Research Article Open Access
Physico-Chemical Stability of Mixtures of Fosaprepitant used in Clinical Practice
Maria Amparo Martínez-Gómez1,2*, Ana Moya Gil1,2, Begoña Porta Oltra2 and Mónica Climente Martí2
1Foundation for the Promotion of Health Biomedical Research and Valencia (FISABIO), C / No 31 Sir Masco, Valencia, Spain
2Pharmacy Service, Hospital Universitario Doctor Peset, Avda. Gaspar Aguilar 90, Valencia, Spain
- *Corresponding Author:
- Maria Amparo Martinez-Gómez
Servicio de Farmaciaacy Department
Hospital Universitario Doctor Peset
Avda. Gaspar Aguilar 90, 46017, Valencia, Spain
Tel: +34961622366
E-mail: martinez_margoma@gva.es
Received date: June 27, 2014; Accepted date: July 18, 2014; Published date: July 22, 2014
Citation: Martínez-Gómez MA, Gil AM, Oltra BP, Martí MC (2014) Physico-Chemical Stability of Mixtures of Fosaprepitant used in Clinical Practice. J Anal Bioanal Tech 5: 197. doi: 10.4172/2155-9872.1000197
Copyright: © 2014 Martínez-Gómez MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Fosaprepitant dimeglumine is a new drug indicated to prevent nausea and vomiting associated with highly emetogenic cisplatin-based and moderately emetogenic cancer chemotherapy in adults. Due to its complexity in managing, since it requires reconstitution and dilution before intraveonous administration, it is necessary to evaluate physico-chemical stability of fosaprepitant at concentrations used in our routine clinical practice and at different conditions of storage to expand the information datasheet (fosaprepitant 150 mg in 150 mL of 0.9 g/dl sodium chloride (NaCl) at ambient conditions and light for 24 hours) and simplify preparation technique conforming to the fluid marketed branches. These studies should be carried out following the recommendations International Conference on Harmonisation (ICH) guidelines for evaluate stability and the criteria of the United States Pharmacopeia to maintain quality and safety of the preparation. So, stability study of fosaprepitant 150 mg in 50, 100 and 250 mL of 0.9 g/dl NaCl at room temperature/refrigerated and protective from/exposed to ambient light has been carried out. An HPLC method has been developed and validated according to ICH guidelines to evaluate chemical stability of fosaprepitant. Physical stability study has been carried out by visual inspection, measure of pH and gravimetry to control evaporation. The results shown in this paper represent the first evidence of the physico-chemical stability of the mixtures of fosaprepitant 150 mg in 50, 100 and 250 mL of 0.9 g/dl NaCl are physico-chemically stable for 7 days at room temperature (27.0 ± 0.9°C) and refrigerated (4.9 ± 1.5°C); and exposed to ambient light, 15 days at room temperature and refrigerated and protected from light and 12 days fosaprepitant reconstituted and stored refrigerated and exposed to light.
Keywords
Fosaprepitant; Physico-chemical stability
Introduction
In hospital clinical practice, most drugs are administered by parenteral route and whose management is very complex since usually require individualization of dose based on patient anthropometric criteria and dilution before administration.
The Guideline update for MASCC (Multinational Association of Supportive Care in Cancer) and ESMO (European Society for Medical Oncology) in preventing nausea and vomiting induced by chemotherapy and radiotherapy recommends the use of receptor antagonists of 5-hydroxytryptamine-3 (5-HT3) (ondansetron, granisetron, tropisetron, dolasetron and palosetron), dexamethasone, aprepitant and fosaprepitant as antiemetic agents to prevent nausea and vomiting from acute and delayed chemotherapy highly or moderately emetogenic induced in adult rather than D2 dopamine receptor antagonists (metoclopramide hydrochloride, domperidone). Before the introduction of aprepitant and fosaprepitant, a combination of a 5-HT3 receptor antagonist plus dexamethasone was the regimen of choice for the prevention of acute nausea and vomiting in cisplatintreated patients. Aprepitant, a potent and selective antagonist of the neurokinin (NK)1 neurotransmitter receptor showed its antiemetic activity when added to a 5-HT3 receptor antagonist plus dexamethasone in several phase II double-blind studies [1].
Fosaprepitant dimeglumine (FOS) is a new drug powder for solution for infusion (Ivemend® for injection, Merck Sharp & Dohme, Spain, S.A.), indicated to prevent acute and delayed nausea and vomiting associated with highly emetogenic cisplatin-based and moderately emetogenic cancer chemotherapy in adults in intravenous administration. It is a phosphorylated prodrug that is rapidly converted to aprepitant, an oral selective neurokinnin-I receptor antagonist approved [1-4]. Each vial contains fosaprepitant dimeglumine equivalent to 150 mg fosaprepitant, which corresponds to 130.5 mg of aprepitant. It is given as part of a combination therapy with a corticosteroid and a 5-hydroxytryptamine-3 (5-HT3) receptor antagonist [2-4]. The recommended dose is 150 mg administered in 150 mL of 0.9 g/dl sodium chloride (NaCl) as an infusion over 20- 30 minutes on day 1, initiated approximately 30 minutes prior to chemotherapy [3]. The plasma fosaprepitant concentrations generally reach steady state by 15 minutes after the start of infusion; although the studies says the maximum concentration is proportional to the infusion time, no clinically important pharmacokinetic differences were noted in the cohorts examined [5] .
According to Ivemend® datasheet, physico-chemical stability of reconstituted FOS (150 mg of FOS in 5 mL of 0.9 g/dl NaCl, 30 mg/ mL) after dilution with 0.9 g/dl NaCl to a concentration of 1 mg/mL is 24 hours at 25°C; however from the microbiologic point of view, Ivemend® should be used immediately or during 24 hours at 2-8°C on the contrary all responsibility is for the usuary [1,6,7].
In a recently published study, compatibility of FOS 1 mg/mL with 0.9 g/dl NaCl, water for injection and 5% dextrose was observed after preparation and for 24 hours of storage at ambient conditions, with a maximum 0.1% of degradate formation in all 3 diluents over 24 hours. Compatibility was evaluated by HPLC for FOS and FOS degradate concentrations, particulate matter and changes in pH of the solution for 24 hours [8].
Thus, the development of appropriately designed stability studies, following the Pharmacopoeia guidelines and the recommendations of the International Committee on Harmonization (ICH), including evaluation of the chemical and physical stability and in some cases, the biological (enzymes, antibodies) and microbiological stability of preparations, may allow know higher stability data than information established by the pharmaceutical industry and with greater adaptability to clinical practice [9,10].
To evaluate physical stability of the drug in solution, the pH variation analysis, the visual inspection of color changes, cloudiness (turbidity) and/or precipitation and the gravimetry to analyze the water loss measurement, are assays methods to check that no critical change has occurred during storage before its administration [11].
To evaluate chemical stability of a drug, there are recommendations of ICH: Stability-indicating assay methods (SIAMS) [9,10]. According to United States-Food and Drug Administration (US-FDA) stability guideline of 1998, SIAMS are defined as “validated quantitative analytical methods that can detect the changes with time in the chemical, physical, or microbiological properties of the drug substance and drug product, and that are specific so that the contents of active ingredient, degradation products, and other components of interest can be accurately measured without interference [12]. Different techniques such as titrimetric, spectrophotometric and chromatographic techniques have been commonly employed in analysis of stability samples. In this sense, High Performance Liquid Chromatography (HPLC) has been very widely employed due to its high-resolution capacity, sensitivity and specificity [13]. The measure of pH of drug solution is a simple method to evaluate chemical stability too, because variations of pH can be due to degradation although it must be confirm with HPLC.
In Europe, the fluid volumes traded are 50 mL, 100 mL, 250 mL and others. However, the information of the datasheet to administrate FOS is 150 mg in 150 mL 0.9 g/dl NaCl, which does not conform making it difficult their management. Furthermore, physicalchemical stability of reconstituted drug is to 24 hours too. This study allows to expand the information of datasheet of FOS and simplify preparation technique in our routine clinical practice conforming to the fluid marketed branches maintaining quality and safety of the preparation.
Experimental
Instrumentation and chromatographic conditions
An Agilent Technologies 1100 liquid chromatograph with a quaternary pump, a diode array detector (DAD), a thermostated column compartment, an autosampler and a HP Compaq computer equipped with Agilent-Chemstation software was used. 10 μL of each solution was injected, by duplicate, into the chromatograph through a Rheodyne valve (Cotati, CA), with a 20 μ loop. Kromasil® C18 column of 5 μm particle size (250×4, 6 mm inner diameter, Análisis Vínicos, Spain) was used. Mobile phase was orthophosphoric acid (0.1%)-acetonitrile (45:55, v/v); the flow rate was set to 1 mL/min, temperature to 20°C and detection to 241 nm. The column was equilibrated for 30 min prior to injection of the drug solution.
Otophosphoric acid and acetonitrile solutions were previously vacuum-filtered through 0.45 μm nylon membranes (Micron Separations, Westboro, MA) and sonicated prior to HPLC analysis.
A pH meter (model 3510, Jenway, UK) connected to a glass pHelectrode and an analytical balance (GF-200, A&D Instruments Ltd, UK) were used to measure the pH and weight, respectively.
Chemicals
Ivemend® 150 mg (powder for infusion; batch H018972, expiration date March 2014; batch J002720, expiration date September 2014) was purchased from Merck Sharp & Dohme Ltd. (Spain). Sodium chloride 0.9 g/dl w/v intravenous infusion BP Viaflo® 50 mL, 100 mL and 250 mL were purchased from Baxter (Spain).
Acetonitrile (Scharlab S.L., Spain), ortophosphoric acid (Fluka Analytical, Sweden) and sterile water for injection (Grifols, Spain) were used to prepare the mobile phase used in chromatographic analysis.
Solutions preparation
Stock solution of FOS was prepared by reconstitution of drug powder content in commercially vial (Ivemend®) with 5 mL of 0.9 g/ dl NaCl, being FOS concentration of 30 mg/mL. For the calibration curve, six calibrators of FOS were prepared by making serial dilutions from stock solution with 0.9 g/dl NaCl over the range 0.15-3.9 mg/mL.
Buffer solution was prepared by diluting 1 mL of ortophosphoric acid with water to a final volume of 1 L.
Samples preparation and storage conditions
12 mixtures were prepared, in the same way as those prepared for hospital clinical practice following the guidelines of “Pharmaceutical Compounding: Sterile Preparations” of the United States Pharmacopeia (USP) [14]. For each mixture, 5 mL of reconstituted FOS (Ivemend®, 30 mg/mL) was injected into Viaflo® bags containing 250 mL (mixtures 1-4), 100 mL (mixtures 5-8) and 50 mL (mixtures 9-12) of 0.9 g/dl NaCl, so the concentrations were: mixtures 1-4, 0.6 mg/mL; mixtures 5-8, 1.5 mg/mL; mixtures 9-12, 3.0 mg/mL.
For each FOS concentration level, 4 mixtures were prepared. Only two of them were introduced in protective bags for ambient light (PL); one of them was stored at room temperature (RT: 20-25°C) and the other at 2-8°C (F). In the case of mixtures exposed to light (L), one of them was stored at RT and the other at F. Table 1 summarizes the storage conditions of each mixture assayed.
| Mixture | Volume (mL) | Storage conditions | |
|---|---|---|---|
| Light | T | ||
| 1 | 250 | L | F |
| 2 | 250 | L | RT |
| 3 | 250 | PL | F |
| 4 | 250 | PL | RT |
| 5 | 100 | L | F |
| 6 | 100 | L | RT |
| 7 | 100 | PL | F |
| 8 | 100 | PL | RT |
| 9 | 50 | L | F |
| 10 | 50 | L | RT |
| 11 | 50 | PL | F |
| 12 | 50 | PL | RT |
T: Temperature; L: Exposition to ambient light; PL: Protection from light
F: Refrigerated (4.9 ± 1.5°C); RT: Room temperature (27.0 ± 0.9°C).
Table 1: Conditions of storage of the mixtures of fosaprepitant assayed
Rests of reconstituted were stored in vial refrigerated and exposed to ambient light.
Chromatographic method validation
The developed chromatographic method was validated for linearity, specificity, accuracy, precision, limit of detection, limit of quantification and robustness as per ICH guidelines [9,10]. The chromatograms were evaluated on the basis of the peak area of FOS.
Linearity: The linearity was determined at six levels of FOS over the range 0.15-3.9 mg/mL. Absorbance of each calibrator was measured and the graph mean absorbance (y-axis) versus concentration (x-axis) was plotted. Correlation coefficient (r), y-intercept and slope of regression line were estimated.
Specificity: The specificity of the method was ascertained by evaluating the presence of interferences at the retention time of FOS.
Accuracy (% Recovery): The accuracy of the method was determined by calculating recoveries of FOS by method of standard additions. Known amount of FOS (0%, 50%, 100%, 150%) were added to a pre quantified sample solution, and the amount of FOS was estimated by measuring the peak areas and by fitting these values to the straight-line equation of calibration curve.
Method precision (Repeatability): Standard solutions of FOS (0.15, 1.50 and 3.00 mg/mL) were analyzed six times and relative standard deviation (%RSD) was calculated for each concentration level.
Intermediate precision (Reproducibility): Variation of results of three different concentrations (0.15, 1.50 and 3.00 mg/mL) within the same day (intra-day) and between days (inter-day) was analyzed. Intraday precision was determined by analyzing FOS for three times in the same day and inter-day precision, by analyzing FOS daily for five days.
Limit of Detection (LOD) and Limit of Quantitation (LOQ): LOD is defined as the lowest concentration of an analyte that can reliably be differentiated from background levels. LOQ of an individual analytical procedure is the lowest amount of analyte that can be quantitatively determined with suitable precision and accuracy. LOD and LOQ were calculated using following equation as per ICH guidelines. LOD=3.3×/S; LOQ=10×σ/S; Where σ is the standard deviation of y-intercepts of regression lines and S is the slope of the calibration curve.
Robustness: Robustness of the method was performed by variation in mobile phase ratio, flow rate and pH.
Physical and chemical stability assessment
Physical compatibility was evaluated daily by: (1) visual inspection of the mixtures for color changes, cloudiness (turbidity), and/or precipitation; (2) loss of volume due to evaporation by gravimetry, weighting each mixture before and after extracting aliquot to HPLC analysis; (3) pH of mixtures was measured at time 0, 12, 24 hours and after that, each 48 hours. For this purpose, at each time, an aliquot of 2.5 mL was removed from each mixture by inserting a new 2.5 mL Terumo® syringe with a needle (Microlance® sterile 19G) into the bag injection port, previous homogenization by double inversion.
Chemical stability of mixtures and reconstituted was evaluated by determining daily the amount of FOS by HPLC. For this purpose, each day, an aliquot of 150 μl was removed from each mixture and from the vial of Ivemend® with reconstituted, in the same way that for the analysis of pH. The pair data FOS concentration and time were adjusted, if it was possible, to a zero- (equation 1) or first-order kinetic equation (equation 2), where C was the drug concentration at a specific time; C0 was the drug concentration at t=0; K0 was the zero-order degradation rate constant and K1, was the first-order degradation rate constant.
C=C0 – k0·t (1)
ln C=ln C0 - k1·t (2)
The parameter T90 was used to establish the caducity of the mixture. This parameter was calculated by using equation 3 and 4, depending on the order of reaction.
T90=0.1·C0/k0 (3)
T90=0.105/k1. (4)
Results and Discussion
In the summary of product characteristics of Ivemend®, it is indicated that the white to off-white amorphous powder of FOS must be reconstituted with 5 mL of 0.9 g/dl NaCl solution and then, diluted prior to administration in an infusion bag filled with 145 mL of 0.9 g/dl NaCl solution for injection to yield a total volume of 150 mL (concentration of FOS: 1 mg/mL). This mixture is stable for 24 hours at 25°C exposed to light. Furthermore, incompatibility of FOS with any solutions containing divalent cations such as Ringer’s injection and lactated Ringer’s injection has been described [1,6,7].
There is a recently study [8] in which diluent compatibility assessment of FOS has been carried out by diluting FOS with 0.9 g/ dl sodium chloride, water for injection or 5% dextrose to a final concentration of 1 mg/mL; no significant changes has been observed in degradate color or clarity and a maximum 0.1% of degradate formation has been quantified in all 3 diluents with minimal change for the FOS assay over 24 hours storage at ambient conditions (25°C, 1 atm, ambient humidity and light).
However, in routine clinical practice in Europe, other infusion volumes are used, 50 mL, 100 mL and 250 mL, being the concentration of FOS 150 mg in solution 3.0, 1.5 and 0.6 mg/mL, respectively. In this sense, Azuma et col. conclude in their pharmacokinetic study that intravenous administration of a single 150 mg dose of FOS at different concentrations (0.6-1.5 mg/mL) and over different infusion times (15- 30 minutes) is safe and well tolerated in healthy Japanese men but they do not evaluated stability [15]. Recently, it has been published original articles about assessing physico-chemical compatibility of FOS with 5-HT3 antagonists and corticosteroids with assays of 24 hours [8,16-18].
Optimization and validation of the chromatographic method
To optimize the chromatographic conditions a C18 HPLC column, ortophosphoric acid solution (0.1%) and acetonitrile mixture were found to be the best stationary phase and mobile phase combination to have a symmetrical and well-resolved peak of FOS in 0.9 g/dl NaCl mixtures. The total runtime for the analysis was 5 min and the retention time of FOS was 3.5 min. No interference with degradation products was observed.
Chromatographic method validation
In the experimental conditions indicated, the analytical performance parameters suggested by ICH guidelines [9-10] were evaluated: linearity, specificity, accuracy, method precision, intermediate precision, limits of detection and quantification and robustness. Because of the simplicity of the procedure, no internal standard was needed.
Linearity: Correlation coefficient (r) was >0.9995, which indicates that the method obeys Beer’s law. The slope (y=473.45·x) but not the intercept value was statistically significant at 95% confidence level.
Specificity: It was adequate since no interferences were observed at retention time of FOS (3.5 min).
Accuracy: Accuracy was determined by calculating the recovery. The method was found to be accurate with percentage of recovery of 96.8%-102.8%.
Precision
a) Repeatability: The % RSD was ≤ 4.5%, which indicated that the method was precise.
b) Intra- and inter-day precision: %RSD was ≤ 1.2% in boths cases, which indicated that the method was precise.
LOD and LOQ: Under the experimental conditions used, the lowest amount of FOS that could be detected (LOD) was 0.1 mg/mL. LOQ was 0.4 mg/mL.
Robustness: %RSD values were <2% after making small deliberate changes in the developed HPLC method, which indicated that the method was robust for the intended purpose.
Figure 1 shows the chromatograms of mixtures of 150 mg FOS at 50 mL, 100 mL and 250 mL just after preparation.
So, since all the criteria were acceptable according to ICH guidelines, the proposed method was adequate to determine FOS in mixtures.
Physical and chemical stability assessment
During the study, room and fridge´s temperature were (27.0 ± 0.9)°C and (4.9 ± 1.5)°C, respectively. At the end of the study, none of the mixtures showed changes in color, precipitation of FOS or measurable losses of volume caused by evaporation at the different storage conditions. So, all mixtures were physically compatible during the time of storage.
For all the mixtures assayed, the pH value was basic; just after preparation; mean value of pH in mixtures with the same FOS concentration was: 8.5 ± 0.3 for mixtures in 50-100 mL and 7.95 ± 0.11 for mixtures in 250 mL. For all the mixtures assayed, except for mixture 11, a decrease in pH value was observed after 7 and 15 days of storage. This decrease was more important in mixtures with a volume of 250 mL (variation of pH from 6.6 to 9.9%) and mixture 5 (-12.6%). Table 2 shows variation of pH during the study for each mixture assayed.
| Mixture | Storage conditions | Variation of pH (%) | Remaining concentration (%) | |||
|---|---|---|---|---|---|---|
| Light | T | 7 days | 15 days | 7 days | 15 days | |
| 1 | L | F | -6.6 | -6.6 | 99.33 ± 1.00 | 98.53 ± 0.15 |
| 2 | L | RT | -9.3 | -10.0 | 100.20 ± 0.70 | 99.40 ± 0.50 |
| 3 | PL | F | -8.7 | - | 97.00 ± 2.00 | - |
| 4 | PL | RT | -9.9 | - | 98.40 ± 1.30 | - |
| 5 | L | F | -12.6 | -13.4 | 99.45 ± 1.02 | 98.30 ± 1.50 |
| 6 | L | RT | -1.9 | -1.7 | 99.09 ± 0.19 | 97.50 ± 2.40 |
| 7 | PL | F | -1.1 | - | 98.00 ± 0.40 | - |
| 8 | PL | RT | -2.9 | - | 99.00 ± 0.60 | - |
| 9 | L | F | -1.8 | -1.9 | 98.64 ± 0.10 | 97.00 ± 2.00 |
| 10 | L | RT | -0.9 | -1.2 | 98.30 ± 0.60 | 97.57 ± 0.06 |
| 11 | PL | F | +0.4 | - | 98.30 ± 0.30 | - |
| 12 | PL | RT | -1.2 | - | 98.60 ± 0.30 | - |
| R | L | F | - | - | 96.80 ± 0.90 | 74.68 ± 1.00 |
T: Temperature; L: Exposition to ambient light; PL: Protection from light
F: Refrigerated (4.9 ± 1.5°C); RT: Room temperature (27.0 ± 0.9°C);
R: Reconstituted
Table 2: Variation of pH and remaining concentration of fosaprepitant in mixtures during storage.
As regards variation of FOS concentration with time, Table 2 shows the percentage of FOS remaining at the different concentrations and conditions of storage assayed. As can be observed, in all mixtures remaining concentration of FOS was ≥97% after 7 days of storage; a slightly decrease in concentration was observed in mixtures exposed to light after 15 days of storage, but anyway remaining concentration was ≥97%. So, it seems to be that temperature, light and FOS concentration do not affect FOS chemical stability.
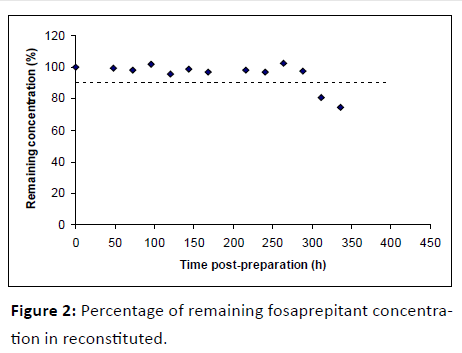
Figure 2 shows variation of remaining FOS concentration in reconstituted during the time of the study; as can be observed, remaining concentration was upper than 90% after 12 days of storage, decreasing from this moment to day 15th up to 74.68%.
To determine the caducity of the mixtures, the pair data FOS concentration and time were adjusted to a zero- and first-order kinetic equations (equations 1 and 2) and the parameter T90 was calculated as indicated (equations 3 and 4). Table 3 shows the calculated degradation constant and T90 values obtained for each mixture. When it was not possible to adjust pair data to kinetic equations, caducity of mixtures was established by considering the maximum time at which remaining FOS concentration determined was ≥90%. So, the results obtained of FOS by HPLC indicated that mixtures of FOS 150 mg in 50 mL, 100 mL and 250 mL of 0.9 g/dl NaCl were chemically stable for 7 days at RT, F, L and PL. Stability up to 15 days can be guarantee under L at both temperatures stored and in all volumes assayed.
| Mixture | Zero-order kinetic | First-order kinetic | ||||
|---|---|---|---|---|---|---|
| K0 ·10-4 (mg·mL-1·h-1) |
R2 | T90 (days) |
K1·10-4 (mg·mL-1·h-1) |
R2 | T90 (days) | |
| 1 | - | - | 15* | - | - | 15* |
| 2 | - | - | 15* | - | - | 15* |
| 3 | 1.38 ± 0.20 | 0.8298 | 18 | 2.30 ± 0.40 | 0.8297 | 19 |
| 4 | - | - | 7* | - | - | 7* |
| 5 | - | - | 15* | - | - | 15* |
| 6 | - | - | 15* | - | - | 15* |
| 7 | - | - | 7* | - | - | 7* |
| 8 | - | - | 7* | - | - | 7* |
| 9 | 2.01 ± 0.25 | 0.7112 | 54 | 0.78 ± 0.11 | 0.7109 | 56 |
| 10 | - | - | 15* | - | - | 15* |
| 11 | 5.10 ± 0.70 | 0.8304 | 20 | 2.10 ± 0.30 | 0.8298 | 21 |
| 12 | 3.30 ± 0.50 | 0.8250 | 13 | 7.99 ± 1.06 | 0.8210 | 13 |
| R | - | - | 12 | - | - | |
R: Reconstituted
*Maximum experimental time at which remaining fosaprepitant concentration measured was ≥90%.
Table 3: Degradation constants and T90 values for mixtures assayed.
On the other hand, a limit of the study is the variation of pH of mixtures, but could not due to instability of FOS and could be explained by considering the flow of CO2 through polyolefin bag and the consequent acidification of solution. Another point to consider is the reconstitution process because when the FOS was reconstituted with 5 mL of 0.9 g/dl NaCl, foaming was observed in 10 assays; after 30 min of reconstitution an increase of 0.5 ± 0.1 mL was observed, which can mean underdosing in 10% in the dose of the clinical practice.
Our study is useful in clinical practice since it provides information on physico-chemical stability of FOS at different concentrations used in routine (150 mg of FOS in 50 mL, 100mL and 250 mL in 0.9 g/dl NaCl) and at different conditions of temperature (RT and F) and light (L and PL). In this sense, versatility of preparation conditions of FOS has been increased respect to Ivemend® product information from the industry and it is possible to preparate FOS mixtures in advance following the criteria of the USP. In this environment, it is important to know the stability of a drug in solution and its compatibility with other drugs. In general, the information contained in the datasheet of the drug is insufficient and it is necessary to consult other databases such as Trissel’s handbook on injectable drugs and Stabilis, although in most cases, information does not meet the conditions in clinical practice [6,7].
The centralization of the preparation of parenteral drugs in the Pharmacy Department, under the supervision of a responsible and trained pharmacist in preparation of drugs, plays an important role in the management of these drugs because it allows its handling in sterile conditions, fulfilling the requirements of good preparation practices. The Chapter 797 of USP and the General Direction of Basic Services of the National Health System and Pharmacy of the Health, Social Services and Equality Government of Spain have the aim to describe conditions and practices to prevent harm, including death, to patients that could result from microbial contamination (non-sterility), excessive bacterial endotoxins, variability in the intended strength of correct ingredients that exceeds either monograph limits for official articles or 10% for nonofficial articles, unintended chemical and physical contaminants, and ingredients of inappropriate quality in compounded sterile preparations (CSPs). CSPs are potentially most hazardous to patients when administered into body cavities, central nervous and vascular systems, eyes, and joints, and when used as baths for live organs and tissues. Despite the extensive attention in this chapter to the provision, maintenance, and evaluation of air quality, the avoidance of direct or physical contact contamination is paramount. To achieve the above conditions and practices, this chapter provides minimum practice and quality standards for CSPs of drugs based on current scientific information and best sterile compounding practices. In this sense, considering the preparations of FOS like CSP compounder under the conditions at a low risk level preparation of contamination (the CSP are compounded with aseptic manipulations entirely within ISO Class 5 or better air quality using only sterile products, components and devices, When involves only compounding the transfer and not more than two entries into any one sterile container or package), the storage periods cannot exceed before administration more than 48 hours at controlled room temperature, more than 14 days at a cold temperature and for 45 days in solid frozen state between -25°C and -10°C [14-19].
The stability studies improve quality in health care effectiveness because they guarantee the chemical integrity and activity of the active ingredients of the preparation, in security because they prevent the formation of precipitates or toxic metabolites that may affect patient safety, in efficiency because they allow reuse remains of medication vials or preparations administered, generating savings to the health system and in quality of life of patients because they advance planning and preparation by reducing waiting times.
Conclusion
Guaranteed physico-chemical stability in preparation of mixture of Fosaprepitant 150 mg, after reconstituted in 5 mL of 0.9 g/dl NaCl stored at 2-8°C and exposed to light for 12 days and after dilution with 50 mL, 100 mL, 250 mL of 0.9 g/dl NaCl after storage at room temperature or at 2-8°C for 7 days under protection from light and for 14 days exposed to light. However, considering these preparations as CSPS risk level, mixtures stored at controlled room temperature have to be administered before 48 hours after preparation. The results from this paper represent the first evidence of the physico-chemical stability of fosaprepitant at different concentrations used in routine clinical practice and different conditions of storage. We hope these results will permit health care professionals to optimize the preparation and administration of fosaprepitant in order to improve the quality of the treatment of oncology patient.
Acknowledgements
The authors acknowledge FISABIO for the financial support.
References
- Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, et al. (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21 Suppl 5: v232-243.
- European Medicines Agency (2014) Ivemend® 150MG-EMEA/H/C/000743 -PSUV/0022. EPAR-Product information.
- Feyer P, Jordan K (2011) Update and new trends in antiemetic therapy: the continuing need for novel therapies. Ann Oncol 22: 30-38.
- Celio L, Ricchini F, De Braud F (2013) Safety, efficacy, and patient acceptability of single-dose fosaprepitant regimen for the prevention of chemotherapy-induced nausea and vomiting. Patient Prefer Adherence 7: 391-400.
- Junichi A, Hiroyuki F (2013) Pharmacokinetics of a single 150 mg intravenous infusion of fosaprepitant: effects of concentration and infusion time in healthy japanese men. Clin Pharmacol Drug Devel 2: 394-399.
- Stabilis (2014) Monographie of fosaprepitant.
- Trissel LA (2013) Handbook of injectable drugs. 17th edition, American Society of Health-System Pharmacists.
- Sun S, Schaller J, Placek J, Duersch B (2013) Compatibility of intravenous fosaprepitant with intravenous 5-HT3 antagonists and corticosteroids. Cancer Chemother Pharmacol 72: 509-513.
- Skrdla PJ, Abrahim A, Wu Y (2006) An HPLC chromatographic reactor approach for investigating the hydrolytic stability of a pharmaceutical compound. J Pharm Biomed Anal 41: 883-890.
- Templeton I, Eichenbaum G, Sane R, Zhou J (2014) Case study 5. Deconvoluting hyperbilirubinemia: differentiating between hepatotoxicity and reversible inhibition of UGT1A1, MRP2, or OATP1B1 in drug development. Methods Mol Biol 1113: 471-483.
- Gómez MA, Arenas VJ, Sanjuán MM, Hernández MJ, Almenar CB, et al. (2007) Stability studies of binary mixtures of haloperidol and/or midazolam with other drugs for parenteral administration. J Palliat Med 10: 1306-1311.
- U.S. Department of Health and Human Services (1998) Guidance for industry: stability testing of drug substances and drug products (Draft guidance). Food and Drug Administration, Rockville, MD.
- Bakshi M, Singh S (2002) Development of validated stability-indicating assay methods--critical review. J Pharm Biomed Anal 28: 1011-1040.
- United States Pharmacopeia (2011) Pharmaceutical compounding: sterile preparations. The United States Pharmacopeial (USP) Convention, edition (USP 35 NF 30).
- Junichi A, Hiroyuki F (2013) Pharmacokinetics of a single 150 mg intravenous infusion of fosaprepitant: effects of concentration and infusion time in healthy japanese men. Clin Pharmacol Drug Devel 2: 394-399.
- Lipp HP, Gfrörer W, Herbst N (2011) Physico-chemical compatibility of palonosetron HCI, fosaprepitant dimeglumine and dexamethasone-21-dihydrogene-phosphate IV admixtures over at least 24 hours. Support Care Cancer 19: 67-370.
- Lipp HP, Gfrörer W (2014) Assessing physico-chemical compatibility of concomitantly diluted antiemetics including palonosetron-HCl and fosaprepitant dimeglumine. Cancer Chemother Pharmacol 73: 435-436.
- Duersch B (2014) Reply to: Assessing physico-chemical compatibility of concomitantly diluted antiemetics including palonosetron-HCl and fosaprepitant dimeglumine. Cancer Chemother Pharmacol 73: 437.
- Spanish National Health (2014) Guide to good practices in preparing medicines in hospital pharmacy services. The Spanish National Health Service. Ministry of Health, Social Services and Equality.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 24083
- [From(publication date):
September-2014 - Jul 04, 2025] - Breakdown by view type
- HTML page views : 18842
- PDF downloads : 5241