Research Article Open Access
Photocatalytic Studies of Tio2/Sio2 Nanocomposite Xerogels
Muhammad Yaseen1, Zeban Shah2*, Renato Veses C2, Silvio Dias LP2, Ã?Â?der Lima C2, Glaydson dos Reis S2, Julio Vaghetti CP2, Wagner Alencar SD2 and Khalid Mehmood11Department of Chemistry, Hazara University Mansehra Dhudial 21130, K.P.K Pakistan
2Federal University of Rio Grande do Sul, Av. Bento Gon�§alves, Porto Alegre, RS, Brazil
- *Corresponding Author:
- Dr. Zeban Shah
Federal University of Rio Grande do Sul
Av. Bento Gon�§alves, Porto Alegre, RS, Brazil
Tel: 555134340123
E-mail: zeban.shah@ufrgs.br or zs_zaib77@yahoo.com
Received date: February 01, 2017; Accepted date: February 16, 2017; Published date: February 25, 2017
Citation: Yaseen M, Shah Z, Veses RC, Silvio Dias LP, Ã?Â?der Lima C, et al.(2017) Photocatalytic Studies if Tio2/Sio2 Nanocomposite Xerogels. J Anal Bioanal Tech 8:348. doi: 10.4172/2155-9872.1000348
Copyright: © 2017 Yaseen M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The use of titania-silica materials in photocatalytic processes has been proposed as an alternative to the conventional TiO2 catalysts, in order to facilitate the separation of products after the reaction. However, despite the large number of research in this field, the mechanism governing the photocatalytic activity of the mixed TiO2/SiO2 oxides is not clear. Titania-Silica nanocomposite xerogels were prepared by sol-gel method. This work has been used to describe the synthesis and the photocatalytic properties of TiO2-SiO2 nanocomposite xerogel. The nanocomposite xerogels were prepared by keeping the molar ratio of TEOS:TTIP:MtOH:DIW at 1: 1:6:14 respectively and the catalysts used were HCl and NH4OH. After the preparation xerogels were characterized by FTIR, XRD, UV and LLS. All these techniques show the amorphous nature of Titania-silica xerogel.
Keywords
Photocatalysis; TiO2-SiO2 mixed oxide; LLS; FTIR; UV; XRD
Introduction
TiO2 is well recognized as a valuable material with application as a white pigment in paints, as filler in paper, textile and in rubber/plastics [1]. Due to low cost, non-toxicity, stability and other best characteristics TiO2 attracts a great attention. TiO2 has wide applications in various fields like antireflection opticals, coatings, waste water purifications, catalyst supporting, ceramics senser element, as a photocatalyst, in electric devices like (in lithium based battery), as a base in high quality paints, paper, plastics [2]. Titania has excellent biocompatibility with respect to bones implants and applications in electrochromic devices [3]. Titania shows good photocatalytic applications due to which it gained tremendous demands and green energy and environmental protection. Many other oxides like iron oxides, zinc oxides etc. also shows the similar behavior due to photocatalytic activity of titanium dioxide it play a wide role in different fields like air, waste water purification, good UV blocking properties weakening of the organic fibers [4]. Silica doped in to the titania matrix increase the photocatalytic activity because the silica doping decrease particle size and also increase the specific surface area and thermal stability of titania particle towards anatase to rutile phase conversion [5]. SiO2- TiO2 materials are used in different fields like as catalyst supporting materials, acidic catalyst for many reactions, selective reduction, as an anti-reflective materials for coatings or sensing nanoimprints photonic crystals [6-9]. Dielectric mirrors and low loss waveguides solids of low thermal expansion coefficient, bioactive solids self-cleaning coatings solids of controlled acidity and photocatalysts [10-14]. The TiO2-SiO2 mixed oxides catalytic activity was studied and observed that the TiO2- SiO2 have better photocatalytic activity as compared to TiO2 and SiO2 which was confirmed through LLS and UV results.
Experimental
Sample preparation
The xerogels can be synthesized by Sol-Gel process in which metal alkoxide is used as a precursor source that undergoes catalyzed hydrolysis and condensation to get nano scale materials of that metal [15]. TTIP was used as a precursor and TEOS was added as an organic solvent. In this synthesis HCl and NaOH were used as catalysts.
TiO2-SiO2 mixed oxide
TiO2-SiO2 xerogels was synthesized by Sol-Gel process. TEOS (Tetraethylorthosilicate) and TTIP (Titanium tetra isopropoxide) were used as precursor. It was observed that the TTIP hydrolysis rate is much faster than TEOS [16]. The synthesis consists of two steps. The first step is the synthesis of SiO2 sol, in which 1:6 TEOS and Methanol were mixed together i.e. 7 ml of TEOS and 43 ml of methanol. TEOS were added drop wise slowly to the methanol with continuous stirring. Then 0.05 mol L-1 HCl was added drop wise to the sol in order to adjust the acidic pH at 2. Then the sol was allowed to stirrer for about 2 an hour in order to get the homogeneous sol. In the second step TiO2 Sol synthesis, 1:14 solution of TTIP and DIW were mixed with continuous stirring until the homogeneous sol of TiO2 were obtained. Then in 1:1 of TiO2 and SiO2 sols were mixed with continuous stirring in order to get homogeneous sol and then 0.05 mol L-1 NH4OH solution was added to this mixture drop wise for the adjustment of pH. The pH of the homogeneous mixtures (sol) was observed by the pH-meter continuously. At last the mixtures (sol) were allowed to stirrer for some time to get the homogeneous mixture (sol).
Gel and xerogel preparation
In this method 3ml of TEOS, the silica precursor was taken in the reaction beaker and 6ml of ethanol was added dropwise with continue stirring. Further 3 ml of water, 4 ml of acetic acid and 3 ml of TTIP (Titania precursor) were added with continuous stirring for 15 min at room temperature. The prepared sol changed into a gel which was placed in the oven at 65 for 1 h which resulted in the conversion of gel in to xerogel.
Characterization
Structure, quality, photocatalytic studies and morphological characteristics of Titania-silica gel were studied by different characterization techniques like, LLS, FTIR, UV and XRD etc.
Results and Discussions
FTIR result
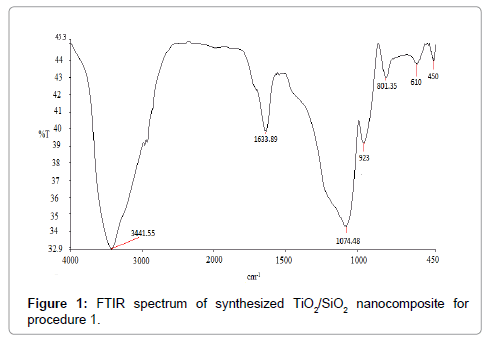
The FTIR spectra of the synthesized nanocomposite (TiO2/SiO2) were recorded by Perkin Elmer series 100 FTIR spectrometer with a 5 cm-1 resolution. FTIR spectrum was recorded at 4000-450 cm-1. The absorption at 1074 cm-1 (Figure 1) is the characteristics peak for Si-O-Si. The peak observed at 801 cm-1 is due to Si-O-Si symmetric stretching. The broad absorption at 1633 cm-1 match with OH bending vibrations and is attributed to chemisorbed water. The well defined peak at 3441 cm-1 shows OH stretching vibrations. The peak observed at 923 cm-1 corresponded Si-O-Ti vibrations. The band observed at 450-610 cm-1 is due to Ti-O Stretching.
Many characteristic FTIR peaks were observed in Figure 2. The bands observed at 3391 cm-1 and 1557cm-1 were due to the OH bending and stretching vibrations respectively. The peak observed at 1165 cm-1 correspond to Si-O-Si antisymmetric stretching vibration. The peak obtained at 923 cm-1 correspond to Si-O-Ti vibration. The peak observed at 801 cm-1 is due to the Si-O-Si symmetric stretching vibration. The absorption at 1410 cm-1 is due to C-H interaction of Si-R structure unit (Figure 3).
XRD measurement
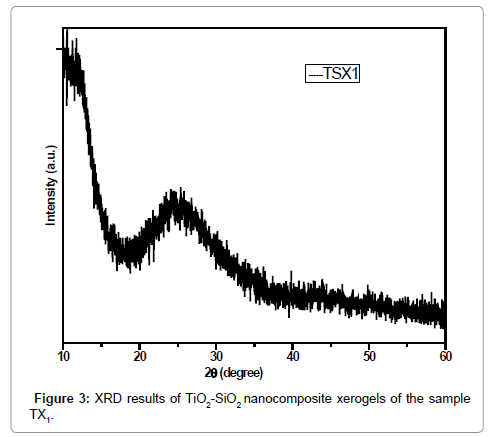
The XRD pattern of synthesized nanocomposites (TiO2/SiO2) was collected in the range of 10-60 2θ (degree) shown in Figure 4. The XRD patterns of the synthesized materials indicate that the TiO2-SiO2 nanocomposites are essentially non-crystalline and have amorphous structure which can be accomplished from the broad characteristic diffraction peak between 2θ ~ 20θ° and 30 θ°.
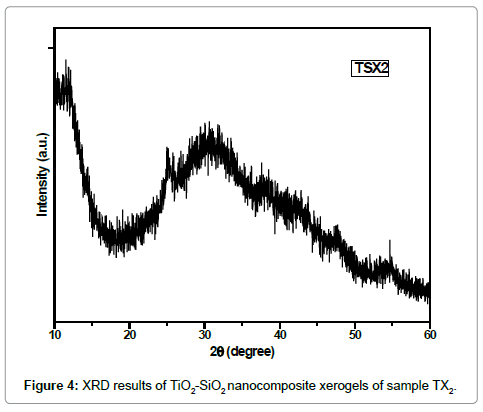
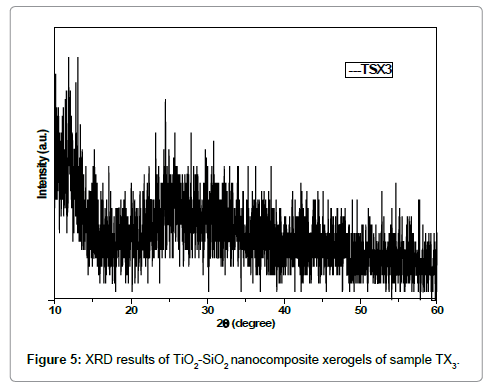
The Figures 5 and 6 also show the same XRD results of the synthesized TiO2/SiO2 nanocomposites. These samples were also synthesized by sol-gel method with different concentrations of precursors and solvents used. The hump in XRD pattern indicate that the TiO2-SiO2 nanocomposites are essentially non-crystalline and have amorphous structure which can be accomplished from the broad characteristic diffraction peak between 2θ ~ 20θ° and 30 θ° while the Figure 2 shows very low crystallinity at 2θ and 25 θ°.
Catalytic activity
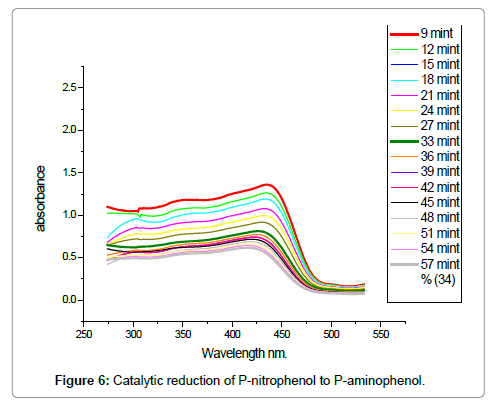
To check the catalytic activity of TiO2-SiO2 the p-nitrophenol was reduced by NaBH4 to p-aminophenol in aqueous medium. The procedure adopted was used as a given amount of mixed oxides was added to 1 ml of p-nitrophenol (0.08 mmol L-1) for initiation of reduction. For mixture preparation 1 ml of aqueous solution of NaBH4 (1.5 mmol L-1) was added to reaction chamber. The p-nitrophenol reduction was determined by studying the absorbance at 440 nm with respect to time. This reaction was selected due to simplicity and formation of single product. By using time dependence uv-vis spectra the reduction process was checked. The appearance of a new peak at 300 nm was observed which confirm the p-nitrophenol reduction to p-aminophenol which is shown in Figure 6.
LLS result (hydrodynamic radii)
The LLS results (hydrodynamic radii) of the three samples are given in Table 1 below. All the three samples have different hydrodynamic radii. The two samples TSX2 and TSX3 have same precursors and solvent but used at different ratio. The sample TSX17 have same precursors but different solvents and precursor ratio were used. The LLS results (hydrodynamic radii) are different because the LLS result (hydrodynamic radius) of the sample TSX3 were measured during the conversion of sol in to gel and the LLS results (hydrodynamic radii) of the TSX2 and TSX17 were calculated after the gel was formed.
| S No | Sample | Hydrodynamic radius (nm) |
|---|---|---|
| 1 | TSX17 | 170.3 |
| 2 | TSX2 | 138.49 |
| 3 | TSX3 | 52.6 |
Table 1: LLS Results (hydrodynamic radii) of the three samples prepared by sol gel process.
SEM results
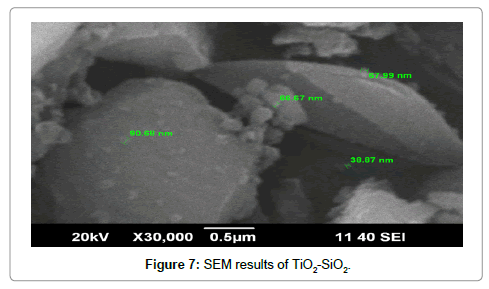
The Figure 7 shows the SEM image of synthesized containing TiO2-SiO2. From SEM image, it is clear that in most part of the sample large particles is obtained, which gives the evidence of the agglorimation of small particles. Along large agglomarited particle small size individual particles can also be absorved.
EDX results
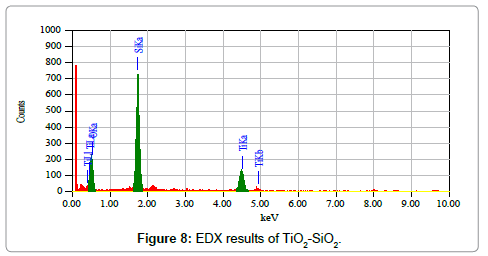
The EDX spectrum is showing the elemental composition of the three elements in different percentage which is silicon (Si) and titanium (Ti) and oxygen (Figure 8). The EDX spectra has confirmed all the constituents that were added in the synthesized sample.
Conclusion
(TiO2/SiO2) nanocomposites were synthesized by sol-gel process where Tetraethylorthosilicate (TEOS) and Titanium tetraisopropoxide (TTIP) were used as precursors in the presence of organic solvent. The XRD results confirmed that the synthesized TiO2-SiO2 mixed oxide xerogel is non-crystalline and having amorphous nature. In the TiO2/ SiO2 nanocomposites, materials, in the amorphous SiO2 matrix TiO2 nanocrystals are present in highly dispersed form. The amorphous SiO2 and Ti–O–Si bond formation and TiO2-SiO2 mixed oxides give rise effectively stability of TiO2 anatase form. It also bound crystallites growth, significantly increase surface area. So, the increase in surface area caused the improvement in photocatalytic activity of TiO2-SiO2 mixed oxides nanocomposi[te xerogels. FTIR spectra show the presence of Ti-O-Si crosslinks and revealed interactions between TiO2 and SiO2 at a molecular scale. Ti-O-Si bonds and interactions may enhance surface properties, catalytic and photoactivity. The TiO2-SiO2 mixed oxides catalytic activity was also studied and observed that the TiO2-SiO2 have better photocatalytic activity as compared to TiO2 and SiO2 which was confirmed through LLS and UV results.
Acknowledgements
The authors acknowledge CNPq for financial support.
References
- Jalava JP (2006) Size and Shape Dependence of the Electronic and Spectral Properties in TiO2 Nanoparticles. Part Syst Charact 23: 159-164.
- Hashimoto K, Irie H, Fujishima A, Jpn J (2005) TiO2 photocatalysis: a historical overview and future prospects. Appl Phys 44: 8269.
- Guo YG, Hu YS, Maier J (2006) Synthesis of hierarchically mesoporous anatase spheres and their application in lithium batteries. Chemical commun 26: 2783-2785.
- Tanaka K, Capule MF, Hisanaga T (1991) Effect of crystallinity of TiO2 on its photocatalytic action. Chemical Physics Letters 187: 73-76.
- Meng X, Qian Z, Wang H, Gao X, Zhang S, et al. (2008) Sol�gel immobilization of SiO2/TiO2 on hydrophobic clay and its removal of methyl orange from water. J Sol-Gel Sci Tech 46: 195-200.
- Wang X, Shen J (2010) Sol�gel derived durable antireflective coating for solar glass. J sol-gel sci tech 53: 322-327.
- Courtney L, Sermon PA, Towers J, Halepoto D, de Namor AFD, et al. (2004) Sol�gel chemistry for Ba 2+ sensors to allow oil production engineered nanometrically. J sol-gel sci tech 32: 229-236.
- Li M, Tan H, Chen L, Wang J, Chou SY (2003) Large area direct nanoimprinting of SiO 2�TiO 2 gel gratings for optical applications. J Vacuum Sci Tech B: Microelectronics and Nanometer Structures Processing, Measurement, and Phenomena 21: 660-663.
- Kanehira S, Kirihara S, Miyamoto Y (2005) Fabrication of TiO2�SiO2 photonic crystals with diamond structure. Journal of the American Ceramic Society 88: 1461-1464.
- Lin W, Wang GP, Zhang S (2005) Design and fabrication of omnidirectional reflectors in the visible range. J Modern Optics 52: 1155-1160.
- Sermon PA, Leadley JG, MacGibbon RM, Ruzimuradov O (2012) Tuning X/(TiO2) x�(SiO2) 100�x (0<x<40) xerogel photocatalysts. Ionics 18: 455-459.
- Miyata N, Kamitakahara M, Kawashita M, Kokubo T, Nakamura T (2003) Mechanical properties of bioactive PDMS-CaO-SiO2-TiO2 and PTMO-CaO-TiO2 hybrids soaked in a simulated body fluid. In Key Engineering Materials, Trans Tech Publications 240: 943-946.
- Miller JB, Ko EI (1997) Control of mixed oxide textural and acidic properties by the sol-gel method. Catalysis today 35: 269-292.
- Yoon KH, Noh JS, Kwon CH, Muhammed M (2006) Photocatalytic behavior of TiO 2 thin films prepared by sol�gel process. Materials Chem Phy 95: 79-83.
- Privman V, Goia DV, Park J, Matijevic E (1999) Mechanism of formation of monodispersed colloids by aggregation of nanosize precursors.J Colloid Interface Sci 213: 36-45.
- Cheng P, Zheng M, Jin Y, Huang Q, Gu M (2003) Preparation and characterization of silica-doped titania photocatalyst through sol�gel method. Materials Letters 57: 2989-2994.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 5298
- [From(publication date):
February-2017 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 4471
- PDF downloads : 827