Research Article Open Access
Phosphorus Stabilization in Constructed Wetlands under Changing Hydroclimatic Conditions
| Pant HK* | |
| Department of Earth, Environmental and Geospatial Sciences, Lehman College, the City University of New York, USA | |
| Corresponding Author : | Pant HK Department of Earth, Environmental and Geospatial Sciences Lehman College, the City University of New York, USA Tel: (718) 960 5859 E-mail: hari.pant@lehman.cuny.edu |
| Rec date: Aug 20, 2014, Acc date: Oct 21, 2014, Pub date: Oct 30, 2014 | |
| Citation: Pant HK (2014) Phosphorus Stabilization in Constructed Wetlands under Changing Hydro-climatic Conditions. J Earth Sci Clim Change 5:229. doi: 10.4172/2157-7617.1000229 | |
| Copyright: © 2014 Pant HK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Earth Science & Climatic Change
Abstract
Although there is a reduction in P reaching aquatic systems from point sources, non-point sources such as urban and agricultural runoffs are bringing substantial amounts of phosphorus (P) into aquatic systems as the storm events are quite common due to climatic changes in various parts of the world, and making eutrophication of surface water a global concern. Constructed wetlands could play important roles in stabilizing P; hence reduce eutrophication of natural aquatic and semi-aquatic ecosystems. However, the selection of the construction site may well determine the effectiveness of a constructed wetland. This study shows that P transformation in soils is crucial for P sequestration in a wetland rather than the amounts of native P. Using 31Phosphorus Nuclear Magnetic Resonance Spectroscopy (31P NMR), previously unreported an active organic P form, phosphorarginine, was identified. This study indicates that constructing wetlands on organic P-enriched sites may not solve the P loading to water bodies as the organic P compounds would not be as stable as they were thought, thus can play a crucial role in eutrophication, after all.
Constructed wetlands are considered as a low cost alternative for treating wastewater from various sources including urban and agricultural runoffs [1]. Phosphorus flux from soils to the overlying water column depends on various factors including physico-chemical characteristics of soils and the nature of the P compounds stored in soils. Although the construction of wetlands on highly fertilized agricultural lands or manure-impacted lands could help to stabilize the potential P runoff from the area to the surrounding water bodies, the construction could also result in solubilization of stored P and release to the water column. A significant portion of the water column P could be removed by biotic and abiotic processes due to dissolution of calcium (Ca)/magnesium (Mg), and iron (Fe)/aluminum (Al) bound P, and hydrolysis and mineralization of organic P during initial stabilization period. Several studies have reported on the potential use of wetlands for removal of nutrients including P from wastewater [2,3] and runoffs, however, wetland soils could function as source or sink for P depending on the quality and quantity of native P.
Animal waste and agricultural runoffs in the watershed are the most important sources contributing P loads to freshwater lakes [4]. Phosphorus loading from the adjacent watershed deteriorates the water quality of lakes and estuaries because of the agricultural runoffs such as Lake Okeechobee, USA, Lake Erie, USA/Canada, and Lake Winnipeg, Canada are prime examples [5]. Construction of wetland has been looked at as an alternative to curtail P influx to the lake, however, forms and amount of P stored in soil could be crucial in the selection of construction sites along with different biogeochemical processes, which occur in wetlands. The mineralization of inorganic P from organically bound P is fundamental to the maintenance of the P cycle in natural and semi-natural ecosystems [6,7]. The biological and pedological transformations of P are well recognized, but the mechanisms of organic P transformation into inorganic P are not well understood. For example, abandonment of dairies/agricultural fields after reaching critical level of P in soils is considered as preventive measures for the reduction in potential threat to the surrounding water bodies. However, study on P forms using 31P NMR showed the transformation of P into different forms would increase its mobility consequently, adversely affect surface and ground water qualities.
Various operationally defined chemical fractionation schemes have been used to categorize P into different pools on the basis of their extractability. However, use of 31P NMR could provide identification of individual P forms, and allow estimation of their stability pertaining to ecological consequences under changing hydro-climatic conditions.
Wet soils (50 g) were extracted twice with 100 mL 0.4 M NaOH, for four hours each, by shaking in an end-over-end mechanical shaker at 20 ± 2°C. After the each extraction, the suspensions were centrifuged for 20 min at 5,000×g. The supernatants were pooled and subjected to gel filtration. The extracts were fractionated using a G-25 Sephadex column (with a fractionation range of 100-5,000 mol wt.; dry bead diameter = 20-80 µm; bed volume = 4-6 mL g-1; column volume = 75 mL) as described by Pant [6] and Pant et al. [8]. The extract (20 mL) was pipetted onto the top of the column and eluted with deionized distilled water by pumping at a rate of 0.6 mL min-1. Eighty, 3 mL fractions were collected using a fraction collector. The fractions containing NaOH were separated, using litmus paper test. To check for loss of any forms of P, the NaOH fractions were tested for the presence of P. No NaOH was found up to the 49th fraction and no P was found after the 49th fraction. The fractions free from NaOH were combined and concentrated (10 times) in a Vacuum Rotatory Evaporator at 35°C.
An aliquot of 4.0 mL of the concentrated extract was scanned in a 12 mm tube at 121.4688 MHz on a NT 300 31P NMR Spectrometer [9] using a 90° pulse with 5.0 sec delay and sampling interval of 0.0000622 sec. To obtain better signal to noise ratio, 8,000 scans were collected, thus, to inhibit any possible microbial activities during the scan, 0.25 mL toluene was mixed with the sample prior to scan collections. The chemical shifts were determined with respect to an external standard of 85% phosphoric acid, and the identification of peaks of various P forms in the NMR spectra were done by comparing the references reported by Pant et al. [8], Gadian [10] and Thebault et al. [11]. Moreover, since the resonances are sensitive to pH and salt concentration [10], samples were spiked with 0.1 mL (10 mg P mL-1) of pyrophosphate (Na4HP2O7), as an internal standard for the conformity in the peak identifications.
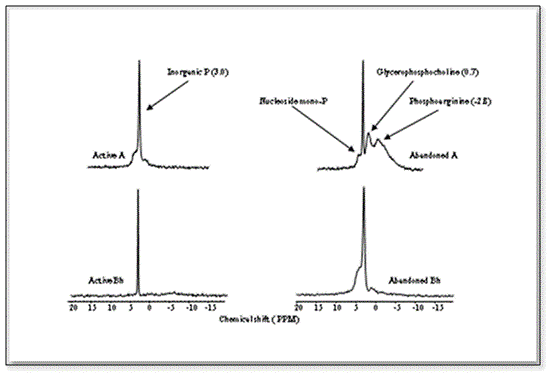
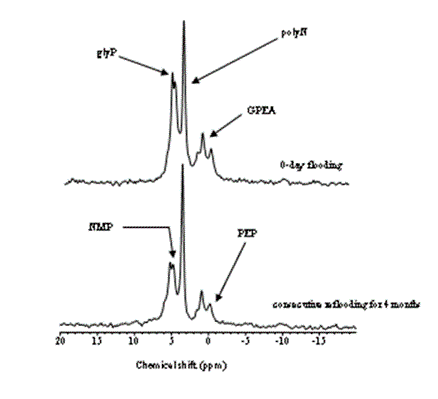
This study showed transformation of P compounds over a period of time in the P-impacted soils. The transformations of highly mobile P into relatively stable P as the abandonment of the impacted land continued. As far as the control of P runoff into the aquatic systems from adjacent farmland is concerned, the transformations of inorganic P to organic P are promising. However, in the long-term, the relatively stable P would mineralize, and ultimately reaches to the adjacent water bodies. The P compounds in the soils, as revealed by 31P NMR, are mainly low stability organic P compounds, and can potentially be hydrolyzed. Signature for phosphoarginine at chemical shift (~ -2.8 ppm), which is never been reported in soils/waters, was obtained. Phosphoarginine, a low stability (a high energy compound associated with eukaryotic cells) and a high adsorptive potential organic compound (due to charge associated with it, Figure 2), could maintain long-term availability of P in farmland, and alter the trophic status of the receiving water bodies.
Thus, the constitution of different P forms, and their mobility in different soil/sediment profiles are much more important than the total P [6,13]. A substantially high composition of inorganic P in the sub-soils may suggest that manure-impacted fields could also be a potential source of P to the groundwater. Moreover, especially in Spodosols, an increase in water table could transport substantial amounts of P into the nearby slough/ditches or water bodies through the eluted E horizon.
References
- Vohla C, Kõiv M, Bavor, HJ, Chazarenc F, Mander U (2011) Filter materials for phosphorus removal from wastewater in treatment wetlands- A review. EcolEng 37: 70-89.
- Kadlec RH, Knight RL (1996) Treatment wetlands. CRC Press, Boca Raton, FL.
- Pant HK, Reddy KR, Lemon E (2001) Phosphorus retention capacity of root bed media of sub-surface flow constructed wetlands. EcolEng 17: 345-355.
- Allen Jr LH (1987) Dairy-siting criteria and other options for wastewater management on high water-table soils. Soil Crop Soc FL 47: 108-127.
- Wines A (2013) Spring Rain, Then Foul Algae in Ailing Lake Erie. The New York Times, March 15, 2013: A1.
- Pant HK, Reddy KR (2001) Hydrologic Influence on Stability of Organic Phosphorus in Wetlands detritus. J Environ Qual 30: 668-674.
- McCulloch J, Gudimov A, Arhonditsis G, Chesnyuk A, Dittrich M (2013) Dynamics of P-binding forms in sediments of a mesotrophic hard-water lake: Insights from non-steady state reactive-transport modeling, sensitivity and identifiability analysis. ChemGeol 354: 216-232.
- Pant HK, Warman PR, Nowak J (1999) Identification of soil organic phosphorus by 31P nuclear magnetic resonance spectroscopy. Comm Soil Sci Plant Anal 30: 757-772.
- Buszko ML, Buszko D, Wang DC (1998) Internet technology in magnetic resonance: a common gateway interface program for the World Wide Web NMR spectrometer. J Magnetic Resonance 131: 362-366.
- Gadian DG (1982) Nuclear magnetic resonance and its applications to living systems. Clarendon Press, Oxford, UK.
- Thebault MT, Kervarec N, Pichon R, Nonnotte G, Le Gal Y (1999) A 31P nuclear magnetic resonance study of the hydrothermal vent tube worm Riftiapachyptila. C R Acad Sci 322: 537-541.
- Palmer-Felgate, EJ, Mortimer, Robert JG, Krom MD, Jarvie HP, et al. (2011) Internal loading of phosphorus in a sedimentation pond of a treatment wetland: Effect of a phytoplankton crash. Sci Total Environ 409: 2222-2232.
- Steinman AD, Ogdahl ME, Weinert M, Thompson K, Cooper MJ, et al. (2012) Water level fluctuation and sediment–water nutrient exchange in Great Lakes coastal wetlands. J Great Lakes Res 38: 766-775.
- Pant HK, Reddy KR (2003) Potential internal loading of phosphorus in a wetland constructed in agricultural land. Water Res 37: 965-972.
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Atmosphere
- Atmospheric Chemistry
- Atmospheric inversions
- Biosphere
- Chemical Oceanography
- Climate Modeling
- Crystallography
- Disaster Science
- Earth Science
- Ecology
- Environmental Degradation
- Gemology
- Geochemistry
- Geochronology
- Geomicrobiology
- Geomorphology
- Geosciences
- Geostatistics
- Glaciology
- Microplastic Pollution
- Mineralogy
- Soil Erosion and Land Degradation
Recommended Journals
Article Tools
Article Usage
- Total views: 13593
- [From(publication date):
November-2014 - Jul 11, 2025] - Breakdown by view type
- HTML page views : 9046
- PDF downloads : 4547
