Research Article Open Access
Phytoextraction Ability of Amaranthus mangostanus L. from Contaminated Soils with Cs or Sr
| Wang Dan1,2*, Zhang Xiaoxue1, Luo Xuegang2 and Tang Yunlai1,2 | |
| 1College of Life Science and Engineering, Southwest University of Science and Technology, China | |
| 2Defense Key Laboratory of the Nuclear Waste and Environmental Security, China | |
| Corresponding Author : | Wang Dan College of Life Science and Engineering Southwest University of Science and Technology, China Tel: +86-816-6089126 E-mail: wangdan@swust.edu.cn |
| Received December 29, 2014; Accepted February 24, 2015; Published February 27, 2015 | |
| Citation: Dan W, Xiaoxue Z, Xuegang L, Yunlai T (2015) Phytoextraction Ability of Amaranthus mangostanus L. from Contaminated Soils with Cs or Sr. J Bioremed Biodeg 6:277. doi:10.4172/2155-6199.1000277 | |
| Copyright: © 2015 Dan W, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The uptake, distribution, and accumulation of 133Cs and 88Sr with its antioxidant responses in Amaranthus mangostanus L plants was studied during cultivation in outdoor potted-soil. The results showed that the uptake capacity of Amaranthus for 133Cs was higher than that for 88Sr at the same molarity treatment where the concentration of 88Sr or 133Cs in the soil was from 0.1 mmol/kg to 5 mmol/kg. The maximum 133Cs and 88Sr uptake of total plant was 3535.2 mg/kg dw and 639.4 mg/kg, respectively. Amaranth had much stronger capacity for absorbing 133Cs than for 88Sr. The 133Cs BCF of amaranth above-ground part and its total plant at the different concentrtion treatments in the soil was higher than for the 88Sr BCF except at the 0.1 mmol/kg treatments. The TF was lower in the lower concentration (0.1 and 0.5 mmol/kg) than that in the higher concentration of 133Cs and 88Sr in the soil (1 and 5 mmol/ kg). Amaranth could be used as a potential candidate plant for phytoremediation of 137Cs and 90Sr. The MDA content under 88Sr stress was higher than that under 133Cs stress, and the activities of CAT under 88Sr stress were less than that under 133Cs stress.
| Keywords |
| Amaranth; Uptake; Accumulation; 133Cs; 88Sr; Phytoextraction |
| Introduction |
| The radionuclides comging from nuclear test or nuclear accident are the main potential nuclear pollutants for environment and are widely paid attention to the phytoremediation technology of radioactive pollution can achieve the goal of clearing radionuclides, remediating or controling contaminated soils. It has many merits, such as low investment, low maintenance cost, convenient manipulation, no the second-time contamination, safety and the function of beautifying the environment [1-4]. The phytoextraction and remediation technology of contaminated soils with low-radioactive nuclides is the popular issues now, and it is considered to be cost-effective for cleaning up sites contaminated with low levels of long half-life radionuclides [5-7]. The selection of hyper accumulators for the different nuclides or metals, their uptake ability and accumulation capacity for the radionuclides is the most important factors which influence the remediation efficiency. Problems such as how to screen a series of plants with appropriate biological characteristics that can rapidly accumulate significant quantities of radionuclides, and how to find a way to enhance the bioavailability of radionuclides to the plants, should be addressed [8-11]. The contamination of soil with 90Sr and 137Cs has long-term radiological and health impacts due to their long half-lives and chemical similarities with two essential elements required for plant growth, Ca2+ and K+, respectively [12-14]. Some research reported that the 88Sr and 134Cs uptake ability of plant from the soil was the same with their 90Sr and 137Cs uptake ability as the stable isotopes of 137Cs and 90Sr. 133Cs and 88Sr can imitate the migration and distribution of 137Cs and 90Sr in the plant soil system very well [15,16]. Amaranthus mangostanus L. was chosen as the research material in this experiment to investigate its characteristics of uptake, transportation, and accumulation and its physiological mechanism in 133Cs and 88Sr polluted soils, and also to lay some theoretical foundations for phytoremediation of soil contamination with radionuclides. |
| Materials and Methods |
| Plant material and treatment |
| The experiment was carried out with outdoor potted plants in the lab block of life science in Southwest University of Science and Technology(SWUST) in Mianyang, Sichuan (E=104°42', N=31°32', elevation: 462 m). The soil in the experiment was yellow earth, coming from the nursery in the garden center of SWUST. The pH of the soil in H2O and KCl were measured with a pH meter. The soil organic matter was measured by using the Tyurin’s method; each gram of grinded and sieved soil was mixed even with 5 ml deionized water, and the electrical conductivity of the soil was measured with a conductivity meter after it was static. The main physical and chemical characteristics of the soil were: pH in H2O:7.06, pH in KCl: 6.18, Organic matter: 1.35%, Electrical conductivity: 1.25 ms/cm. |
| Each pot (diameter 25 cm, height 20 cm) was put into 4.5 kg of soil and some calcium superphosphate and clean water was sprayed before sowing. The plants of Amaranthus mangostanus L were transplanted into each treatment group when the plant had 5-6 leaves. There were 3 plants for each pot, 3 pots for each treatment, with three replications. Since the radionuclides were set on the soil surface by sedimentation and enters the inside with the time being, 133CsCl or 88Sr(NO3)2 solution was sprayed separately and evenly on the soil surface of each pot based on the concentrations in Table 1 in the next day after transplanting. The treatments of 133Cs and 88Sr were divided into two groups. |
| The plants were watered regularly during the experiment and maintained a field water capacity of 70%. The management level for each treatment field was the same when maturation the plants were harvested to be measured. |
| Measuring the physiological and biochemical indexes |
| The leaves from the plants were taken away and each physiological index was measured after harvesting. The MDA content was measured with the TBA colorimetry [17]. The total chlorophyll content was measured at 652 nm with 95% ethanol colorimetry and calculated [17]. The activity of CAT was measured with the ultraviolet absorption method [17]. The activity of the POD and SOD was measured with Li’s method [17]. The experiment results were the average values of three repeated tests. |
| Measuring the 133Cs and 88Sr contents |
| The plants of Amaranthus mangostanus L were harvested after maturation and divided into two parts, viz. roots or above-ground parts. The weeds and stones in the air-dried amaranth plant sample were removed and passed through 2 mm sieve, then rinsed in deionized water. They were dried to a constant weight in an oven and then measured for dry weight. Different parts of plants were pulverized with a stainless steel blade. The sample plants were washed with distilled water, weighed exactly 0.5 g after-sample and placed in the conical flask, in addition, 10 mL acid mixture (volume ratio of nitric acid: perchloric acid was 3:1) was added to it. Having been covered for an overnight, the liquid samples were washed into the Kjeldahl flask and dispelled on the electric stove until it began to give out white smoke. The digestive juice was colorless and transparent. It was kept into the constant volume of 50 ml with 0.5 mol/ L nitric acid. The graphite furnace atomic absorption spectroscopy (AA700, Perkin Elmer Company, USA) was used to measure the content of 133Cs and 88Sr. |
| Measuring the biomass |
| The whole plants were used for biomass measurement after harvesting. The plant samples were washed with distilled water and separated into the above-ground parts and the root parts with scissors. Firstly, samples were air-dried in cool and well-ventilated places, then were oven dried (68ΓΆΒ?Β? ± 2ΓΆΒ?Β?, 24 h). The dry weight of each part was weighed to calculate the biomass. |
| Determination of the TF value and CF |
| TF (transfer factor) refers to nuclide content in the above-ground parts/nuclide content in the root parts. BCF (concentration factor) refers to nuclide content in the above-ground parts or total plant/ nuclide content in the soil. |
| Data analysis |
| Microsoft Excel 2003 (U.S, Microsoft), DPS3.1 Software (China), and the Origin 6.0 mapping software (U.S., Microcal) were used for data analysis. All detecting data were repeated three times, and results were expressed in the mean ± standard deviation (Mean ± SD) form. |
| Results |
| The 133Cs or 88Sr absorption and distribution in amaranth |
| The 133Cs or 88Sr contents in the plants were measured after harvesting and the results were shown in Table 2. |
| It can be found from the Table 2 that the content of 133Cs and 88Sr in the control plants was smaller than the minimum detectable amount. The total contents of 133Cs in the plant rose with the increase of the 133Cs concentration in the soil and there were very significant differences between all the treatments (p<0.01). The highest 133Cs content of total plant was 3535.2 ± 150.5 μg.g-1 dw, which was in the 5 mmol/kg treatment. The 133Cs content in the above-ground parts of the plant was rising with the increase of the 133Cs concentration in the soil and there were also very significant differences between all the treatment (p<0.01), too. The 133Cs content in the 5 mmol/kg treatment was the highest, which was 1820.1 ± 170.1 μg g-1dw. The variation trend of the 133Cs content in the root part was the same with as that in the above-ground part. It showed that 133Cs concentration in the soil was the most important factor which affected the 133Cs content in the plant of Amaranthus. The 88Sr distribution and transportation were the same trend as they were in the 133Cs treatment. The highest 88Sr contents of all the plant was 639.4 ± 21.9 μg.g-1 dw, which was in the 5 mmol/kg treatment, too. |
| The 133Cs or 88Sr contents in the above-ground part was lower than it in the root in 0.1mmol/kg and 0.5mmol/kg treatment and the number of their transfer factor (TF) were lower than 1. In the high level trearment (1mmol/kg and 5mmol/kg), however, the situations were opposite and the number of their TF was higher than 1. It showed that the 133Cs or 88Sr could be transported easily from the root to the aboveground parts when the 133Cs concentration in the soil was high. The TF of 133Cs or 88Sr in amaranth was similar. |
| BCF (concentration factor) can reflect the nuclide or heavy metal phytoextraction ability of plant from the soil. The Table 2 showed that the amount of 133Cs or 88Sr BCF trend of amaranth above-ground and its total plant was similar, which declined with the rise of 133Cs or 88Sr concentration in the soil., which indicated that it was more dificulty to extrat 133Cs or 88Sr from the soil in the higher concentration treatment by the amaranth than in the lower concentration. Except in the 0.1 mmol/kg treatment, 133Cs BCFs of above ground and total plant was higher than the BCFs of 88Sr which means the 133Cs phytoextraction ability were higher than 88Sr phytoextraction ability of amaranth in the higher 133Cs or 88Sr concentration in the soil. But in the 0.1 mmol/kg 133Cs or 88Sr treatment, the 133Cs or 88Sr CF of total plants was 21.69 and 28.29, respectively. The 133Cs phytoextraction ability was lower than 88Sr phytoextraction ability of amaranth. |
| Effects of 133Cs or 88Sr on the growth and biomass distribution of amaranth |
| The biomass of the amaranth plants was measured after harvesting, and the results were showed in Table 3. It can be found from the Table 3 that the difference of total biomass was not significant to the control in the low 133Cs concentration treatments (0.1 and 0.5 mmol/ kg), while in the high concentration treatments (above 1 mmol/kg), it was significantly lower than the control and the low concentration treatments. The treatments of different 133Cs concentrations had the same trends of effects on the above-ground and root biomass of the amaranth plants. |
| For different concentrations of 88Sr treatments, the above-ground, root and total biomass all decrease with the increase of the 133Cs concentration in the soil and there were significant difference (p<0.05) except between the 0.5 and 1 mmol/kg treatment. It could be found that high concentrations of 88Sr had stronger effect on the biomass of amaranth than that of 133Cs stress. |
| Table 3 also demonstrates the biomass distribution of the aboveground and root parts of amaranth. The results showed that the 133Cs or 88Sr treatment had no significant influence on the proportion of biomass of above-ground parts and root parts to the total biomass. The biomass of above-ground parts took up 82-88% of the total biomass. The biomass of root parts took up 12-18% of the total biomass. It was universal that the biomass of the roots was lower than that of the above-ground parts. |
| The 133Cs or 88Sr extraction efficiency of the amaranth |
| The 133Cs or 88Sr extraction efficiency of amaranth in 133Cs or 88Sr treatments was calculated and the results are showed in Table 4. The Table 4 shows that the 133Cs or 88Sr concentration in the soil, concentration in the plant, contents in the plant and contents in a pot were rising gradually with the increasing concentration in the soil, but the biomass of total plant and the ratio of contents in the plant with the contents in a pot were fall gradually. The ratio of 133Cs or 88Sr contents in the plant with the contents in a pot was the highest in the 0.1 (mmol/ kg) treatments, which was 19.91% and 23.97%, respectively. When in 5(mmol/kg) treatment, the ratio was lowest, which was 3.66% or 0.76%, respectively. That means it was more difficult to uptake 133Cs or 88Sr from the soil when 133Cs or 88Sr concentration in the soil was high and it would take more time to apply amaranth to remediate high 133Cs or 88Sr concentration contaminated soil. In this test, when the 133Cs concentration in the soil was 0.1mmol/kg or 13.29 mg/kg and the ratio of content in the plant with contents in a pot was the same every time, it needed to grow amaranth plants approximately 5 times (if to grow amaranth plants 2 seasons in a year, it needed 2.5 years) theoretically to uptake all the 133Cs from the soil. But when the 133Cs concentration in the soil is 5 mmol/kg or 664.5 mg/kg, it needed to grow amaranth plants approximately 28 times (if to grow amaranth plants 2 seasons in a year, it needed 14 years). When the 88Sr concentration in the soil is 5mmol/kg or 438.10mg/kg it needed to grow amaranth plants approximately 132 times (if to grow amaranth plants 2 seasons in a year, it needed 66 years). |
| Physiological response of amaranth to 133Cs or 88Sr stress |
| The effect of 133Cs or 88Sr on the chlorophyll and MDA content of amaranth: The chlorophyll and MDA content of amaranth under the stress of 133Cs and 88Sr was shown in Figure 1. It can be found from the Figure 1 that the effects of 133Cs and 88Sr stress were similar, i.e. the chlorophyll content rose at first and then fell with the increasing of 133Cs and 88Sr content, it was the highest at the 0.5 mmol/kg treatment with the 133Cs and 88Sr treatment and the chlorophyll content decreased at the 1 mmol/kg treatment and it was the lowest at the 1 mmol/kg treatment. Moreover, the chlorophyll contents of all the treatments under the stress were significant difference (p<0.05) comparing to the control under the stress of 133Cs and 88Sr. |
| Figure 1 also showed the changes of the MDA content in amaranth leaves after different 133Cs and 88Sr treatments. It can be found from the Figure 1 that the after harvesting, the MDA content rose continually with the increasing 133Cs and 88Sr treatment concentration and all was higher than the control except at the 0.1 mmol/kg 133Cs treatment. For both 133Cs and 88Sr treatments, the highest values of their MDA content appeared at the 5 mmol/kg treatment. The MDA content of amaranth in 88Sr treatments was higher than in 133Cs treatments in different concentration133Cs and 88Sr treatments. The MDA content of all the treatments was significant difference (p<0.05) with the control under stress of 133Cs and 88Sr. It can be found that high concentrations of 133Cs and 88Sr strengthened the peroxidization of the membranes of amaranth. |
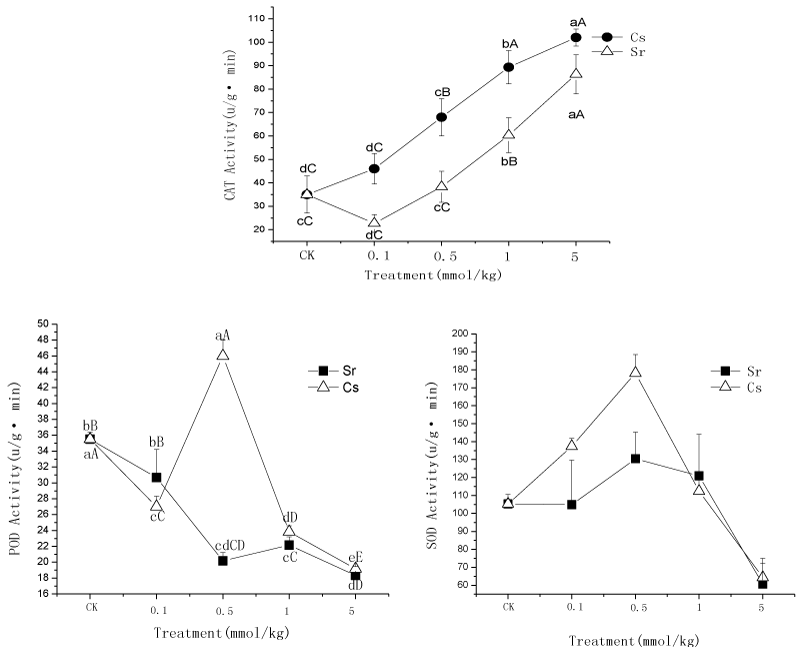
| The effect of 133Cs or 88Sr on the activity of CAT, POD, SOD of amaranth: Figure 2 showed the changes of CAT, POD, SOD activity of amaranth leaves in different 133Cs and 88Sr treatments. It can be found from the Figure 2 that after the harvest, the CAT activity rose continually with the increasing 133Cs and 88Sr concentration. While the CAT activity declined firstly in 88Sr 0.1 mmol/kg, then rose with the increasing 88Sr concentration. Both of the highest values appeared at the 5 mmol/kg treatments, the CAT activity was 102 and 86.33 U/g min in 133Cs and 88Sr treatments, respectively, the CAT activity of the control was 35 U/g min. Except the control, the CAT activity of amaranth in 88Sr treatments was lower than that in 133Cs treatments among different concentration of 133Cs or 88Sr t treatments. The result indicated that high concentrations of 133Cs and 88Sr strengthened the the CAT activity of amaranth. |
| As Figure 2 showed, the effects of 133Cs and 88Sr stress on POD activity were different. After the 133Cs treatment, the POD activities first declined then rose and decline again. It was the highest at the 0.5 mmol/kg treatments, which was 45.1 u/g.min and the lowest at the 5 mmol/kg treatments, which was 17.2 u/g.min. For the 88Sr treatments, the POD activities of all treatments were lower than the control, and declined continually with the increasing 88Sr treatments concentration. Besides, the 5 mmol/kg treatment was the lowest. |
| Figure 2 also showed that the effects of 133Cs and 88Sr stress on POD activity were different. After the 133Cs treatment, the POD activities first rose and then declined. It was the highest at the 0.5 mmol/kg treatments, which was 175.9 u/g.min and the lowest at the 5 mmol/kg treatments, it was 57.6 u/g.min and lower than the control. For the 88Sr treatments, the POD activities of all treatments were not significantly different (p<0.05) from the control, except at the 5 mmol/kg treatment, when it was the lowest. |
| Analysis of the correlation between the 133Cs and 88Sr contents and each physiological index |
| Table 5 were acquired after analyzing the correlation of the indexes including the total element content in the plant, MDA content, chlorophyll content, POD-CAT and SOD activity tested after the harvest for 133Cs and 88Sr treatments. In Table 5, the 133Cs content was positively correlated with the CAT activity (r=0.9240, p<0.05). This means the increase of the 133Cs content in the plant activated CAT activity increasing and alleviated the degree of 133Cs stress to the plant. The chlorophyll content was also positively correlated to the SOD activity (r=0.9617, p<0.01), too. Which means the increase of SOD activity accelerate chlorophyll synthesis. The correlation between other indexes was not significant. In Table 5, the 88Sr content was positively correlated to the MDA content (r=0.9697, p<0.01) and negatively correlated to the POD activity. (r=-0.8966, p<0.05) It means the increase of the 88Sr content in the plant activated MDA content increase and reduced the POD activity. The chlorophyll content was positively correlated to the SOD activity. (r=-0.9094, p<0.05) and the MDA content was positively correlated with the CAT activity. |
| Discussion and Conclusion |
| Plants vary in their abilities to uptake, translocate and sequester radionuclides [7,18,19]. The uptakes of 133Cs and 88Sr by the plants of amaranth were different when the treatment concentrations were the same in the soil. Generally, the amaranth absorbed more 133Cs than 88Sr at the same molarity treatment in the soil. The maximum 133Cs uptake of the total plant was 3535.2 mg kg-1 dw when the 133Cs concentration was 5 mmol/kg (664.5 mg/kg) in the soil, and the minimum was 301.9 mg/kg.dw when the 133Cs concentration was 0.1 mmol/kg (13.92 mg/kg) in the soil. The maximum 88Sr uptake of total plant was only 639.4 mg/kg. dw when the 88Sr concentration was 5 mmol/kg (438.7 mg/kg)in the soil, and the minimum was only 247.8 mg/kg. dw when the 88Sr concentration was 0.1 mmol/kg (8.76 mg/kg) in the soil. The result in this study was opposite to Dan Wang’s previews reported that accumulation of 88Sr was recorded higher than 134Cs in radish plantlets [20]. It showed that amaranth had much stronger capacity of absorbing 133Cs than 88Sr. Based on the effects of the 133Cs and 88Sr stress on the biomass of the plants, the maximum uptake of 133Cs or 88Sr by amaranth reached 36.437 mg and 5.00 mg per plant, respectively. The 133Cs BCF of the above-ground parts and the total plant of amaranth in different concentration treatments in the soil were higher than the 88Sr BCF except in the 0.1 mmol/kg treatment. The TF trend of amaranth for 133Cs and 88Sr were the same and at the lower 133Cs and 88Sr concentration (0.1and 0.5 mmol/kg), the accumulation of 133Cs and 88Sr was found higher in roots compared with when it was above –ground parts, while at higher 133Cs and 88Sr concentration (1 and 5 mmol/kg), 133Cs and 88Sr accumulation was more when it was in above -ground parts than it was in roots. The result was the same as Shraddha Singh’ result [6], which means the transfer capacity of 133Cs and 88Sr to the above-ground parts, was similar. Some researchers reported many plant species uptake more Sr than Cs when the concentration of Cs or Sr is same in the soil or hydroponic medium [6,16,20]. Which means it is more difficult to remediate Cs pollution than Sr with phytoremediation technology. The stronger uptake capacity of amaranth for 133Cs in low concentration was favorable for the restoration of the soils of low level Cs pollution. The present study suggests that amaranth can be used as a potential candidate plant for phytoremediation of 137Cs. |
| The biomass of the amaranth plants gradually decreased with the increase of 133Cs and 88Sr content in the soil. But it was not more obvious in the 133Cs treatment than in the 88Sr treatment. |
| The chlorophyll content in leaves is a most important physiology index in the growth and metabolism of plants. Under 133Cs and 88Sr stress, the chlorophyll content in amaranth firstly increase and then decreases with the increasing of 133Cs and 88Sr content in the soil. This means lower 133Cs and 88Sr contents in the soil activate the chlorophyll content and higher 133Cs and 88Sr content stress can decrease chlorophyll content. This can lead to lower photosynthesis ability and decrease the biomass of plants. |
| Malondialdehyde (MDA), one of the decomposition products of polyunsaturated fatty acids of membrane is considered as a reliable indicator of oxidative stress [13]. In this experiment, the MDA content under 88Sr stress was higher than that under 133Cs stress, and the activities of CAT under 88Sr stress was lower than that under 133Cs stress. It is the important physiological reason why amaranth has better tolerance to 133Cs stress and uptake more 133Cs than 88Sr. |
| Acknowledgements |
| The authors gratefully acknowledge the financial support from the State Administration of Science Technology and Industry for Defence, China under the defense fundamental research projects b3120110001. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14277
- [From(publication date):
March-2015 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 9674
- PDF downloads : 4603
