signatures of benthic invertebrates in arctic lakes indicate limited coupling to pelagic production. Limnol oceanogr 51: 177-188.
- Carpenter SR, Kitchell JF, Cottingham KL, Schindler DE, Christense DL, et al. (1996) Chlorophyll variability, nutrient input, and grazing: evidence from whole-lake experiments. Ecology 77: 725-735.
- Fitzgerald SA, Gardner WS (1993) An algal carbon budget for pelagic-benthic coupling in Lake Michigan. Limnol oceanogr 38: 547-560.
- Strayer DL (1991) Perspectives on the size structure of lacustrine zoobenthos, its causes, and its consequences. J N Am Benthol Soc 10: 210-221.
- Lindegaard C (1994) The role of zoobenthos in energy flow in two shallow lakes. Hydrobiologia 275: 313-322.
- Wildish D, Kristmanson D (1984) Importance to mussels of the benthic boundary layer. Canadian J Fisheries and Aquatic Sciences 41: 1618-1625.
- Kelly JR, Berounsky VM, Nixon SW, Oviatt CA (1985) Benthic-pelagic coupling and nutrient cycling across an experimental eutrophication gradient. Marine Ecology Progress Series 26: 207-219.
- Zanden MJV, Rasmussen JB (1999) Primary consumer d13C and d15N and the trophic position of aquatic consumers. Ecology 80: 1395-1404.
- Vander Zanden MJ, Vadeboncoeur Y (2002) Fishes as integrators of benthic and pelagic food webs in lakes. Ecology 83: 2152-2161.
- Wetzel RG (1990) Land-water interfaces: metabolic and limnological regulators. Internationale Vereinigung fuer Theoretische und Angewandte Limnologie Verhandlungen IVTLAP 24:
- Ryding S-O, Rast W (1989) Control of eutrophication of lakes and reservoirs. Manual the biosphere series 1:
- Harper D (1992) Eutrophication of freshwaters: principles, problems and restoration. Chapman & Hall, London 2:364.
Relevant Topics
- Aquatic Ecosystems
- Biodiversity
- Conservation Biology
- Coral Reef Ecology
- Distribution Aggregation
- Ecology and Migration of Animal
- Ecosystem Service
- Ecosystem-Level Measuring
- Endangered Species
- Environmental Tourism
- Forest Biome
- Lake Circulation
- Leaf Morphology
- Marine Conservation
- Marine Ecosystems
- Phytoplankton Abundance
- Population Dyanamics
- Semiarid Ecosystem Soil Properties
- Spatial Distribution
- Species Composition
- Species Rarity
- Sustainability Dynamics
- Sustainable Forest Management
- Tropical Aquaculture
- Tropical Ecosystems
Recommended Journals
Article Tools
Article Usage
- Total views: 14291
- [From(publication date):
March-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 9730
- PDF downloads : 4561