Research Article Open Access
Physiological Parameters of Clinical Yeasts Growth and Isolation of Specific Antigens
| Arzumanyan Vera1*, Shmeleva Olga1, Serdiuk Olga2 and Michailova Natalia1 | |
| 1Mechnikov Research Institute for Vaccines and Sera, Moscow, Russia | |
| 2Institute for Diagnostics and Prophylaxis of Social Diseases, Moscow, Russia | |
| *Corresponding Author : | Vera Arzumanyan Mechnikov Research Institute for Vaccines and Sera. Moscow, Russia Tel: 8-916-495-20-21 Fax: 8-495-917-49-00 E-mail: veraar@mail.ru |
| Received July 29, 2013; Accepted August 22, 2013; Published August 26, 2013 | |
| Citation: Arzumanyan V, Shmeleva O, Serdiyk O, Michailova N (2013) Physiological Parameters of Clinical Yeasts Growth and Isolation of Specific Antigens. Biochem Physiol 2:116. doi:10.4172/2168-9652.1000116 | |
| Copyright: © 2013 Arzumanyan V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
At present time 7 genera of clinically important yeasts are known - Candida, Malassezia, Saccharomyces, Cryptococcus, Trichosporon, Geotrichum and Rhodotorula. Most of them can cause deep and superficial mycoses, but some are associated with allergic diseases. Development of diagnostic preparations from yeast Candida was started many years ago and is constantly improving. Commercial kits with antigens of yeast Malassezia has appeared recently. The known methods of antigens extraction based on the disruption of yeasts cells (mechanic or ultrasonic). This approach coupled with the presence of cross reactive determinants exists in many fungi. Aim of this work was to develop the specific antigenic preparations from all listed yeast genera. We have used the following yeast strains from our institute collection - Candida albicans № 927, Geotrichum candidum № 1206, Malassezia furfur № 1451, Rhodotorula mucilaginosa № 132, Cryptococcus neoformans № 3465, Trichosporon cutaneum № 18; and Saccharomyces cerevisiae Y-375 (Russian Collection, Pushchino). Yeasts were cultured in liquid defined media up to the end of exponential growth phase; maximal growth rate and doubling time were calculated. After that cultures were centrifuged, and supernatants were lyophilized, and cells were used for mice immunization and antigen extraction. White mice were immunized with cell suspension for 5 times with increasing doses (interval 1 week), after that sera were obtained. Antigen preparations № 1 were obtained from cells by rapid extraction with sodium dodecyl sulfate solution – in this case cell walls were not disrupted, and extract consisted in general from superficial proteins. Preparations № 2 are lyophilized supernatants (see above). To estimate preparation specificity dot-blot analysis of each preparation with each mouse serum was carried out. From this analysis it was demonstrated that all preparations № 1 contained the specific antigens, i.e. each preparation reacted with better extent with corresponded serum. However in some extent all these preparations were polluted with cross-reactive proteins (spots with other sera). Moreover the preparation from Malassezia furfur reacted with control sera, maybe because of this yeast is the resident of healthy animal skin. At the same time preparations № 2 were free from cross-reactive proteins, except preparation from Malassezia furfur – no one sera, included control sera (pure mouse) reacted with this preparation. We can come to conclusion, that in the case of Malassezia one should carry out special purification of culture liquid, but in the case of other yeasts cells free culture supernatants are preferable for creating of antigen diagnostic kits.
| Keywords |
| Candida; Malassezia, Saccharomyces; Cryptococcus; Trichosporon; Geotrichum; Rhodotorula; Physiological parameters; Antigens |
| Introduction |
| Yeasts are ascomycetous or basidiomycetous fungi, which in vegetative stage propagated by gemmation or division that resulted in single-celled growth; their sexual structures do not put in fruit bodies [1]. Now it is known more than 200 species of yeasts, and some of them are clinically important. Number of yeast genera/species is to be found in clinical practice progressively increase: In 1980 it was 4 genera of 10 species [2], in 1987 – 5 genera of 18 species [3], in 1998 – 9 genera of 36 species [4], and in 2011 – 19 genera of 80 species [5]. Such situation can be explain on the one hand by increasing (discovering) of new species partly due to creating new methods of fungal identification, but on the other hand by extension of individuals with depressed immunity. It is important however those 19 genera may be reducing to 7 basic genera of yeasts, because most of them are teleomorphs (sexual stage) of these 7 genera: Candida, Malassezia, Rhodotorula, Cryptococcus, Trichosporon, Geotrichum and Saccharomyces. Type species of these genera are C. albicans, M. furfur, R. mucilaginosa (old name – rubra), Cr. neoformans, T. cutaneum, G. candidum and S. cerevisiae. |
| The data concerning of habitats and diseases associated with clinically important yeasts we summarized on the base of different publications (Table 1) [4,6-13]. Most of them can cause deep and superficial mycoses, but some of them are associated with allergic diseases. Therefore use of diagnostic kits in this case in very actual. Now the three main methods of microbial diagnostics are known – physiological/biochemical, concerned with ability of yeasts to grow on different nutritional substrates; immunological, interrelated with reaction of serum antibodies with specific yeasts antigens; and molecular, namely method polymerase chain reaction (PCR), concerning the search of specific sites of yeast DNA. Today immunological method remains the simpliest for clinical use, however to get more authentic results all three methods should be use. |
| Development of immunological diagnostic preparations from yeast Candida albicans and Cryptococcus neoformans was started many years ago and constantly improved [14,15]. Recently the commercial kit with antigens of yeast Malassezia has appeared. So only for three yeast genera of 7 the diagnostic kits exist. |
| The known methods of antigens extraction are based on the mechanic or ultrasonic disruption of yeasts cells. This approach coupled with the presence of cross reactive determinants exists in many fungi. Moreover the main method of yeasts cells growing now is the periodic cultivation on solid media in Petri dishes. It is rather archaic approach, because in this case it is not known exactly the physiological stage (growth phase) of resulted culture. Growth phase is very important because it is known that more specific antigens are characteristic for exponential growth phase (young culture), but cross-reactive antigens – for stationary phase (old cells) [16]. |
| Thus the aim of this work was to study of yeasts growth phases in liquid defined media for further determination of specific antigens localization. |
| Materials and Methods |
| Yeasts strains were obtained from institute collection - Candida albicans № 927, Geotrichum candidum № 1206, Malassezia furfur № 1451, Rhodotorula mucilaginosa № 132, Cryptococcus neoformans № 3465, Trichosporon cutaneum № 18; and Saccharomyces cerevisiae Y-375 from Russian Collection, Pushchino. |
| Cultivation of non-lipophilic yeasts was held at 30°C and speed of rotation 150 per min in 500 ml flasks contained 100 ml of defined medium (g /l): glucose–20, asparagine- 15, (NH4) 2 SO4 – 1.4, Mg SO4x 7 H2O – 0.5, NaCl-0.1, CaCl2 -0.02; 10 ml of 1.2 M phosphate buffer ph 5.5; trace elements, antibiotic [17]. Lipophilic yeast Malassezia furfur was cultivated in the same flasks in liquid defined medium, created earlier and contained (g/l): Tween 40–10, asparagin-1, sodium taurocholic -10; the same salts, buffer and antibiotic (see above) [18]. |
| Periodically probes were sterile collected and optical density (OD) was measured at wave-length 530 nm and cuvette thickness 0.5 cm. Maximal growth rate (μmax) and doubling time (Td) was calculated using formula [19]: |
| μmax = (dx/dt) × (1/x); 1/h |
| dx = x2 – x1; OD units |
| dt = t2 – t1; h |
| x = (x1 + x2)/2; OD units |
| Td = 0.693/μmax; h |
| Maximal OD corresponded to OD in stationary phase. |
| Immunization of mice |
| Yeasts cells from the end of exponential phase were harvested by centrifugation, suspended in 0.02 % sodium mertiolate at different concentrations, exposed 2 hours at room temperature for killing cells and frozen at -25°C untill immunized. For one experiment for each yeast strain 10 white outbred mice were used (in sum – 70 mice), and 10 such mice were used as a control. As a whole 3 immunization experiments were held. Altogether 5 immunization cycles were carried out within each experiment: at first step each mouse was intraperitonealy introduced by 0.5 ml of yeast suspension with concentration 250 μg protein /ml; at second step – a 1 week later - 500 μg protein /ml; at third step – 1 week later – 1000 μg protein/ml; at forth step – 1 week later – 2000 μg protein/ml; at fifth step – 1 week later – again 2000 μg protein/ ml; 1 week after total mice antisera were collected and pooled according to yeasts strains and controls. |
| Dot-blot analysis |
| Antigen preparations № 1 were obtained from cells of the late exponential growth phase by rapid extraction with 1 % sodium dodecyl sulfate (SDS) solution during 10 min at 40°C – in this case cell walls were not disrupted, and extract consisted in general from superficial proteins. |
| Antigen preparations № 2 are lyophilized supernatants (culture liquids) obtained from the same cultures. All preparations were dissolved in buffered physiological solution (BPS) up to protein concentration 10 mg/ml. As negative control the solution contained 2 mg/ml bovine serum albumin (BSA) was used; as positive control – mouse serum, dissolved with BPS at the ratio 1:3. |
| 1 μl of preparation was applied on the nitrocellulose membrane (pore diameter 22 μ, “Advanced Microdevices PVT. LTD”, India) and dried. After that method Towbin H. was used [20]. Membrane was washed during 30 min with 0,1 % Tween 20, dissolved in physiological solution, after that – 3 times during 1min with distilled water. Blocking of nonspecific binding: 30 min in BPS with 10 % of fetal bovine serum – 1000 μl per one strip. |
| First incubation of strips with mice antisera was carried out during night at room temperature and agitation (50 μl of serum in 1000 μl BPS with 10 % of fetal bovine serum). Washing was made during 10 min with 0,1 % Tween 20, dissolved in PS, after that – 3 times with distilled water during 1 min. |
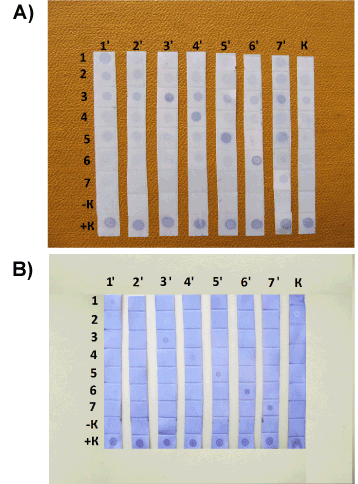
| Second incubation: 1 h at room temperature and agitation with conjugate of monoclonal antibodies to mouse IgG with peroxidase dissolved in BPS with 10 % of fetal bovine serum in ratio 1: 1000. Washing method sees above. Development: exposition of strips during 15 min in solution of precipitating monocomponent tetramethyl benzidine with hydrogen peroxide (NPO “BioTest Systems”, Moscow, Russia). Horizontal lines (Figure 2) show the results of interaction of given yeast antigen with different mice antisera; vertical lines – interaction of given mice antisera with different yeasts antigens. |
| Results |
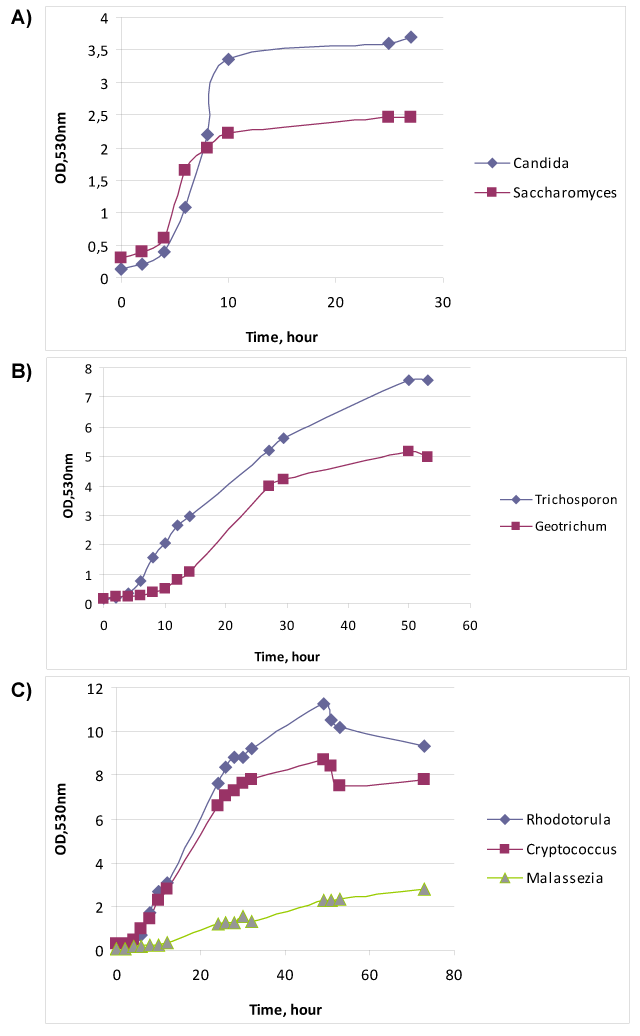
| The relationship of growth phase with specific antigens [16] makes us to study the physiological parameters of yeasts cultures. Figure 1A demonstrates the growth curves of two ascomycetous fungi – Candida albicans № 927 and Saccharomyces cerevisiae Y-375. Exponent of C. albicans last from 4th to 10th hour of cultivation, whereas growth of S. cerevisiae realized by two exponents (diauxic growth) – from 4th to 6th hour and from 6th to 10th hour. Most likely the first fungus consumed two substrates – glucose and asparagine – simultaneously, but the other fungus used asparagine after glucose. Furthermore the μmax of S. cerevisiae (Table 2) was 1.4 times over than μmax of C. albicans. However, S. cerevisiae made less biomass (OD), than C. albicans, probably due to more intensive catabolism of glucose with ethanol formation. |
| Figure 1B shows the cultivation of Trichosporon cutaneum № 18 and Geotrichum candidum № 1206. These two fungi belong to the different groups – T. cutaneum is basidiomycete, but G. candidum is ascomycete, however their metabolism and outward are very similar. Under the simple microscopy one can mix up these two fungi, only the urease test may differentiate them: T. cutaneum is urease positive, but G. candidum – urease negative. As one can see from the Figure 1B both yeasts had diauxic growth, T. cutaneum – from 6th hour to 12th hour and from 12th hour to 30th hour, but G. candidum – from 14th hour to 27th hour and from 27th hour to 50th hour. The μmax and biomass of T. cutaneum was 2.3 and 1.5 times over than μmax and biomass of G. candidum. |
| Figure 1C demonstrates the growth of three basidiomycetes - Rhodotorula mucilaginosa № 132, Cryptococcus neoformans № 3465 and Malassezia furfur № 1451. The first two fungi showed very similar diauxic growth curves:R. mucilaginosa had first exponent from 4th to 26th hour, and the second one from 26-th to 49-th hour; and Cr. neoformans – from 4th to 26th hour, and from 30th to 49th hour. These fungi had almost the equal μmax. M. furfur as obligate lipophilic yeast was cultivated in Tween 40 contained medium and had relatively slow growth with long exponent from 10th to 53rd hour and low μmax. |
| It is clear that for technological purposes it is convenient the fast growth, short exponent, and large biomass at the end of first exponent. The first two indexes related with the rate of process, and biomass will direct correlate with antigen yield. The comparison of these parameters among studied yeasts showed that the most “rapid” was S. cerevisiae, but its biomass was very low. However, R. mucilaginosa, in spite of relatively long exponent, demonstrated largest harvest of cells. Most “low” and not very fruitful was M. furfur. |
| We subscribe to an opinion that specific antigens are located predominantly on the surface of yeast cell [21]. Hence the first method of antigens obtaining was based on the careful extraction of surface proteins with SDS (see above – preparation № 1). For such extraction were used yeasts cultures at the end of exponential phase, because exactly this phase should provide us the most specific antigen complex and enough biomass [16]. The results of interaction of preparations № 1 with mice antisera shown on Figure 2A. Apparently that each yeast extract reacted better with corresponding antiserum, however it is clear that some preparations reacted with other antisera too. This may be explained in the following way: such treatment with SDS leads to partial extraction of some cross-reactive (may be internal) proteins. Moreover the preparation from M. furfur reacted with control sera, maybe because of this yeast is the resident of healthy animal skin. |
| We expected that specific proteins secreted to the extracellular medium during the yeast growth, therefore decided to collect exponential cultures, eliminate cells and carry out the dot blot analysis with cell-free cultural liquids and mice antisera (see above – preparation № 2). The appropriate results demonstrated on Figure 2B. Within the limits of sensitivity of present method we can establish that each antigen preparation № 2 reacted only with corresponded mice antisera, except the M. furfur. Most likely Tween 40, which was the constituent of defined medium for this fungus, prevented the interaction between yeast proteins and nitrocellulose. In this case one should carry out special purification of the preparation № 2, or use the preparation of late stationary phase then all Tween 40 will be consumed by cells (although in this case the cross-reacted proteins may be secreted to the medium). |
| Summarize the results we conclude that clinical yeast growth was studied and comparative estimation of growth phases, growth rates and biomass accumulation was done. Moreover the most convenient approach for subsequent development of specific yeasts diagnostic preparations was selected – it is the use of cell-free cultural liquids derivable after cultivation of yeasts in defined liquid media. |
| Acknowledgement |
| Authors express their thanks to Tamara Artemieva and Liubov Butovchenko for technical support and permanent care. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 14413
- [From(publication date):
November-2013 - Jul 13, 2025] - Breakdown by view type
- HTML page views : 9832
- PDF downloads : 4581
