Phytochemical Screening and In-vitro Antioxidant and Antiproliferative Activity of Aqueous Leaf Extract of Ximtenia americana against Non- Small Cell Lung Cancer
Received: 29-Jun-2018 / Accepted Date: 24-Jul-2018 / Published Date: 30-Jul-2018 DOI: 10.4172/2573-542X.1000117
Keywords: Ximenia americana; Phytochemicals; Total flavanoids content; In-vitro antioxidant; MTT assay
Introduction
Cancer is a general term applied of series of malignant diseases that may affect different parts of body. These diseases are characterized by rapid and uncontrolled formation of abnormal cells, which may mass together to form a tumor or proliferate throughout the body by the process of metastasis. The main forms of cancer treatment for cancer in humans are surgery, radiation and drugs (chemotherapeutic agents) can often provide temporary relief of symptoms, prolongation of life and occasionally cures. Cancer continues to represent the largest cause of mortality in the world and claims over 6 million lives every year [1]. In developing countries since from following decades, the numerous of people with cancer will continue to increase may be due to life style, nutrition and environmental conditions [2-4]. In many countries cancer is 2nd leading cause of death after heart diseases [5]. Lung, colorectal and stomach cancer are among the five most common cancers in the world for both men and women [6]. Lung cancer is the leading cause of cancer deaths worldwide. American cancer society estimated in 2016, about 1 of 4 cancer deaths are from lung cancer. Every year, more people die of lung cancer than of colon, breast and prostate cancers. Furthermore if you consider 5 year survival rate for lung cancer patients it drops from 54% to 4% in patients with metastatic lung cancer [7].
However, most of the anticancer drugs currently used such as doxorubicin, paclitaxel give rise to undesirable side effects such as cardio toxicity and tumor drug resistance [8]. Since from ancient time’s plant secondary metabolites and their semi synthetic derivatives continue to play an important role in the treatment of cancer as novel drugs [9,10] and 60% of currently used anticancer agents are derived in one way or another from natural sources [11]. Plant derived natural products such as flavonoids, terpenes, alkaloids and phenols are gaining more importance due to their diverse pharmacological properties including cyto-toxic and cancer chemo protective effects [12].
Plants are the rich sources of secondary metabolites such as alkaloids, phenols, flavonoids, tannins, saponins, glycosides, terpenoids etc. that possess a wide array of biological properties including antibacterial, antifungal, antioxidant and anticancer [13]. Phytochemicals and even the whole plant extracts are known to prevent arrest or reverse the cellular and molecular processes of carcinogenesis due to its multiple intervention strategies [14] because of these reason herbal medicines making an impact on both world health and international trade. Medicinal plants continue to play a central role in the health care system of the large proportions of the world’s population [15].
Already large number of new drugs derived from plants secondary metabolites have been applied in treatment and prevention of cancer [16]. In many countries the use of medicinal plants to treat diseases is quiet common due to two main factors i.e. easy access and low cost with less side effects [17,18].
In the present study Ximenia americana Linn. Plant belonging to Olacaceae family was selected. X. americana is a small tree or shrub, native to tropical area of Africa and seen distributed in many parts of the world. This species is used in treatment of wide variety of ailments by many rural communities in Africa and Asia. This is commonly known as wild olive or sour plum or yellow plum and extensively used as herbal remedy in treatment of malaria, leproutic ulcer, and skin infections [19].The leaves are reported to have antibacterial activity and also used in the treatment of fever, tuberculosis, tooth decay and wounds [20].
Many investigations have validated the use of roots in the treatment of leprosy, syphilis, dysentery, and wounds. The stem bark has been reported to have anti-trypanosmal activity. The root bark and leaf of Ximenia americana is used as herbal medication for the cure of many ailments by Northern part of Nigeria [21]. In our previous studies the aqueous and methanolic leaf extracts of X. americana showed significant antioxidant and anti-inflammatory activities [22].
However, till-date a systematic study on biological activities of chemical constituents present in X. americana is still not agreeable [23,24]. The extensive literature survey exposed that only few reports exist on this plant leaves, but no information are available on anticancer activity in particular with lung cancer. Henceforth, present study was undertaken and made an attempt to identify Phytochemicals and invitro antioxidant and antiproliferative activity of aqueous extract of Ximenia americana.
Materials and Methods
Plant collection
Ximenia americana leaves were collected from Karnatak University Campus, Dharwad, India in the month of June, 2017. The leaves were identified and authenticated by Dr. Kotresha K., Department of Botany, Karnatak Science College, Dharwad, Karnataka, India. A voucher specimen (N0-01/2016) was deposited at the Department of Botany, Karnatak Science College, Dharwad, Karnataka. Fresh disease free plant material was washed under running tap water, shade dried and pulverized to fine powder using mechanical grinder. The powder was stored in airtight containers at room temperature for further use.
Chemicals
3-(4,5–dimethyl thiazol–2–yl)–5–diphenyltetrazolium bromide (MTT), Fetal Bovine serum (FBS), Phosphate Buffered Saline (PBS), Dulbecco’s Modified Eagle’s Medium (DMEM) and Trypsin were obtained from Sigma Aldrich Co, St Louis, USA.EDTA, Glucose and antibiotics from Hi-Media Laboratories Ltd., Mumbai. Dimethyl Sulfoxide (DMSO) and Propanol from E.Merck Ltd., Mumbai, India.
Cell lines
A549 &NCI-H460non small cell lung cancer cell lines were procured from National Centre for Cell Sciences (NCCS), Pune, India. Stock cells were cultured in DMEM supplemented with 10% inactivated Fetal Bovine Serum (FBS), penicillin (100 IU/ml), streptomycin (100 μg/ml) and amphoteri¬cin B (5 μg/ml) in a humidified atmosphere of 5% CO2 at 37°C until confluent.
Crude extraction
The 100 g of dried X. americana leaf material was extracted with distilled water using Soxhlet apparatus for 4-6 hrs at 40-500°C. The extractant solvent was evaporated using rotary evaporator and the resultant slurry of crude extract was thoroughly dried and weighed. The extract was freeze-dried at −200°C and stored at 40°C until use. The yield was found to be 8% w/w with reference to the air dried plant material.
Phytochemical analysis
The crude powder of Ximenia americana was qualitatively tested for different phytochemical constituents namely alkaloids, flavonoids, glycosides, phenols, lignin’s, saponins, sterols, tannins, anthraquinone and reducing sugar by following the standard procedure [25].
Estimation of flavonoids content
The flavonoids content in the plant extracts was estimated according [26] with quercetin as reference standard. It is an aluminium chloride colorimetric method in which each extract (0.5 ml) separately mixed with 1.5 ml of methanol, 0.1 ml of 10% aluminium chloride, 0.1 ml of 1M potassium acetate and 2.8 ml of distilled water. The reaction mixture was kept at room temperature for 30 min; the absorbance of the reaction mixture was measured at 415 nm using a UV-VIS Spectrophotometer. The value of optical density was used to calculate the flavonoids content present in the sample and the calibration curve was plotted by using quercetin solutions at concentrations 12.5 to 100 μg/ml in methanol.
Determination of Antioxidant Activity by Using In-vitro Method
Hydrogen peroxide scavenging assay
The antioxidant activity of Ximenia americana was evaluated with ascorbic acid as a standard based on their ability to scavenge the hydrogen peroxide [27]. 0.6 ml of 4mM H2O2 solution in phosphate buffer (pH-7.4) was added to 0.5 ml of known concentration of standard ascorbic acid and to tubes containing different concentrations ranging from 100 μl to 500 μl of plant extracts in phosphate buffer (pH-7.4). Absorbance of the solution was measured at 230 nm after 10 min against the blank solution containing phosphate buffer without hydrogen peroxide. Control was prepared by replacing the sample or standard with phosphate buffer. All samples were assayed in triplicates. The percentage of inhibition was calculated by using formula method.
Percentage of inhibition %=Ac-At/Ac × 100
Cell Viability Assay
Culturing of cells
The cells were dissociated with TPVG solution (0.2% trypsin, 0.02% EDTA, 0.05% glucose in PBS). The stock cultures were grown in 25 cm² culture flasks and all experiments were carried out in 96 microtitre plates (Tarsons India Pvt. Ltd., Kolkata, India).
Preparation of test solutions
For Cytotoxicity studies, each weighed test drugs were separately dissolved in DMSO and volume was made up with DMEM supplemented with 2% inactivated FBS to obtain a stock solution of 1 mg/ml concentration and sterilized by filtration. Serial two fold dilutions were prepared from this for carrying out cytotoxic studies.
Determination of cell viability by MTT Assay
The effect of aqueous extract of leaves extract of Ximenia americana on the viability of non-small cell lung cancer (A549 &NCI-H460) cells was determined using the standard colorimetric MTT assay using the 3-(4,5-dimethylthiazol- 2-yl)-2,5-dimethyl tetrazolium bromide dye (Sigma, St. Louis, MO, USA) [28]. The monolayer cell culture was trypsinized and the cell count was adjusted to 1.0 × 105 cells/ml using DMEM containing 10% FBS and seeded to 96-well microtiter plates (Falcon, Becton– Dickinson, Franklin Lakes, NJ, USA). After 24 h of plating, cells were serum starved for 24 hr. Respective concentrations of aqueous leaves extract of Ximenia americana were added to serum free medium and the assay was terminated after 48 h. Medium was removed and 200 μl of DMSO was added and the amount of formazan formed was measured at 595 nm on a Model 680 Microplate Reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The percentage growth inhibition was calculated using the following formula and concentration of test drug needed to inhibit cell growth by 50% (IC50) values is generated from the dose-response curves for each cell line. This assay is based on the reduction of MTT by the mitochondrial dehydrogenase of intactcells to a purple formazan product [29].
Statistical analysis
All experiments were performed in triplicates (n=3) and the data are presented as the mean ± standard deviation and standard error. Differences between the means of the individual groups were analyzed using the analysis of variance procedure of SPSS software 20 Version (IBM). The significance of differences was defined at the P<0.05 and P<0.01 level.
Results
Phytochemical analysis
In the present study, aqueous extract of Ximenia americana was screened for different Phytochemicals by following the standard procedure of various different biochemical tests. The results revealed that aqueous extract of Ximenia americana contains broad spectrum of secondary metabolites which mainly include phenols, tannins, flavonoids, alkaloids and glycosides (Table 1).
| Tests | Aqueous extract |
|---|---|
| A) Alkaloids | |
| Iodine | - |
| Waganer’s | + |
| Dragendroff’s | + |
| B) Flavonoids | |
| Pew’s Test | ++ |
| Shinoda Test | ++ |
| NaoH Test | ++ |
| C) Glycosides | |
| K-K Test | + |
| Glycoside Test | + |
| Conc. H2SO4 Test | + |
| Molish Test | + |
| D) Phenols | |
| Ellagic acid Test | + |
| Phenols Test | + |
| E) Lignin | |
| Lignin Test | - |
| Lobat Test | - |
| F) Saponins | |
| Foam Test | + |
| Haemolysis Test | ++ |
| G) Sterols | |
| L-B Test | - |
| Salkowsk Test | - |
| H) Tannins | |
| Gelatin Test | + |
| Lead acetate Test | ++ |
| I) Anthraquinone | |
| Bomtrager’s Test | - |
| J) Phlobatanin | - |
| K) Oils and Fats | |
| Filter paper Test | ++ |
| Saponification Test | + |
Table 1: Phytochemical constituents present in theXimenia americanaleaves.
Total flavonoids content
In the present study, aqueous extract of Ximenia americana leaves was determined by aluminum chloride colorimetric method [26] and expressed as quercetin equivalents (QE) per gram of plant extracts.
Aqueous extract of Ximenia americana exhibited highest amount of flavonoids content 68.49 ± 0.12245 mg/gof quercetin equivalent [by using equation Y=0.018X+0.229 & R2=0.982].
Hydrogen peroxide scavenging assay
In hydrogen peroxide radical scavenging assay known concentration (100 μg) of aqueous extract of Ximenia americana was subjected along with ascorbic acid as a standard. The results revealed that aqueous extract of Ximenia americana showed highest scavenging activity than standard with scavenging percentage 82.84 ± 0.3848. The values are tabulated in Table 2.
| Sl. No. | Concentration | Treatment | % Inhibition |
|---|---|---|---|
| 1 | 100 µg | Standard | 74.4633 ± 0.13051** |
| 2 | 100 µg | X.americana aqueous extract | 82.8464 ± 0.3848** |
Results are expressed as Mean±SD (n=3); **significant at the p<0.01.
**Correlation is significant at the 0.01 level (2-tailed)**
*Correlation is significant at the 0.05 level (2-tailed)*
Table 2: H2O2 Assay.
Cytotoxic activity of aqueous extract from leaves of Ximenia americana against non-small cell lung cancer cells (A549 &NCI-H460)
Cell Viability &Morphological observation
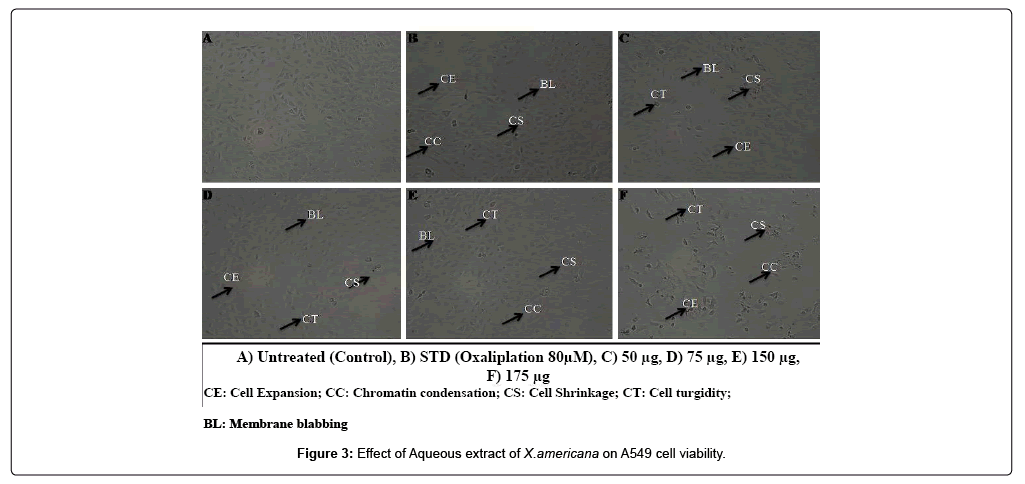
In the present study untreated A549 and NCI-H460 cell lines taken as control group, A549 and NCI-H460 cell lines treated with standard drug Oxaliplatin considered as positive control group whereas aqueous extract of X americana Figure 1 treated A549 and NCI-H460 cell lines taken as treated group. In the present study different concentration of standard drug and aqueous extract of X. americana were taken to study morphological changes as well as cell growth inhibition in both A549 and NCI-H460 non-small cell lung cancer cell lines Figure 2. Morphological studies revealed that compared with control group, treated group and positive control group showed significant increase in detached cells in culture medium. The cells displayed as turgid and shrunken in shape compared to untreated control cells. Morphological changes in nucleus representing apoptosis. Whereas normal cells appeared as regular and normal in shape, in case of treated group chromatin condensation, elongation of cells and decrease in cell count and density were observed which are the characteristic features of apoptosis. Microscopic examination revealed that morphological changes and shrinkage of cells leading to cell apoptosis induced by the aqueous extract of Ximenia americana (Figures 3 and 4).
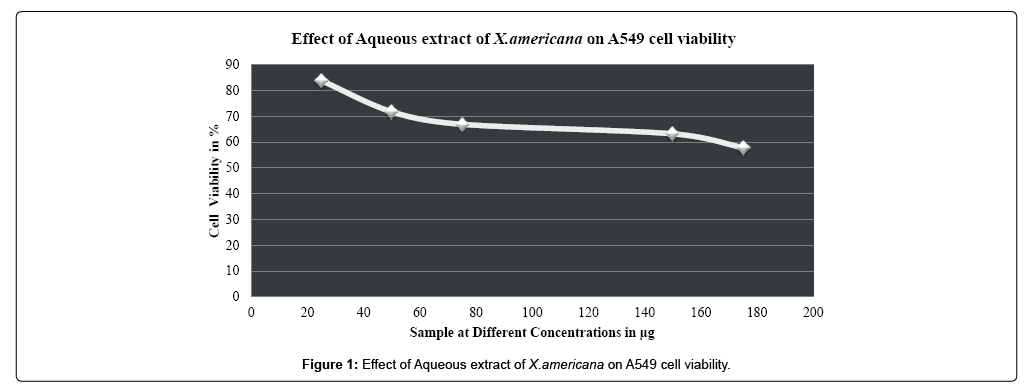
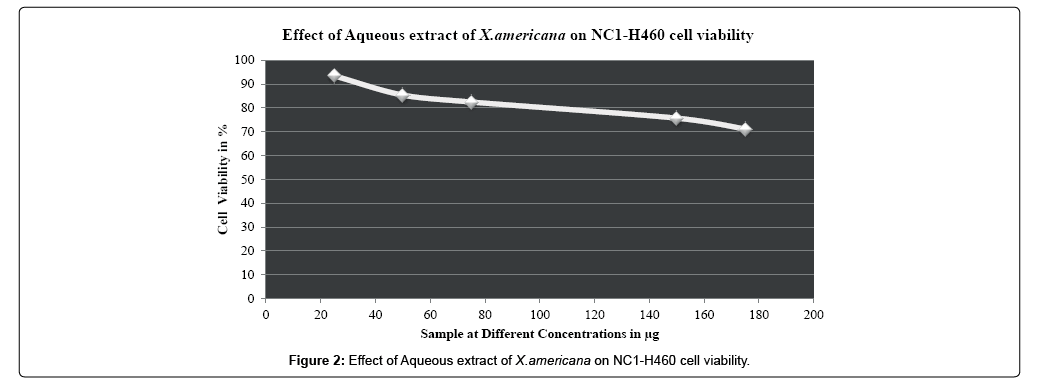
While consideration of antiproliferative activity and growth inhibition studies untreated A549 and NCI-H460 non-small cell lung cancer cells were taken as control. A549 and NCI-H460 both cell lines were treated with different concentrations (25 μg-175 μg) of aqueous leaf extract of Ximenia americana and standard drug Oxaliplatin (20 μM-80 μM).The cell viability of the aqueous extract Ximenia americana was compared to standard chemotherapeutic drug Oxaliplatin. Increase in the cell viability was observed at minimum concentration only, as the concentration increases viability of cells was decreased. In case of both aqueous extract and standard drug increase in concentration above 50 μg & 40 μM respectively causes the decreases in cell count as well as division of cells as compared to untreated control group and 50% of cell viability was observed to be 229.20 μg and 338.30 μg for A549 and NCI-H460 respectively and for standard drug Oxaliplatin it was observed to be 54.17 μM &72.04 μM for A549 and NCI-H460 respectively. IC50 values for standard and extract were calculated and depicted in Table 3. Overall Aqueous extract from leaves of Ximenia americana has strong dose dependent anticancer activity against nonsmall cell lung cancer cells especially against A549 with IC50 value 229.20 μg and it was observed to be more i.e.338.30 μg with NCI-H460 cell line.
| Treatment | Cell Line | Concentration in µg | Cell Viability in Percentage (%) | IC50 in µg |
|---|---|---|---|---|
| Aqueous extract of Ximenia americana | A549 | 25 µg | 83.7588 ± 0.39960 | 229.20 µg |
| 50 µg | 71.7021 ± 0.40277 | |||
| 75 µg | 66.8439 ± 0.73276* | |||
| 150 µg | 63.1560 ± 0.31514* | |||
| 175 µg | 57.8014 ± 0.25570** | |||
| NC1-H460 | 25 µg | 93.4439 ± 0.25429 | 338.30 µg | |
| 50 µg | 85.3838 ± 0.36428 | |||
| 75 µg | 82.4745 ± 0.15276 | |||
| 150 µg | 75.7097 ± 0.36425 | |||
| 175 µg | 71.1181 ± 0.40421* | |||
| Standard Drug Oxaliplatin | A549 | 20 µM | 65.2127 ± 0.46370* | 54.17 µM |
| 40 µM | 55.7092 ± 0.47707* | |||
| 60 µM | 46.8085 ± 0.12283* | |||
| 80 µM | 39.4326 ± 0.18764** | |||
| NC1-H460 | 20 µM | 69.6109 ± 0.43777* | 72.04 µM | |
| 40 µM | 62.2502 ± 0.32123* | |||
| 60 µM | 51.805 ± 0.30556* | |||
| 80 µM | 48.5804 ± 0.32124** |
Results are expressed as Mean±SE (n=3); **significant at the p<0.01.
**Correlation is significant at the 0.01 level (2-tailed)**
*Correlation is significant at the 0.05 level (2-tailed)
Table 3: Comparison of Effect of Aqueous extract of X.americana and Standard Drug Oxaliplatin on A549 and NCIH-460 cell viability.
Discussion
Cancer is the leading cause of death worldwide and overall statistics study proven that compared to other diseases death rate of cancer is getting very much high per every year and it is one of the most common devastating disease affecting millions of people per year. Cancer has been a leading cause of global morbidity due to its rapid progression and poor diagnosis [30-34]. Lung cancer is the leading cause of cancer deaths in men and 2nd in leading cause of deaths in women [35]. Many synthetic drugs are available to treat cancer but those are provided with severe side effects as well as cost effective. According to world health organization (WHO) approximately 65-80% of developing countries including the India depend on traditional medicine for their health care due to difficulties of accessing modern medicines [36]. Medicinal plants play a significant role in the treatment of cancer [37-39]. Natural products or derivatives have been demonstrated to have significant anticancer activities due to their ability to inhibit tumor growth, angiogenesis and metastasis without any side effects [40-44]. It was reported that 40% of anticancer agents between 1940 and 2002 were derived from natural products or their mimics, including vinca alkaloids, Taxus diterpenes, camptotheca alkaloids etc. [45].
In the present study Ximenia americana Linn. Plant belonging to Olacaceae family was selected for phytochemical screening and evaluation of its antioxidant and anticancer activity against nonsmall cell lung cancer. Phytoconstituents were evaluated by different quantitative biochemical tests. Phytochemical results revealed the presence of different secondary metabolites such as alkaloids, saponins, terpenoids, tannins, flavonoids and phenols in the aqueous extract of Ximenia americana. These secondary metabolites are considered as natural source of antioxidant, antimicrobial and anti-inflammatory agents which have been shown to reduce the risk and progression of many diseases such as cancer and diabetes [46,47].
Especially phenolic compounds are high potent radical terminators by donating hydrogen atom to radical and inhibit lipid oxidation. Polyphenolic compounds present in plant are known to have several biological activities like antitumor, anti-inflammatory, antioxidant and anticancer [48,49]. Several studies show that alkaloids, glycosides are known to possess antimicrobial properties [50]. Saponins are considered as key ingredient to produce inhibitory effect on inflammation, they also used as dietary supplements and nutraceuticals. Different physical and biological properties of saponins made them useful drugs such as antimicrobial, anti- inflammatory and hemolytic agent [51,52]. Tannins are considered to be possessing medicinal properties like antiviral, antibacterial, antiparacitic, anticancer, antiulcer and antioxidant agents. It also investigated that this was able to inhibit HIV replication [26,53,54]. Even the phenols and flavonoids are considered to be diverse and broad group of natural components which possess broad spectrum of biological activities including antioxidant activity [55]. Overall phytochemical study showed that aqueous extract of X. americana possess broad spectrum of secondary metabolites.
In the present study aqueous extract of X. americana exhibited total flavonoids content 68.49 ± 0.12245 mg/g of quercetin equivalent. Flavonoids can play the role of antioxidant through different mechanisms including terminating free radicals, reducing the oxygen concentration, transforming primary products of oxidation into non oxidant molecules and acts as metal chelators [56].
Free radicals enhances the abnormal uncontrolled oxidation reaction in the body which leads to the failure of antioxidant defense mechanism and causes damage to the cell structures which increases the risk factors for many diseases such as Alzheimer’s disease, Parkinson disease, cardiovascular disorders, liver disease, inflammation and cancer [57]. Cancer occurs due to excessive free radical damage which causes damage in the genetic material DNA, proteins and lipids which further leads to the mutations which cause conversion of normal cell into cancer cell [58]. Antioxidants are compounds that protect cells against the damaging effect of reactive oxygen species [25]. Recently natural antioxidants are in high demand because of their potential in health promotion and disease prevention. H2O2 assay is one of the common methods used to investigate the antioxidant capacity of the extracts. In the present study aqueous extract of Ximenia americana subjected to H2O2 assay. In H2O2 assay strong oxidizing agent which inactivate certain enzymes directly and also react with metal ions like Fe+2, Cu+2 and leads to the toxic effects [59]. In the present study, aqueous extract of Ximenia americana exhibited highest scavenging activity with the 82.84 ± 0.3848% percentage of inhibition on comparing with standard.
During cancer development many genetic, epigenetic and abnormal changes will occur with the cells. These changes results in the sustained cell proliferative signaling, insensitivity to growth suppressors, evading apoptosis, metastasis and angiogenesis that may develop into malignant phenotype [1].
Using in-vitro assay system for the screening of potential anticancer drug has been common practice almost since the beginning of cancer therapy in 1946. There is several numbers of advantages in in-vitro test using cell cultures which includes analysis of specificity, feasibility of using small amount of test substance for studies. A novel anticancer drug should possess Cytotoxicity of low concentration against cancerous cell lines and should be safe against normal cell lines even at higher concentration [60].
In the present study aqueous extract of X. americana was subjected to evaluating anticancer activity against non-small cell lung cancer cell lines A549 and NCI-H460 by using in-vitro MTT cell viability assay. In this study A549 and NCI-H460 cells were incubated with different concentration (25 μg-175 μg) 48 hrs-72 hrs. Morphological studies revealed that compared with control group, treated group and positive control group showed significant increase in detached cells in culture medium and as the concentration was increasing it causing observable changes in the growing cells such as the cells displayed as turgid and shrunken in shape compared to untreated control cells. Morphological changes in nucleus representing apoptosis. Whereas normal cells appeared as regular and normal in shape, in case of treated group chromatin condensation, elongation of cells and decrease in cell count and density were observed which are the characteristic features of apoptosis. Microscopic examination revealed that morphological changes and shrinkage of cells leading to cell apoptosis induced by the aqueous extract of Ximenia americana. Significant decrease in cell viability was observed with increase in concentration in both cell lines. Strong dose dependent anticancer activity was observed against nonsmall cell lung cancer cells, with the maximal inhibition of cell growth (<40%) obtained at 175 μg. Aqueous extract of Ximenia americana was most effective in inhibiting the growth of cells at a concentration of 175 μg/mL for a single time of 72 h. The results were compared with those obtained with the standard Oxaliplatin drug which successively inhibited the growth in both cell lines at low concentration i.e.40 μM/ mL. Variation in IC50 value was observed between extract and standard drug; standard drug exhibited 54.17 μM and 72.04 μM for A549 and NCI-H460 respectively whereas aqueous extract exhibited 229.20 μg and 338.30 μg for A549 and NCI-H460 respectively. Microscopic examination revealed that morphological changes and shrinkage of cells leading to cell apoptosis induced by the aqueous extract of Ximenia americana.
Surgical resection, radiation or systemic chemotherapy is the main types of treatment for most cancers, but in case of lung cancer post treatment reoccurrence is quite frequent and although the cessation of smoking is important for lung cancer prevention [61]. The preventive mechanisms of tumor promotion by natural Phytochemicals range from the inhibition of genotoxic effects, increased antioxidants and anti-inflammatory activity, inhibition of cell proliferation, protection of intracellular communications to modulate apoptosis and signal transduction pathways [62,63].
Many bioactive compounds from medicinal plants and other living organisms such as bacteria and fungi [64], even the present study correlates with this study and aqueous extract of Ximenia americana proven to have significant antiproliferative activity against lung cancer. The bioactive compounds from medicinal plants are provided with a wide variety of chemical structures with various biological activities and also bioactive compounds from plants can able to suppress or prevent the initial phases of carcinogenesis [65] that provides important prototypes for the development have already proven to have antitumor potential against lung cancer [66] of novel drugs [67-70]. Statistically the antioxidant assay and MTT assay were observed significant difference (P<0.01 and P<0.05) in extract and Standard drug.
Conclusion
Phytochemical analysis of aqueous extract Ximenia americana plant showed that it contains broad spectrum of bioactive compounds that included alkaloids, glycosides, saponins, tannins, phenols and flavonoids. These bioactive compounds already reported to have several medicinal properties which include anticancer, anti-proliferative, antimicrobial, antioxidant, anti-tuberculosis, antimicrobial, antiinflammatory, hemolytic agent and antiviral. The present study also showed high flavonoids content in aqueous extract of Ximenia americana. In context of antioxidant assay aqueous extract exhibited significant antioxidant activity over standard ascorbic acid. In case of anticancer activity, aqueous extract of Ximenia americana showed significant noticeable antiproliferative activity by inhibiting <50% cells growth in A549 &NCI-H460 non-small cell lung cancer cell lines and results were compared with standard drug Oxaliplatin. Thus the present study concludes that aqueous extract of Ximenia americana exhibited higher antioxidant as well as antiproliferative. However further studies are needed to find out the structural of bioactive compounds and investigate mechanisms of antioxidant and anticancer activities of the bioactive compounds in in-vivo animal study.
Conflict of Interest
We wish to confirm that there are no known conflicts of interest associated with this publication.
References
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646-74.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61: 69-90.
- Marmot M, Atinmo T, Byers T, Chen J, Hirohata T, et al. (2007) Â Food, nutrition, physical activity, and the prevention of cancer: a global perspective. World Cancer Research Fund/American Institute for Cancer Research, Washington DC, United States.
- Veer P, Kampman E (2007) Food, nutrition, physical activity and the prevention of cancer: a global perspective. World Cancer Research Fund/American Institute for Cancer Research, Washington DC, Unites States.
- Dahab RA, Afifi F (2007) Antiproliferative activity of selected medicinal plants of Jordan against a breast adenocarcinoma cell lines (MCF7). Sci Pharm Inc 75: 121-136.
- Shoeb M (2006) Anticancer agents from medicinal plants. Bangladesh J Pharmacol1 1: 35-41.
- Siegel RL, Miller KD, Jemal A (2016). Cancer Statistics. CA Cancer J Clin 66: 7-30.
- Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, et al. (2009)Â Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem 16: 3267-3285.
- Pan L, Chai H, Kinghorn AD (2010) The continuing search for antitumor agents from higher plants. Phytochem Lett 3: 1-8.
- Indap MA, Radhika S, Motiwale L, Rao KVK (2006) Quercetin: antitumor activity and pharmacological manifestations for increased therapeutic gains. Indian J Pharm Sci 68: 465-469.
- Cragg GM, Grothaus PG, Newman DJ (2009) Impact of natural products on developing new anti-cancer agents. Chem Rev 109: 3012-3043.
- Kviecinski MR, Felipe KB, Schoenfelder T, de Lemos Wiese LP, Rossi MH, et al. (2008) Study of the antitumor potential of Bidenspilosa (Asteraceae) used in Brazilian folk medicine. J Ethnopharmacol 69: 75-117
- De Fatima A, Modolo LV, Conegero LS, Pilli RA, Ferreira CV, et al. (2006) Lactones and their derivatives: biological activities, mechanisms of action and potential leads for drug design. Curr Med Chem 13: 3371-3384.
- Mehta RG, Murillo G, Naithani R, Peng X (2010) Cancer chemoprevention by natural products: how far have we come? Pharm Res 27: 950-61.
- Akerele O (1988) Medicinal plants and primary health care: An agenda for action. Fitoterapia 59: 355–63.
- Conforti F, Ioele G, Statti GA, Marrelli M, Ragno G, et al. (2008) Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food ChemToxicol 46: 3325-3332.
- Borges R, Nascimento MVM, Carvalho AGV, Valadares MC, Paula JR, et al. (2013) Antinociceptive and anti-inflammatory activities of the ethanolic extract from Synadeniu mumbellatum Pax (Euphorbiaceae) leaves and its Fractions. Evid -Based Compl Alt.
- Orlanda JFF, Vale VV (2015) Phytochemical analysis and photoprotective activity of theethanolic extract of Euphorbia tirucalli Linneau (Euphorbiaceae). Rev Bras Pl Med 17: 730–736.
- Voss C, Eyol E, Berger MR (2008) Unit of Toxicology and Chemotherapy. Deutsches Krebs for schungszentrum, E100 ImNeuenheimer Feld, Heidelburg, Germany.
- Ogunyleye DS, Ibitoye SF (2003) Â Â Â Studies of antimicrobial activity and chemical constituents of Ximenia americana. Trop J Pharm Res 2: 239-241.
- Maikai VA, Nok AJ, Alawa C (2008) In vitro anti-trypanosomal activity of aqueous and methanolic crude extract of stem bark of Ximenia americanaon Trypanosoma congolense. J Med Plant Res 2: 55-58.
- Arun KS, Kotresha K, Kaliwal BB, Vedamurthy AB (2015) Evaluation of in vitro antioxidant and anti-inflammatory activities of Ximenia americana extracts. Asian Pac J Trop Dis 5: 918-923.
- Monte FJ, De Sousa Gomes E, De Araujo MR, De Lemos TL (2012) Ximenia americana: Chemistry, Pharmacology and Biological Properties, a Review. INTECH Open Access Publisher.
- James DB, Abu EA, Wurochekke AU, Orji GN (2007) Phytochemical and Antimicrobial Investigation of the Aqueous and Methanolic Extracts of Ximenia americana. Journal of Medical Science 7: 284-288.
- Deepti K, Umadevi V, Vijayalakshmi G, VinodPolarao B (2012) Antimicrobial activity and phytochemical analysis of Morindatinctoria Roxb Leaf extracts. Asian Pac J Trop Biomed 2: S1440-2.
- Chang C, Yang M, Wen H, Chern J (2002) Estimation of total flavonoids content in propolis by two complementary colorimetric methods. Journal of Food Drug Analysis 10: 178–182.
- Akiyama H, Fujii K, Yamasaki O, Oono T, Iwatsuki K (2001) Antibacterial action of several tannins against Staphylococcus aureus. J Antimicrob Chemother 48: 487-491.
- Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB (1987) Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res 47: 936–942.
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63.
- Gulcin I, Buyukokuroglu ME, Oktay M, Kufrevioglu OI Â (2002) On the invitro antioxidant properties of melatonin. J Pineal Res 33: 167-71.
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN. Int. J. Cancer 136: E359-E386.
- Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, et al. (2015) Â Annual Report To The Nation on the Status of Cancer, 1975e 2011, Featuring Incidence of Breast Cancer Subtypes by Race/ Ethnicity, Poverty, and State. J. Natl Cancer Inst 107: djv048.
- Torre LA, Siegel RL, Ward EM, Jemal A (2015) Global Cancer Incidence and Mortality Rates and Trends- An Update. Cancer Epidemiol Biomark Prev 25: 16-27.
- Bello B, Fadahun O, Kielkowski D, Nelson G (2011) Trends in lung cancer mortality in South Africa: 1995-2006. BMC Public Health11: 209.
- Shibuya K, Mathers CD, Boschi-Pinto C, Lopez AD, Murray CJ (2002) Global and regional estimates of cancer mortality and incidence by site: II. Results for the global burden of disease 2000. BMC Cancer 2: 37.
- Parkin DM (2001) Global cancer statistics in the year 2000. Lancet Oncolology 2: 533–543.
- Calixto JB (2005) Twenty-five years of research on medicinal plants in Latin America: a personal view. J Ethnopharmacol 100: 131-134.
- Ansil PN, Wills PJ, Varun V, Latha MS (2014) Cytotoxic and apoptotic activities of Amorphophalluscampanulatus (Roxb.) Bl. Tuber extracts against human colon carcinoma cell line HCT-15. Saudi J Biol Sci 21: 524-531.
- Roslen NA, Alewi NA, Ahamada H, Rasad MS (2014) Cytotoxicity screening of Melastoma malabathricum extracts on human breast cancer cell lines in vitro. Asian Pac J Trop Biomed 4: 545-548.
- Butler MS (2004) The role of natural product chemistry in drug discovery. J Nat Prod 67: 2141-2153.
- Fridlender M, Kapulnik Y, Koltai H (2015) Plant derived substances with anti-cancer activity: from folklore to practice. Front Plant Sci 6: 799.
- Dall'Acqua S (2014) Natural products as antimitotic agents. Curr Top Med Chem 14: 2272-85.
- Biersack B, Schobert R (2012) Indole compounds against breast cancer: Recent Developments. Curr Drug Targets 13: 1705-19.
- Negi AS, Gautam Y, Alam S, Chanda D, Luqman S, et al. (2015) Natural antitubulin agents: importance of 3,4,5-trimethoxyphenyl fragment. Bioorg Med Chem 23: 373-89.
- Vindya NG, Sharma N, Yadav M, Ethiraj KR (2015) Tubulins-the target for anticancer therapy. Curr Top Med Chem15: 73-82.
- David JN, Gordon MC, Kenneth MS (1999) The influence of natural products upon drug discovery. Nat Prod Rep 17: 215-234.
- Pham-Huy LA, He H, Pham-Huy C (2008) Free radicals, antioxidants in disease and health. Int J Biomed Sci 4: 89-96.
- Philips A, Philips S, Arul V, Padmakeerthiga B, Renju V, Â et al. (2010) Free radical scavenging activity of leaf extracts of Indigofera aspalathoides - An in vitro analysis. J Pharm Sci Res 2: 322-328.
- Amir M, Javed SA, Kumar H (2010) Design and synthesis of 3-[3- (substituted phenyl)-4-piperidin-1-ylmethyl/-4-morpholin-4- ylmethyl-4,5-dihydro-isoxazol-5-yl]-1H-indoles as potent anti-inflammatory agents. Med Chem Res 19: 299-310.
- Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, et al. (2005) In vitro proliferative, apoptic and antioxidant activities of plinicalgin, eliagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols found in pomegranate juice. J NutriBiochem 16: 360-367.
- Balandrin MF, Klocke JA (1988) Medicinal, aromatic and industrial materials from plants. Bajaj Springer-Verlag Berlin, Heidelberg 4: 1-36.
- Francis G, Kerem Z, Makkar HP, Becker K (2002) The biological action of saponins in animal systems: a review. Brit J Nutr 88: 587-605.
- Xu R, Zhao W, Xu J, Shao B, Qin G (1996) Studies on bioactive saponins from Chinese medicinal plants. Adv Exp Med and Biol 404: 371-382.
- Lu L, Liu SW, Jiang SB, Wu SG (2004) Tannin inhibits HIV-1 entry by targeting gp41. Acta Pharmacol Sin 25: 213-218.
- Kolodziej H, Kiderlen AF (2005) Antileishmanial activity and immune modulatory effects of tannins and related compounds on Leishmaniaparasitised RAW 264.7 cells. Phytochemistry 66: 2056-2071.
- Blois MS (1958) Antioxidant determination by the use of a stable free radical. Nat 181: 199-1200.
- Shahidi F, Naczk M (2004) Phenolic in Food and Nutraceutical. CRC Press, Boca Raton, United States.
- Rajkumar V, Guha G, Kumar RA, Mathew L (2010) Evaluation of antioxidant activities of Bergenia ciliaterhizome. Rec Nat Prod 4: 38-48.
- Roszkowski K (2014) Oxidative DNA damage-the possible use of biomarkers as additional prognostic factors in oncology. Front Biosci (Landmark Ed) 19: 808-817.
- Bhatia L, Bishnoi H, Chauhan P, Kinja K, Shailesh S (2011) In-vitrocomparative antioxidant activity of ethanolic extracts of Glycosmispentaphyllaand Bauhinia variegate. Recent Res SciTechnol 3: 1-3.
- Gurib-Fakim A (2006) Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects Med 27: 1–93.
- King HW, Osborne MR, Brookes P (1979) The in vitro and in vivo reaction at the N7-position of guanine of the ultimate carcinogen derived from benzolalpyrene. Chem Biol Interact 24: 345-353.
- Zhao X, Liu X, Su L (2014)Â Parthenolide induces apoptosis via TNFRSF10B and PMAIP1 pathways in human lung cancer cells. J Exp Clin Cancer Res 33: 3.
- Soobrattee MA, Bahorun T, Aruoma OI (2006) Chemopreventive actions of polyphenolic compounds in cancer. Biofactors 27: 19-35.
- Yohana F, Hernandez F, Khandual S, Guadalupe I, Lopez R (2017) Cytotoxic effect of Spirulinaplatensis extracts on human acute leukemia Kasumi-1 and chronic myelogenous leukemia K-562 cell lines. Asian Pac J Trop Biomed 7: 14-19.
- Piccolella S, Pacifico S (2015) Plant-derived polyphenols: a chemopreventive and chemoprotectant worth-exploring resource in toxicology. Advance in Molecular Toxicology: 161-214.
- Monteiro Lde S, Bastos KX, Barbosa-Filho JM, de Athayde-Filho PF, Diniz Mde F, et al. (2014) Medicinal plants and other living organisms with antitumor potential against lung cancer. Evid Based Complement Alternat Med: 604152.
- Cragg GM (1998) Paclitaxel (Taxol) a success story with valuable lessons for natural product drug discovery and development. Med Res Rev 18: 315-331.
- Verpoorte R (1998) Exploration of nature's chemodiversity: the role of secondary metabolites as leads in drug development. Drug Discov Today 4: 232-238.
- Vuorelaa P, Leinonenb M, Saikkuc P, Tammelaa P, Rauhad JP (2004) Natural products in the process of finding new drug candidates. Curr Med Chem 11: 1375-1389.
Citation: Vedamurthy AB, Shettar AK, Madagi SB (2018) Phytochemical Screening and In-vitro Antioxidant and Antiproliferative Activity of Aqueous Leaf Extract of Ximenia americana against Non- Small Cell Lung Cancer. Cancer surgery (Los Angeles, Calif.) 3: 117. DOI: 10.4172/2573-542X.1000117
Copyright: © 2018 Vedamurthy AB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6145
- [From(publication date): 0-2018 - Nov 29, 2025]
- Breakdown by view type
- HTML page views: 5073
- PDF downloads: 1072