Research Article Open Access
Phragmitis australies Growth and Tolerance to Crude Oil Contamination in Mangrove Swamp Soil
| Odokuma LO1 and Ubogu M2* | |
| 1Department of Microbiology, University of Port Harcourt, Nigeria | |
| 2Department of Biological Science, Federal University of Agriculture Makurdi, Nigeria | |
| Corresponding Author : | Ubogu M Department of Biological Science Federal University of Agriculture Makurdi, Nigeria Tel: +234-706-437-1271 E-mail: ubomon@yahoo.co.uk |
| Received September 25, 2014; Accepted October 17, 2014; Published October 20, 2014 | |
| Citation: Odokuma LO, Ubogu M (2014) Phragmitis australies Growth and Tolerance to Crude Oil Contamination in Mangrove Swamp Soil. J Bioremed Biodeg 5:256. doi:10.4172/2155-6199.1000256 | |
| Copyright: © 2014 Odokuma LO, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The growth and tolerance of Phragmitis ausralies to 0, 1, 3 and 6% w/w crude oil contamination in mangrove swamp soil in the Niger Delta was investigated by stem propagation for 120 days period in a green house set-up. The plant recorded 100 % germination in all crude oil concentrations including control. Germination time was, 5.0, 5.3, 5.3 and 7.0 days for the various concentrations of crude oil respectively. While plant height decreased with increased concentrations of crude oil from 3% and above, increased concentrations did not show significant effects on the root length, leaf area and girth growth of plant (p<0.05). However, while there was no significant difference between 0 (control) and 3%, 1% produced an increase as against 6%, which produced a decrease in the fresh and dry weights of plant (p<0.05). The following hydrocarbon utilizing bacteria and fungi, Pseudomonas aeruginosa, Micrococcus luteus, Klebsiella sp. and , Aspergillus niger, A. flavus, Penicillium oxalicum , Mucor sp respectively, were isolated from the rhizosphere of Phragmitis ausralies with the highest crude oil contamination (6 % w/w) using Oil Mineral Salt Agar (OMSA). The total hydrocarbon utilizing bacterial and fungal counts were 7.1 × 106 ± 4.6 × 105 cfu/g (21.7%) and 4.5 × 105 ± 2.6 × 104 cfu/g (11.2%) respectively. Analysis of the baseline properties of soil sample for plant propagation indicate TPH level of 397.5 mg/kg, TOC, 0.06%, pH,5.05 and porosity, 32.0% . In this study, P. australies grew and survived in all concentrations of crude oil contaminated mangrove swamp soil with a high percentage population of hydrocarbon utilizing bacteria and fungi in its rhizosphere without any form of exogenous stimulation or augmentation, it is therefore a potential candidate for rhizoremediation.
| Keywords |
| Phragmitis ausralies; Crude oil; Contamination; Tolerance |
| Introduction |
| Soil pollution resulting from petroleum exploration and production activities in the Niger Delta has been a major source of concern as persistent oil pollution threatens the food security, health and environmental wellbeing of the region. In order to mitigate the devastating effect of crude oil pollution and preserve this rich but fragile environment of the Niger Delta for future generation and sustainable development, there must be a proactive search for effective, economical and environmentally friendly remediation techniques. |
| Convectional clean-up techniques have been shown to be insufficient [1]. Hence the development of alternative technologies for in situ applications especially those based on biological remediation capacity of plant and microorganisms [2,3]. Although, reports on the use of plants and their associated rhizosphere microorganisms in the clean-up of contaminated soils are well documented, little is known about tropical species that could serve for cleanup of oil contamination [4]. The success of any phytoremediation/rhizoremediation approach will depend largely on the growth and survival of the designated plant species in the crude oil contaminated soil. Hence, screening studies will be important in selecting the most useful plants. Given the great number of candidates, a relatively limited number of plants have been investigated [5]. |
| Grasses are known to be the most suitable plants for phytoremediation/rhizoremediation because of their multiple ramified root system [4], which provide room for more microbial activity and growth around the root zone [6]. One common grass species that is prevalent in the mangrove swamp of the Niger Delta is Phragmitis australies. The screening of suitable plants for phytoremediation/ rhizoremediation of pollutants should start from local species as they are prevalent and more adapted to the local environment for which they are to be employed. It is on these premises that the growth and tolerance of Phragmitis australies to crude oil contamination in the mangrove swamp of Niger Delta was investigated. |
| Materials and Methods |
| Soil sample collection and treatment |
| Mangrove swamp soil for plant propagation was collected at ebb tide at depth of 0-15 cm from Ugboroke, Uvwie local government council area, Delta State in March, 2013. 40 g (48.9 ml), 120 g (146.7 ml) and 240 g (293.4 ml) of crude oil with specific gravity of 0.818 g/cm3 obtained from SPDC (Forcados Terminal) were added to plastic pots measuring 25 cm in diameter and 14 cm deep containing 4000 g of soil to obtain a concentration of 1, 3 and 6% w/w crude oil contamination respectively. 4000 g of soil devoid of crude oil was also placed in plastic pots (which serve as control 0% w/w). All treatments including control were made in three replicates. |
| Plant propagation |
| Stems without leaves of P. australies measuring 30 cm in length and 0.8 ± 0.1 cm in diameter were planted (two per pot). Pots together with plants were then regularly watered to maintain flooded state for 120 days period. |
| Determination of percentage germination and germination time |
| P. australies germination time was regarded as time taken for leaves to emerge from cut tips of stems or aerial nodes. While the percentage germination of plants for each concentration of crude oil contaminated soil was calculated as: |
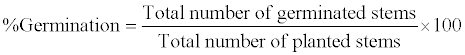 |
| Measurement of plant height, leaf area and girth growth |
| Growth in height, leaf area and girth of plants were taken at day- 120. Plant heights were determined by adapting the method of Omosun et al. [7]. Using a meter rule, plant height was taken from soil surface level to the tip of the tallest / youngest leaf. Girth measurement was taken using a meter tape. While leaf area obtained by taking entire perimeter of leaf and plotting against leaf length × breadth readings. The slope used as the multiplying factors for subsequent leaf length × breadth readings [8]. |
| Measurement of root length, fresh and dry weight of plants |
| Root length, fresh weight and dry weight of plants were determined at day-120 of propagation. Root length was determined using a meter rule by taking measurement from the base of plant to the tip of the longest root. Fresh and dry weights were determined using electronic weighing balance. For fresh weight, whole plant (roots and shoots together) weighed after washing off soil from roots and air-drying for one hour. While dry weight determined after drying plants to constant weight in hot-air oven at 60°C. |
| Collection of soil from rhizosphere for microbial analysis |
| Soil from rhizosphere was collected at day-120 of planting by uprooting each plant from plastic pots as per the method described elsewhere [9,10]. Plant roots together with adhering soil were allowed to drain for about 3-5 min and then shaken to free lightly adhering soil after which about 5-10 g soil was collected along with some adhering roots in a sterile polyethylene bag. This was allowed to airdry for 5-6 hours at laboratory room temperature. Three replicate rhizosphere samples were collected and respectively pooled together and homogenized for microbial analysis. Soil analyses were carried out within 24 h of collection from the greenhouse to the laboratory. |
| Media for the isolation of culturable heterotrophic and hydrocarbon utilizing microorganisms |
| Potato dextrose agar (PDA) was used for the isolation and enumeration of total heterotrophic fungi. The medium was constituted according to the manufacturer’s instructions (Oxoid). Thirty nine (39) grams of the powdered medium dissolved in 1 L of sterile distilled water and autoclaved for 15 min at 121°C, 15 psi. The medium was allowed to cool to about 45°C, tetracycline was added (to prevent the growth of bacteria and permit the selective isolation of fungi), mixed thoroughly under sterile condition [11-13]. The medium was aseptically dispensed into sterile petri dish to set. |
| Nutrient agar (NA) was used for the isolation and enumeration of total heterotrophic bacteria. Medium was constituted according to manufacturer’s instruction (Titan Biotech Ltd.). Twenty eight (28) grams of the powdered medium dissolved in 1 L of sterile distilled water and autoclaved as described above. Medium allowed cooling to 45°C; this was then dispensed into sterile petri dishes and allowed to set. |
| The isolation and enumeration of total hydrocarbon utilizing bacteria and fungi was carried out using Oil agar medium (OAM). The medium was prepared according to the mineral salts medium (MSM) composition of Mills et al. [14] as modified by Okpokwasili and Okorie [15]. The composition of the medium was NaCl, 10.0 g; MgSO4.7H2O, 0.42; KCl, 0.29 g; KH2PO4, 0.83 g; Na2HPO4, 1.25 g; NaNO3, 0.42 g, agar 20 g; distilled water, 1 L and pH of 7.2. Medium was autoclaved as earlier described. This was allowed to cool to about 45°C, 1% v/v of crude oil sterilized with 0.22 μm pore sized Millipore filter paper Moslein France [16] was added (as the sole source of carbon) and thoroughly mixed aseptically with MSM. This was dispensed into sterile petri dishes to set for the isolation of bacteria and fungi (tetracycline was added to plates for the selective isolation of fungi at about 45°C). |
| Isolation and enumeration of total culturable heterotrophic and hydrocarbon utilizing bacteria and fungi |
| The total culturable heterotrophic and hydrocarbon utilizing bacteria and fungi in the rhizosphere and bulk soils were estimated using the soil dilution plate count method. Sterile physiological saline (0.85% w/v sodium chloride) was used as diluents for inoculum preparation [17]. One gram of the homogenized soils for each sample was transferred into a sterile test tube containing 9.0 mL of diluents aseptically, using flame-sterilized steel spatula. Five-fold serial dilutions (10-5) were subsequently prepared from this. 0.1 mL aliquot of each dilution of soil sample was aseptically removed with a sterile pipette and separately spread plated with flame-sterilized glass spreader on well dried agar plates. Triplicate plates were prepared for each dilution on NA, PDA, OMA (with tetracycline for hydrocarbon utilizing fungi) and OMA (without tetracycline for hydrocarbon utilizing bacteria). All plates were incubated at room temperature. |
| Total heterotrophic bacterial and fungal counts taken after 48 h and 5 days respectively. While total hydrocarbon utilizing bacterial and fungal counts taken after 72 h and 5-7 days of incubation on OMA (without tetracycline) and OMA (with tetracycline incorporation) respectively. Colonies that grew on plates were counted and expressed as colony forming unit per gram of soil (cfu/g). |
| Identification and characterization of hydrocarbon utilizing bacteria and fungi associated with 6% w/w crude oil contaminated soil in the rhizosphere of P. australies at day- 120 |
| Pure culture of HUB and HUF isolated from 6% w/w crude oil contaminated soil in the rhizosphere of P. australies at day-120 were obtained by subculturing isolates from mixed culture plates on Oil Mineral Salt Agar onto Nutrient Agar (NA) and Potato Dextrose Agar (PDA) plates and incubating at 28 ± 2°C for 3-4 days and 7- 10 days respectively. |
| Ability of isolates to utilize petroleum hydrocarbon was further confirmed using the Vapour Phase Transfere method [18]. Each bacterial and fungal isolate were separately streaked on Mineral Salt Agar (without oil) in petri dish. Filter paper (Whatman, No. 1) was saturated with filter-sterilized crude oil which was then placed on the Petri dish cover to serve as sole carbon source. All plates were incubated in inverted position at 28 ± 2°C for 3-5 days and 7-14 days for bacteria and fungi respectively. Uninoculated plates served as control [17]. Bacterial and fungal colonies that grew on Mineral Salt Agar were further purified on NA and PDA plates. Agar slants were prepared from purified cultures on NA and PDA respectively for bacterial and fungal isolates from which subsequent studies were carried out. |
| Characterization of bacterial isolates was carried out using cultural, morphological, and biochemical properties. Identification of isolates was based on Bergey’s Manual of Determinative Bacteriology [19,20] .While fungi identification was carried out using cultural and morphological properties following the identification scheme described by Barnett and Hunter [21], Rifai [22], Humber [23] and Ellis [24]. |
| Determination of percentage hydrocarbon utilizing bacteria and fungi in rhizosphere soil |
| The percentage rhizosphere hydrocarbon utilizing bacteria and fungi was determined by the method of Walker and Colwell [13]. The counts of hydrocarbon utilizing bacteria and fungi on incubated plates were calculated and expressed as a percentage of the total heterotrophic bacterial and fungal populations respectively. |
| Physicochemical Analysis of Soil Samples |
| The experimental soil used for this study was analyzed for its physiochemical properties. Using the method of Black [25], soil pH was determined with a pH meter electrode (JENWAY, 3505 pH meter). Soil mineral fractions (texture) were determined using the soil hydrometer method as reported by Aliyu and Oyeyiola [26]. The total organic carbon was determined using the method of Black [25] and Mc Leod [27]. Total petroleum hydrocarbon (TPH) was determined by method 8015C of US EPA [28], using Gas Chromatograph (DANI MASTER GC), equipped with capillary column, identification and data processing software (Data Apex Clarity). Available soil phosphorus was determined by the method of Bray and Kurtz [29]. The method of Ezzati et al. [30] was adapted in the determination of soil porosity. The micro-Kjeldahl procedure as described by Hesse [31] was followed in the determination of total soil nitrogen. |
| Statistical Analysis |
| Data obtained from replicate samples were analyzed using measure of central tendency (mean), dispersion (standard deviation), Student’s t-test and Analysis of Variance (ANOVA) at P<0.05 |
| Results |
| The results of the effect of different concentrations of crude oil in mangrove swamp soil on the percentage germination of Phragmitis australies showed 100% germination for propagated stems in all three concentrations including control. The germination time for propagated stem in mangrove swamp soil was 5.0, 5.3, 5.3 and 7.0 days for 0, 1, 3 and 6% w/w crude oil concentrations respectively (Table 1). Crude oil concentrations had effect on plant germination time only at 6% w/w crude oil contamination as other lower concentrations did not differ significantly from the control (p<0.05). |
| Plant height measurements at day 120 were 207.3, 208, 199 and 187.3 cm at 0, 1, 3 and 6% concentrations respectively. With the exception of 1% concentration where plant height did not differ significantly as compared to the control (0%), plant height decreased with increased concentrations from 3 to 6% w/w crude oil contamination (p<0.05) (Table 1). |
| Leaf areas of plant at day 120 were 84.5, 64.4, 62.1 and 62.2 cm2 for 0, 1, 3 and 6% crude oil concentrations respectively (Table 1). Though the leaf area measurement for the control appears to be higher than all other three concentrations, it did not differ statistically from the three other concentrations (p<0.05). Similarly, the girth growth and the root length measurement at day 120 for all three concentrations did not differ from the control (p<0.05) (Table 1, Figures 1 and 2) respectively. |
| The fresh and dry weights of plant in control were comparatively the same with 3% concentration. However, 1% yielded significantly higher fresh and dry weights of plant than all other concentrations including control. While, 6% concentration yielded the lowest fresh and dry weight measurement (p<0.05) (Table 1). |
| Pseudomonas aeruginosa, Micrococcus luteus and Klebsiella sp. were the hydrocarbon utilizing bacteria, while Aspergillus niger, A.flavus, Penicillium oxalicum and Mucor sp. were the hydrocarbon utilizing fungi isolated from the rhizosphere of P. australies receiving the highest concentration of crude oil contamination (6% w/w) at day 120 of the study (Table 2). Furthermore, the total heterotrophic bacterial and fungal population in the rhizosphere of the of plant receiving 6% w/w oil at day 120 were 7.1 × 106 cfu/g of soil and 4.5 × 105 cfu/g of soil , while their percentage hydrocarbon utilizing population were 21.7 and 11.2 % respectively (Table 3). |
| The base line property of the mangrove swamp soil used for this study shows a low TPH level (397 mg/kg). The soil is acidic and compact with a porosity of 32.0% (Table 4). |
| Discussion |
| In this study, Phragmitis australies showed 100% germination for propagated stems in all three concentrations (1-6%) including control. In a related study, Ogbo et al. [4] demonstrated that the plant Paspalum scrobiculatum germinated and grew in concentrations of 0-15% crude oil contaminated soils tested. Similarly, alfalfa seed has been shown to germinates in soils contaminated with up to 50 g crude oil per kg (5% w/w) [32]. One way of knowing whether the plant being considered for phytoremediation will germinate successfully is to carry out germination tests in the contaminated soil prior to planting [33].The ability of plant species to germinate in hydrocarbon contaminated soil is often the first step in screening for tolerance [34]. Oil contamination in soil is known to slow the rate of germination in plant, this effect could be due to the oil which acts as a physical barrier preventing or reducing access to water and oxygen [4]. While studying the germination of wheat and soybean in kerosene contaminated soil (0.34% w/w), Dibble and Bartha [35] demonstrated that germination was delayed in contaminated soil when compared to uncontaminated soil. The overall percentage germination however, was the same after 10 days in both soils. Though the germination time of Phragmitis australies was delayed by two days at crude oil concentration of above 3% w/w, the high percentage germination of the plant in the contaminated soil is an indication of its tolerance. |
| Growth in height of Phragmitis australies decreased with increased concentration of crude oil above 1%. Similar decrease in the height growth of Paspalum scrobiculatum [4], Amaranthus hybridus [7], Capsicum annum and Lycopersicon esculentum [36], Bromous mermis, Linum ussitasimum, Medicago truncatular and Triticum sativa [37] with increase concentration of crude or spent oil in soil were also reported. The decrease in plant height with increased concentration of crude oil may be attributed to the stressed imposed on the plant by the presence of higher amount of oil in the soil. Hydrocarbon degrading microorganisms in soil compete with plant for oxygen and mineral nutrients. Oxygen exhaustion can create anaerobic conditions which may bring about microbial generation of phytotoxic compounds, such as H2S. The oil also affects the physical structure of the soil, decreasing its capacity to store moisture and air [38]. |
| Although, other workers have reported decreased in leaf area and girth growth with increased concentration of crude oil in soil [4,7,39], the leaf area and stem girth growth of Phragmitis australies was not affected by crude oil concentrations in this study. This again signifies tolerance to crude oil contamination. |
| Similarly, increase in crude oil concentrations in soil did not affect the growth in root length of Phragmitis australies at day-120 of planting as compared to control. Gaskin [34], reported that while there was no significant difference in the root length of Brachiaria decumbens in soil contaminated with 0.5 and 1% w/w diesel/ oil as compared to 0% w/w at week 12, Cymbopogon ambiguous produced a higher root length growth in contaminated soil than control. This appears to suggest that plant root growth response to oil contamination in soil is species related. The absence of any significant effect of increased crude oil concentrations on the root growth of Phragmitis australies is a positive indication of it tolerance to crude oil contamination. The most serious impediment to successful rhizoremediation is its limitation to the deepth of the root zone as many plants have relatively shallow root zones [5]. Conditions favouring degradation of organic pollutants decreases with increasing soil depth [40]. It has been stated that effective oxygen diffusion for desirable rates of biodegradation extends only about 30 cm into the soil profile [41]. The root length of Phragmitis australies at day 120 ranged from 42.0 cm to 46.8 cm in the crude oil contaminated soil. This implies that the plant can serve as a suitable candidate for rhizoremediation. Grasses have been shown to be more efficient in phytoremediation because of their fibrous root system with extensive root surface area for microbial colonization and dense rhizoshere [42]. Although, crude oil spills in soil affect plants adversely by creating conditions which make essential nutrients like nitrogen and oxygen used for plant growth unavailable to them [4], plant roots can act as a substitute for the tilling of soil to incorporate additives (nutrients) and to improve aeration [6,43]. Therefore, plants with well-developed root system such as popular, willow [44] and reed (Phragmites australies), possessing specialized root vessels, aerenchyma which can release oxygen into the rhizosphere in deep soil layer [44,45] are preferentially selected for rhizoremediation. |
| Phragmites australies biomass (fresh and dry weight) decreased only at concentration above 3% w/w crude oil. However, 1% w/w of oil significantly improved plant biomass over control. There are several conflicting reports regarding effect of crude oil on plant biomass. While some workers reported decrease in plant biomass with corresponding increase in oil concentrations in soil [4,7,37,46] even at low concentrations of 0.5% w/w [46], others like Vwioko and Fashemi [39], reported increase at 1% w/w and decrease as from 2-6% w/w; Merkl et al. [47], reported increase in biomass up to 3 and 5%. Very low hydrocarbon levels may actually stimulate plant growth and crop yield [48]. The findings in this study agree with the later reports. The increase in plant biomass may be due in part to petroleum components acting as growth hormones [49]. |
| The hydrocarbon utilizing microorganisms such Pseudomonas aeruginosa, Micrococcus luteus, Klebsiella sp. , Aspergillus niger, A.flavus, Penicillium oxalicum and Mucor sp. isolated from the rhizoshere of P. australies receiving the highest contamination (6% w/w) of crude oil, have also been reported by other workers as hydrocarbon degraders in rainforest and mangrove swamp soil [17,50-52]. |
| In this study, P. australies did not only grew and survived in crude oil contaminated mangrove swamp soil of up to 6% w/w for the 120 days period of its evaluation, but also haboured high percentage population of hydrocarbon utilizing bacteria and fungi (21.7 and 11.2% respectively) in its rhizosphere without any form of exogenous stimulation or augmentation. These findings are of great significance as it presents the possible potential the plant holds as a rhizoremediation tool if properly exploited. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14004
- [From(publication date):
December-2014 - Jul 15, 2025] - Breakdown by view type
- HTML page views : 9405
- PDF downloads : 4599
