Research Article Open Access
Phylogenetic Position of Psittacula Parakeet Bird from Enggano Island, Based on Analyses of Mitochondrial Cytochrome B Gene
Astuti D*, Ashari H and NPrijono SResearch Centre for Biology, Indonesian Insitute of Sciences, Gd, Widyasatwaloka, CSC, Jl. Jakarta-Bogor KM, Cibinong, West Jawa, Indonesia
- *Corresponding Author:
- Astuti D
Research Centre for Biology, Indonesian Insitute of Sciences
Gd, Widyasatwaloka, CSC, Jl. Jakarta-Bogor KM 46
Cibinong, West Jawa, Indonesia
Tel: +33 1 45 25 03 29
E-mail: wiek002@yahoo.com
Received date June 03, 2016; Accepted date July 02, 2016; Published date July 08, 2016
Citation: Astuti D, Ashari H, NPrijono S (2016) Phylogenetic Position of Psittacula Parakeet Bird from Enggano Island, Based on Analyses of Mitochondrial Cytochrome B Gene. J Ecosys Ecograph 6:195. doi:10.4172/2157-7625.1000195
Copyright: © 2016 Astuti D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Ecosystem & Ecography
Abstract
Enggano Island of Indonesia has Psittacula parakeet bird; namely Psittacula longicauda modesta. Phylogenetically, the position of the bird has not been studied yet. The present study used DNA sequences of mitochondrial cytochrome b (cyt b) gene to analyze phylogenetic relationships within Psittacula parakeet birds; especially to reveal the phylogenetic position of Psittacula longicauda modesta. Blood samples were collected from two Psittacula species; Psittacula alexandri from Jawa island, and Psittacula longicauda (Psittacula longicauda modesta from Enggano island and P. l. defontainei from Natuna island). Blood samples were taken from each bird and DNA was extracted from each blood sample. PCR was performed to amplify a single fragment of cyt b gene, by using a pair of nucleotide primer. The DNA targets were then be sequenced. Totally 868-bp of cyt b was used to calculate genetic divergence within and between Psittacula parakeet, and to construct phylogenetic trees. DNA sequence data from others Psittacula species were taken from GenBank. Columba livia, Accipiter, and Cacatua were used as outgroup species. The mean genetic divergence within Psittacula longicauda was 2.16% for P. l. modesta vs P. l. defontainei, 2.37% for P. l. modesta vs P. l. longicauda, and 1.51% for P. l. defontainei vs P. l. longicauda. The mean genetic divergences within Psittacula were 0.0512 ± 0.0051. Both Phylogenetic (NJ and ML) trees showed that P. l. defontainea (Natuna is.) and P. l. longicauda grouped together and to be sister group, while the position of Psittacula parakeet from Enggano island (P. l. modesta) was distant from and as a sister group of (P. l. defontainei and P. l. longicauda). P. longicauda and P. alexandri group together and appeared to be sister group.
Keywords
Psittacula parakeet; Enggano island; DNA sequence; DNA mitochondrial; Cyt b; Genetic divergence; Phylogenetic relationships
Introduction
Enggano Island is one of the outer islands of Indonesia, located in Indian Ocean, approximately 100 km South West of the mainland Sumatra Island, 5°17’ - 5°31’ S and 102° 05’ - 102° 25’ E. It is separated from Sumatra Island by marine basin with a depth of 2000 m. Biologically, it has high endemicity and a wealth of biodiversity. Based on Enggano expedition on 2015 there were many animal species that were found, including around 27 bird species. One of the birds is parrot bird; named Psittacula longicauda modesta ; a subspecies of Psittacula longicauda and as an endemic bird in Enggano Island [1,2].
Other subspecies of P. longicauda are P. l. tytlery , P. l. nicobarica , and P. l. longicauda [3], P. l. defontainei , and P. l. modesta [1]. Each subspecies is distributed in the other islands and outside of Indonesia. In Indonesia, P. l. longicauda is occured in Sumatra, Borneo, Riau Islands, while P. l. defontainei is occured in Natuna Island [1].
Psittacula parakeet bird belongs to parrot bird (order: Psittaciformes ). In the world, there are 15-16 species of Psittacula parakeets which are Psittacula eupatria , P. wardi , P. krameri , P. echo , P. exsul , P. (himalayana) himalayana, P. (himalayana ) finschii, P. intermedia, P. cyanocephala, P. roseata, P. columboides, P. calthropae, P. derbiana, P. alexandri, P. caniceps, and P. longicauda [1-4].
Some previous authors have studied on relationships and the evolutions of Psittacula parakeet birds based on spot colors on the head [5] and molecular data [5,6]. However, there has been no philogeny research report that reveals the position of the parakeet from Enggano Island (P. l. modesta ) either by genetical or morphological character.
Mitochondrial DNA is DNA markers that have been used and recognized to have characters that can reveal the problems of taxonomic species [7]. Mitochondrial DNA consisted of 22-proteincoding genes; one of them is cyt b gene. The gene was chosen in this study because it has been used in animal to reveal taxonomic problems in Parrot birds (e.g. Groombridge et al. [5]; Kundu et al. [6]; Schweizer et al. [8]), and ease to amplify the gene by PCR.
Therefore, through the analyses of mitochondrial cyt b gene sequences, this study was conducted to know 1) phylogenetically, where the position of P. l. modesta from Enggano island is, 2) genetically, whether Psittacula from Enggano is separated from others subspecies of Psittacula longicauda , 3) how far the differgencies (sequence divergences) between Psittacula from Enggano and others subspecies of Psittacula longicauda are, 4) how far the difergencies (sequence divergences) between species of Psittacula are, 5) genetically, whether Psittacula from Enggano is as subspecies of P. longicauda or become a new species.
Methods
Sampling
Genetic materials in form of bloods were collected from each live Psittacula parakeet bird in its origin locations. Totally 13 bird blood samples consisted of 5 samples of Psittacula longicauda defontainei from Natuna Is., 4 samples of P. l. modesta from Enggano Is., and 4 samples of P. alexandri from Java Is. Each blood sample was preserved separately in the 96% of ethanol absolute in 2 ml tube.
DNA extraction
DNA was extracted from each blood sample using QIAGEN DNA Mini Kit by following manufacture protocol. Then, each DNA sample was visualized in the electrophoresis process and ultravioled photo. This process was performed to check the DNA content, quality, and quantity.
Gene fragment amplification
Polymerase Chain reaction (PCR) was performed to amplify a single DNA fragment target of cyt be gene by using a pair of oligonucleotide primers; L14841 AAAAAGCTTCCATCCAACATCTCAGCATGATG AA and H 15767 ATGAAGGGATGTTCTACTGGTTG[9]. PCR condit ions were: 95°C, 35 X (94°C- 30 sec., 52°C-30 sec, 72°C- 60 sec), 72°C-7 min. Each PCR product was containing the DNA fragment target, then to be purified and sequenced. DNA sequencing was done in Fist Base Company.
DNA sequence data analyses
All DNA sequence data’s of each bird and gene were aligned using ProSEq software. DNA variations, substitutions, base compositions, etc. were analysed using MEGA5 version 4 software. Each sequence data was translated to the amino acid to check and examine the absence of pseudo gene and stop codons. DNA sequence data of others Psittacula parakeet and outgroup species (Columba, Accipiter, and Cacatua) were adopted from GenBank. The species name and accession numbers from Genbank are P. columboides (AY220108), P. krameri (AY220114.1), Psittacula roseata (KJ456438.1), Psittacula cyanocephala (GQ996508.1), Psittacula finschi (KJ456435.1), Psittacula himalayana (KJ456436.1), Cacatua alba (AB177973.1), Cacatua galerita (AB177977.1), Columba livia (KC811464.1), and Accipiter bicolor (AY987307).
Phylogenetic analyses
From around 1100-bp of DNA fragment targets of cyt b gene, in this study we decided to analyze only 868-bp to construct NJ and ML trees using MEGA5 version4 [10]. NJ tree was performed using 1000 replicates with Kimura 2-parameters distance, and calculated all codon positions. Model Test was analysed in Mega5 version 4 software models with the lowest BIC scores (Bayesian Information Criterion) was considered to describe the substitution pattern the best, and resulted HKY+G model [11] is the best model for cyt b data in constructing ML tree in this study.
Results
Profiles of DNA sequences in the cyt b gene of Psittacula Parakeets
DNA sequences of cyb be gene in this study were no insertions and deletions, no stop codons, and no pseudogenes. The sequence data were divided into three codon positions and consisted of 287 amino acids. Base compositions in the 868-bp of cyt b gene were presented in Table 1. Composition of C+G was 48.97% less than A+T (51.03%). Cytosine (C) was the highest (35.62%) and Guanine was the lowest (13.17%). Thymine was the highest at second codon position, C and A was the highest at third codon position, and Guanine (G) was the lowest at third codon position.
| Codon position | Thymine (T) | Citosine (C) | Adenin (A) | Guanine (G) | Total base |
|---|---|---|---|---|---|
| % | % | % | % | ||
| 1st position | 23.55 | 29.67 | 24.66 | 22.11 | 290 |
| 2nd position | 37.23 | 28.50 | 20.24 | 14.03 | 289 |
| 3rd positions | 10.32 | 48.66 | 37.65 | 3.37 | 289 |
| All positions | 23.68 | 35.62 | 27.52 | 13.17 | 868 |
Table 1: Mean base composition in the cyt b gene sequences of Psittacula parakeets examined in the current study.
Table 2 presented that nucleotide substitutions were occurred in the sequence data of cytb gene in Psittacula parakeets that were examined in the present study. The nucleotide substitutions were the highest in the third codon position (34 pairs) followed by first (5 pairs) and second codon positions (3 pairs). Among to the 868-bp, the numbers of transitional substitutions (37 pairs) were more abundant than transitional substitutions (5 pairs), and transitional: transversional ratio (R) was 7.40.
| Codon position | Identical pairs (ii) | Transitional pairs(si) | Transversional  pairs (sv) |
si/sv ratio (R) |
|---|---|---|---|---|
| 1st position | 285,00 | 4,00 | 1,00 | 5,03 |
| 2nd position | 286,00 | 2,00 | 1,00 | 3,11 |
| 3rd positions | 256,00 | 31,00 | 3,00 | 10,09 |
| All positions | 827,00 | 37,00 | 5,00 | 7,40 |
Table 2: Nucleotide substitution pairs in the 868-bp of cyt b sequences of Psittacula.
Nucleotide substitution between thymine (T) and cytosine (C) (T ←---→ C) were the highest (26 bases) followed by A←→G (11 bases). But, there were no substitutions between T ←-→ A, C ←→G, and T ←→ G (Table 3). Among to the 868-bp of cyt b, the number of invariable was 580 sites, and variable was 228 sites which 183 sites were phylogenetically informative.
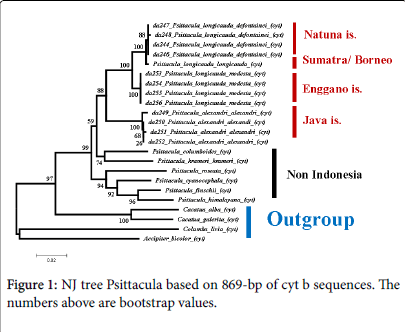
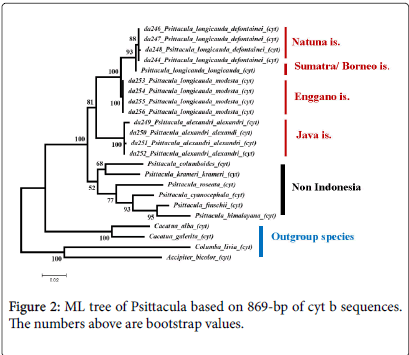
Intraspecific variations were found in the cyt b sequence data of Psittacula . Low intraspecific variation resulted very low intraspecific genetic distances/genetic divergence in the Psittacula, which ranged from 0.00% (P. l. modesta ) to 0.40% (P. a. alexandri ), while for P. l. defontainea was 0.2% (Table 4). The intraspecific differences in Psittacula not affected to the profiles of both phylogenetic NJ and ML trees, because every birds of the same species were grouped together into same clade that supported by 100% bootstrap values (Figures 1 and 2).
| S No. | Species | Distance |
|---|---|---|
| 1 | P.l. defontainea | 0,0023 ± 0,0012 |
| 2 | P.l. modesta | 0.0000 ± 0.0000 |
| 3 | P. alexandrialexandri | 0,0026 ± 0.0016 |
Table 4: Intraspecific distance calculated from cyt b gene sequences.
| Codon position | Base pair | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TT | GG | CC | AA | TC | CA | AG | TA | CG | |
| 1st position | 67,00 | 63,00 | 84,00 | 71,00 | 3,00 | 0,00 | 1,00 | 0,00 | 0,00 |
| 2nd position | 107,00 | 40,00 | 81,00 | 58,00 | 1,00 | 1,00 | 1,00 | 0,00 | 0,00 |
| 3rd positions | 19,00 | 5,00 | 129,00 | 103,00 | 22,00 | 3,00 | 9,00 | 0,00 | 0,00 |
| All positions | 193,00 | 109,00 | 294,00 | 232,00 | 26,00 | 4,00 | 11,00 | 0,00 | 0,00 |
Table 3: Base Pairwise substitution in the 868-bp of cyt b gene sequences of Psittacula.
Overall, mean distance between species of Psittacula based on cyt b gene was 0.0512 ± 0.0051, from the lowest which is around 0.0380 ± 0.00567 (P. finschii vs P. himalayana ) to the highest which is around 0.1003 ± 0.0123 (P. himalayana vs P. alexandri), whereas mean distance within P. longicauda was around 0.0151 ± 0,0085 (P. l. longicauda vs P. l. defontainei ), 0,0216 ± 0,0080 (P. l. modesta vs P.l. defontainei ), and 0,0237 ± 0,0058 (P. l. modesta vs P. l. longicauda ) (Table 5).
| S No. | Species name | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P. columboides | 0,0122 | 0,0121 | 0,0095 | 0,0096 | 0,0108 | 0,0096 | 0,0096 | 0,0096 | 0,0095 | |
| 2 | P. finschii | 0,0926 | 0,0057 | 0,0078 | 0,0119 | 0,0106 | 0,0106 | 0,0099 | 0,0105 | 0,0103 | |
| 3 | P. himalayana | 0,0937 | 0,0380 | 0,0092 | 0,0111 | 0,0109 | 0,0123 | 0,0107 | 0,0116 | 0,0116 | |
| 4 | P. cyanochepala | 0,0749 | 0,0437 | 0,0651 | 0,0098 | 0,0105 | 0,0109 | 0,0107 | 0,0103 | 0,0104 | |
| 5 | P. krameri | 0,0595 | 0,0855 | 0,0764 | 0,0767 | 0,0121 | 0,0112 | 0,0105 | 0,0102 | 0,0111 | |
| 6 | P. roseata | 0,0808 | 0,0780 | 0,0851 | 0,0665 | 0,0825 | 0,0112 | 0,0106 | 0,0122 | 0,0114 | |
| 7 | P. alexandrialexandri | 0,0661 | 0,0900 | 0,1003 | 0,0888 | 0,0712 | 0,0825 | 0,0081 | 0,0047 | 0,0033 | |
| 8 | P. longicaodadefontainei | 0,0640 | 0,0866 | 0,0911 | 0,0878 | 0,0665 | 0,0712 | 0,0615 | 0,0080 | 0,0085 | |
| 9 | P. longicaodamodesta | 0,0652 | 0,0844 | 0,0896 | 0,0832 | 0,0667 | 0,0665 | 0,0547 | 0,0216 | 0,0058 | |
| 10 | P. longicaudalongicauda | 0,0665 | 0,0886 | 0,0978 | 0,0874 | 0,0738 | 0,0852 | 0,0684 | 0,0151 | 0,0237 |
Table 5: Genetic distances (below diagonal) and standrat error (above diagonal) between species of Psittacula, based on 868-bp of cyt b sequences.
Phylogenetic relationships
Based on cyt b gene data, the filogenetic analyses using Neigbornjoining (NJ) and Maximum-Likelihood (ML) revealed the same topologies of both NJ and ML trees. All birds from the same species were clustered in the same clade and supported by 100% bootstrap value. For instance, four individuals of P. longicauda defontainea were clustered together in the same clade, four individuals of P. l. modesta were grouped together in the same clade, this was also occurred for P. alexandri .
In both NJ and ML trees, P. l. longicauda and P. l. defontainea were grouped together in the same clade and supported by bootstrap values 100% for NJ and 93% for ML. Whereas, P. l. modesta appeared to be distant from and to be sister group of P. l. longicauda and P. l. Defontainea that supported by bootstrap values 100% (NJ) and 100% (ML). P. alexandri appeared to be a sister group of P. longicauda with bootstrap values 88% (NJ) and 81% (ML).
Others species of Psittacula from outside of Indonesia (P. columboides , P. krameri , P. roseata , P. cyanocephala , P. finschii , P. himalayana ) were grouped in different clade. In ML tree, they were very clearly separated from Psittacula of Indonesia (P. longicauda and P.alexandri ) with bootstrap value was 100%. In both NJ and ML trees P. columboides and P. krameri were grouped together in same clade with 74% (NJ) and 68% (ML) bootstrap values. Other clade consisted of P. roseata , P. cyanocephala , P. finschii , and P. himalayana with 94% (NJ) and 77% (ML) bootstrap values, respectively. Relationship between clade consisted of (P. columboides and P. krameri ) and clade consisted of (P. columboides , P. krameri , P. roseata , P. cyanocephala , P. finschii , P. himalayana ) was not stable. The NJ tree clade of (P. roseata , P. cyanocephala , P. finschii , and P. himalayana ) was separated from clade of P. columboides and P. krameri ) by 99% bootstrap value. However according to ML tree, they appeared to be a sister group though only supported by 51% bootstrap value.
Discussion
The present study was using blood, tissue samples, and analysing mitochondrial DNA. Occasionally, blood samples mitochondrial DNA was contaminated by nuclear pseudogene, consequently DNA sequences of the cyt b gene were also contained pseudogene. Whereas, mitochondrial DNA from tissue samples relatively had low density of copy of mt DNA. In the present study, there was no indication of pseudogene because there were no stop codons. At the moment we translated DNA sequences to the amino acid, each of three nucleotides constructed an amino acid. Cyt b gene data of the present study had C +G 48.97% of C+G lest than A+T (51.03%) and low in Guanine. It was typically or often occurred in the mitochondrial DNA [12] as well as the mitogenome of A. fasciata and B. lagopus A + T content of 54.2% and 55.0%, respectively [13].
There is no intraspecific variation in the cyt b of P. l. modesta from Enggano Island which indicated that the four birds of P. l. modesta were identical sequences. It was assumed that it might be caused by inbreeding value [14], small population [15], habitat fragmentation [16], effect of endemism [17], or isolated population [18].
P. l. longicauda and P. l. defontainea were grouped in the same clade by 1.51% genetic divergence. It was relevant to the genetic divergence of cyt b from two subspecies of Pyrrhula pyrrhula (1.0 % to 1.5% of genetic divergence). It was indicated that the status of P. l. longicauda and P. l. defontainea were comfortable as subspecies of P. longicauda . In case of P. l. modesta , its genetic divergence from P. l. defontainea was 0.216% and 0.237% from P. l. longicauda . It was relatively higher than genetic divergence between P. l. longicauda + P. l. defontainea . In Pyrruhula, genetic divergences between species were range from 2.6% to 7.5% [19]. Even though genetic divergence between P. l. modesta and P. l. defontanea or P. l. longicauda was more than 1.5 % but it was less than 2.6% to 7.5% [19], 10.1% to 12.8% (Catharthes aura vs C. burrovianus ) [20], and also less than 5.12% (3.80% to 10.03%) of mean genetic distance/divergence between others species of Psittacula that this study examined. Based on the genetic/sequence divergence resulted in the present study, we assumed that P. l. modesta from Enggano Island was still as a subspecies of P. longicauda .
According to Wink [21] the genetic divergence might be useful in some instances to discuss the taxonomic position of the species. P. alexandri appeared as a sister group of P. longicauda with genetic divergence range from 5.47% to 6.84%. While, subspecies of P. longicauda were grouped together with genetic devergence range from 1.51% to 2.37%. If we follow Shields and Wilson [22] and Avise [23] that 2% sequence divergence is equivalent to 1 MY or sequence divergence 2% per MY, we assumed that might be P. alexandri from Java island separated from P. longicauda around 3 – 3.5 MYA, and P. l. modesta (from Enggano Island) was separated from others species of P. longicauda around 2 MYA [24-26].
Conclussion
The present study concluded that 1) Phylogenetically, the position of Psittacula from Enggano Island (Psittacula longicauda modesta) was distant from and as a sister group of others subspecies of Psittacula longicauda (P. l. longicauda and P. l. defontainea ), 2) sequence divergence within Psittacula longicauda were 2.37% for P. l. modesta vs P. l. longicauda , 2.16% for P. l. modesta vs P. l. defontainea, 1.51% for P. l. longicauda vs P. l. defontainea . 3) The mean sequence divergence between species of Psittacula was 5.12% ranged from 3.80% (P. finschii vs P. himalayana ) to 10.03% (P. himalayana vs P. alexandri ). Based on sequence divergence, we conclude that Psittacula from Enggano Island (P. l. modesta ) is a subspecies of P. longicauda or is not a new species of Psittacula.
Acknowledgment
We would like to thank to staff members of the Zoological Division, Research Centre for Biology, and The Indonesian Institute of Sciences for their kind help in doing DNA analyses for the present study. Especial thank to Dr. Amir Hamidy (Coordinator of Enggano Island Expedition 2015) for his suggestions and financial supports. This research was supported by Enggano Expedition Project, R.C. for Biology, Indonesian Institute for Sciences.
References
- Forshaw JM (1989)Parrots of the world. Princeton University Press, USA. pp: 336.
- Juniper T,Parr M (1998) A guide to parrots of the world.Yale University Press, New Haven and London. pp: 584.
- Dickinson EC, Bahr N, Dowsett R, Pearson D, Remsen V (2003) The howard and moore complete checklist of the birds of the world.Londen: A & C Black, London. pp: 1039.
- Sibley CG , Monroe JB (1990) Distribution and taxonomy of birds of the world. Yale University Press.London. pp: 1111.
- Groombridge JJ, Jones CG, Nichols RA, Carlton M, Bruforda WW (2004) Molecular phylogeny and morphological change in the Psittacula parakeets. Molecular Phylogenetics and Evolution 31: 96âÂ?Â?108.
- Kundu S, Jones SG, Prys-Jones RP, Groombridge JJ (2012) The evolution of the Indian Oceanparrots (Psittaciformes) Extinction, adaptive radiation and eustacy. MolecularPhylogenetics and Evolution 62: 296âÂ?Â?305.
- Avise JC, Arnold J, Ball RM, Bermingham E, Lamb T, et al. (1987) Intraspecific phylogeography the mitochondrial DNA bridge between population genetics and systematics. Annu Rev EcolSyst 18: 489-522
- Schweizer M, Gu¨ntert M, Stefan T,Hertwig(2012) Phylogeny and biogeography of the parrot genus Prioniturus Aves Psittaciformes.
- Edwards SV, Arctander P, Wilson AC (1991) Mitochondrial resolution of a deep branchin the genealogical tree for perching birds. The Royal Society 243: 99-107
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. MolBiolEvol 28: 2731-2739.
- Nei M, Kumar S(2000) Molecular evolution and phylogenetics. Oxford University Press, New York.pp: 3-39.
- Helbig AJ, Kocum A, Seibold I, Braun MJ (2005) A multi-gene phylogeny of aquiline eagles Aves Accipitriformes reveals extensive paraphyly at the genus level. Molecular Phylogenetics and Evolution 35: 147-164.
- Jiang L, Chen J, Wang P, Ren Q, Yuan J, et al. (2015)The Mitochondrial Genomes of Aquila fasciata and Buteolagopus (Aves, Accipitriformes): Sequence, Structure and Phylogenetic Analyses. PLoS ONE 10: 0136297.
- Frankham R, Ballou JD, Briscoe DA (2010) Introduction to conservation genetics.Cambridge University Press, USA. pp: 617.
- Furlan E , Stoklosa J, Griffiths J, Gust N, Ellis R, et al. (2012) Small population size and extremely low levels of genetic diversity in island populations of the platypus, Ornithorhynchusanatinus. Ecology and Evolution 2: 844-857.
- Dixo M, Metzger JP, Morgante JS, Zamudio KR (2009) Habitat fragmentation reducesgenetic diversity and connectivity among toad populations in the Brazilian Atlantic Coastal Forest. Biological Conservation 142: 1560âÂ?Â?1569.
- Frankham R (1997) Do island populations have less genetic variation than mainland populations? Heredity78: 311-327.
- Meffe GK, Caroll R (1994) Genetics conservation of diversity within species. In Sinauer Associates,Principles of Conservation Biology, Sunderland. pp: 143-178.
- Töpfer T, Haring E, Birkhead TR, Lopes RJ, Severinghaus LA, et al. (2011) A molecular phylogeny of bullfinches PyrrhulaBrisson, 1760 Aves Fringillidae. Molecular Phylogenetics and Evolution58:271âÂ?Â?282.
- Tavera JJ, Acero AP, Balart EF, Bernardi G (2012) Molecular phylogeny of gruntsteleostei,haemulidae, with an emphasis on the ecology, evolution, and speciation history of New World species. BMC Evolutionary Biology 12: 57.
- Wink M (1995) Phylogeny of old and new world vultures (Aves and Cathartidae) inferred from nucleotide sequnces of the mitochondrial cytochrome b gene. ZeitschriftfürNaturforschung C50: 868-882.
- Shields GF, Wilson AC(1987) Calibration of mitochondrial DNA evolution in gees. J MolEvol 24: 212-217.
- Avise JC, Nelson WS, Sibley CG (1994) DNA sequences support for a close phylogenetic relationship netwee some storks and New World vultures.ProcNatlAcadSci USA 91: 5173-5177.
- Aragon S, Moller AP, Soler JJ, Soler M (1999) Molecular phylogeny of Cuckoos supports a polyphyletic origin of brood paratism. J EvolBiol 12: 495-506.
- Nitta JH,OâÂ?Â?Grady PM (2008) Mitochondrial phylogeny of the endemic Hawaiian cranefliesDiptera, Limoniidae, Dicranomyia Implications for biogeography and species formation. MolPhylogenetEvol 1-9.
- White NE, Phillips MJ, Gilbert TP, Alfaro-Núñez A, Willerslev E, et al. (2011) Molecular Phylogenetics and Evolution 59: 615âÂ?Â?622.
Relevant Topics
- Aquatic Ecosystems
- Biodiversity
- Conservation Biology
- Coral Reef Ecology
- Distribution Aggregation
- Ecology and Migration of Animal
- Ecosystem Service
- Ecosystem-Level Measuring
- Endangered Species
- Environmental Tourism
- Forest Biome
- Lake Circulation
- Leaf Morphology
- Marine Conservation
- Marine Ecosystems
- Phytoplankton Abundance
- Population Dyanamics
- Semiarid Ecosystem Soil Properties
- Spatial Distribution
- Species Composition
- Species Rarity
- Sustainability Dynamics
- Sustainable Forest Management
- Tropical Aquaculture
- Tropical Ecosystems
Recommended Journals
Article Tools
Article Usage
- Total views: 12028
- [From(publication date):
June-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11095
- PDF downloads : 933