Research Article Open Access
Phase II Study Evaluating the Effect of Concomitant Ramucirumab on the Pharmacokinetics of Docetaxel in Patients with Advanced Solid Tumors
Mark N Stein1*, Laura Q M Chow2, David C Smith3, Dale R Shepard4, Ding Wang5, John Powderly6, Archana Chaudhary7, Yong Lin7 and Ling Gao8
1Rutgers Cancer Institute of New Jersey, New Brunswick, NJ, USA
2University of Washington, Seattle, WA, USA
3University of Michigan, Ann Arbor, MI, USA
4Cleveland Clinic Foundation, Cleveland, OH, USA
5Henry Ford Hospital, Detroit, MI, USA
6Carolina Biooncology Institute, Huntersville, NC, USA
6Eli Lilly and Company, Indianapolis, IN, USA
7Eli Lilly and Company, Bridgewater, NJ, USA
- Corresponding Author:
- Stein MN
Rutgers Cancer Institute of New Jersey
195 Little Albany Street New Brunswick, NJ, USA
Tel: 17322356031
Fax: 17322356797
E-mail: mark.stein@rutgers.edu
Received Date: July 20, 2016; Accepted Date: August 31, 2016; Published Date: September 06, 2016
Citation: Stein MN, Chow LQM, Smith DC, Shepard DR, Wang D, et al. (2016) Phase II Study Evaluating the Effect of Concomitant Ramucirumab on the Pharmacokinetics of Docetaxel in Patients with Advanced Solid Tumors. Clin Pharmacol Biopharm 5:161. doi: 10.4172/2167-065X.1000161
Copyright: © 2016 Stein MN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Background: Ramucirumab is a human IgG1 monoclonal antibody that specifically targets the vascular endothelial growth factor receptor-2. The primary objective of this study was to investigate the effect of concomitant ramucirumab on the pharmacokinetics of docetaxel. Methods: Patients with metastatic or locally advanced malignant solid tumors resistant to standard therapy or for which no standard therapy was available were recruited. Patients received docetaxel 75 mg/m2 and ramucirumab 10 mg/kg on day 1 of a 3-week cycle. In cycle 1, docetaxel was administered alone; in cycle 2 and subsequent cycles, ramucirumab was administered followed by docetaxel. Blood was drawn immediately before and at regular intervals after infusions for cycles 1 and 2 to determine docetaxel and ramucirumab concentrations. Results: Docetaxel pharmacokinetic parameters were assessed in 18 patients. The dose-normalized area under the plasma concentration versus time curve from time zero extrapolated to infinity and maximum plasma drug concentration of docetaxel during cycle 2 were similar to those when docetaxel was administered alone during cycle 1, with geometric least squares means ratios of 0.97 (90% CI: 0.84, 1.10) for the area under the plasma concentration versus time curve from time zero extrapolated to infinity and 1.14 (90% CI: 0.84, 1.55) for the maximum plasma drug concentration. Of the 22 patients who received any dose of study drug, the most commonly reported treatment-emergent adverse events included nausea (12 patients, 54.5%), fatigue, leukopenia, and neutropenia (each in nine patients, 40.9%). The most commonly reported grade ≥ 3 treatment-emergent adverse events were leukopenia and neutropenia (each in seven patients, 31.8%). Conclusions: Coadministration of ramucirumab had no effect on the pharmacokinetics of docetaxel. The incidence and severity of treatment-emergent adverse events were consistent with the known safety profiles of docetaxel and ramucirumab.
Keywords
Ramucirumab; Docetaxel; Drug-drug interactions; Pharmacokinetics
Abbreviations
AUC(0-∞): Area under plasma concentration-time curve, Area under the plasma/serum concentration versus time curve from time zero extrapolated to infinity; AUCtlast-∞: Plasma/serum concentration versus time curve from time zero until the time of last measurable concentration to infinity; CI: Confidence Interval; CL: Clearance; Cmax: Maximum Plasma/Serum Drug Concentration; DDI: Drug-Drug Interaction; ECOG PS: Eastern Cooperative Oncology Group Performance Status; IgG1: Immunoglobulin G Subclass 1; LQC: Lower Quality Control; LS mean: Least Squares Mean; mAbs: Monoclonal Antibodies; NSCLC: Non-Small Cell Lung Cancer; SAE: Serious Adverse Event; t1/2: Terminal Half- Life; TEAE: Treatment-Emergent Adverse Event; VEGF: Vascular Endothelial Growth Factor; VEGFR-2: VEGF Receptor-2; Vss: Volume of Distribution at Steady State
Introduction
Angiogenesis is required for invasive tumor growth and metastasis, and as such, is a key target for control of cancer progression [1]. Vascular endothelial growth factor (VEGF) and VEGF receptor-2 (VEGFR-2) are important mediators in tumor-associated angiogenesis [2]. Blockade of VEGF-and VEGFR-2-mediated signaling inhibits the formation of new blood vessels and tumor growth [3].
Ramucirumab is a human immunoglobulin G subclass 1 (IgG1) monoclonal antibody that selectively binds with high affinity to VEGFR-2, blocking binding of all VEGF ligands and receptor activation [4]. The results from REVEL, a randomized phase 3 trial in patients with previously treated non-small cell lung cancer (NSCLC) showed that treatment with ramucirumab plus docetaxel significantly improved survival compared to treatment with docetaxel alone (median overall survival of 10.5 months for patients treated with ramucirumab plus docetaxel versus 9.1 months for patients treated with placebo plus docetaxel [hazard ratio 0.86, 95% confidence interval (CI) 0.75- 0.98; P=0.023] [5]. The results of this trial led to U.S. Food and Drug Administration approval for ramucirumab as second-line therapy in patients with advanced NSCLC.
Docetaxel belongs to the class of taxane antineoplastic agents that act by prevention of microtubule depolymerization leading to cell cycle arrest, apoptosis, and cytotoxicity, and it is metabolized in the liver by cytochrome P450 3A isozymes [6]. Docetaxel has activity against various types of malignancies, including breast, lung, ovarian, prostate, and head and neck cancer. It is used as a single agent as second-line therapy in patients with advanced NSCLC and in combination with cisplatin in chemotherapy-naïve patients with metastatic NSCLC [6].
The primary objective of this study was to assess the effect of concomitant ramucirumab on the pharmacokinetics of docetaxel in patients with advanced malignant solid tumors resistant to standard therapy or for which no standard therapy was available.
Materials and Methods
Study design
This was a multicenter, open-label, single-arm, cross-comparison study investigating the potential of concomitant ramucirumab to affect the pharmacokinetics of docetaxel. This study was conducted in accordance with the Good Clinical Practices, the Declaration of Helsinki, and approval by the medical institutions’ Ethical Review Board. Patients provided written informed consent prior to inclusion. This trial was registered with ClinicalTrials.gov (NCT01567163).
Eligible patients were ≥ 18 years of age, had metastatic or locally advanced malignant solid tumors that were resistant to standard therapy or for which no standard therapy was available, had adequate organ and hematologic function, had no history of uncontrolled hypertension or bleeding, and had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0-2. Patients were required to have had 0-1 prior taxane-containing treatment regimens (including taxane monotherapy), which must have been completed at least 6 months before the first dose of study drug. Prior treatment with bevacizumab was allowed.
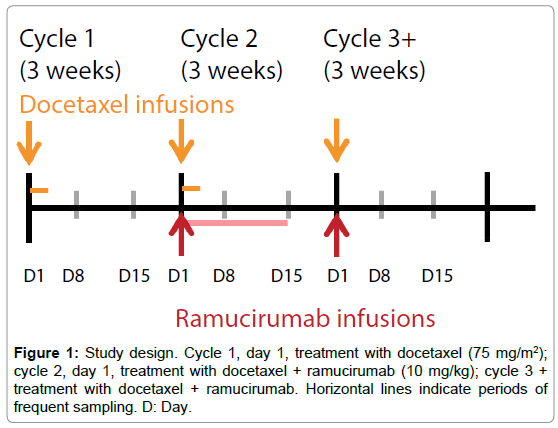
In cycle 1, docetaxel 75 mg/m2 only was administered on day 1 of a 3-week cycle. Combination treatment with ramucirumab 10 mg/kg and docetaxel 75 mg/m2 every 3 weeks began on day 1 of cycle 2 and continued through subsequent cycles until treatment ended (Figure 1). Patients could continue treatment until disease progression, the development of intolerable toxicity, or other reasons for study withdrawal. The study design and data analysis followed US FDA guidance [7].
Pharmacokinetic sampling and assay
Docetaxel: Blood samples for docetaxel analysis were drawn immediately before (0 h) and after (1 h) the docetaxel infusion, and at 1.5, 2, 3, 5, 7, 24, 48, and 72 h after the start of the docetaxel infusion in cycle 1 (monotherapy) and cycle 2 (coadministered with ramucirumab). Docetaxel concentration in plasma was determined using a validated liquid chromatography with tandem mass spectrometric detection method (Covance Laboratories Inc., Madison, Wisconsin, USA). The lower limit of quantification was 5.00 ng/mL. The inter-assay accuracy (deviation of mean from theoretical%) during validation ranged from -4.7-6.7%. The inter-assay precision (%relative standard deviation) during validation ranged from 4.4-7.6%. Testing to assess the potential for coadministered ramucirumab to interfere with the assay was performed. One replicate of pure solution of ramucirumab (2000.00 ng/ mL), and duplicate blank matrix samples and three replicates of lower quality control (LQC) samples spiked with ramucirumab (2000.00 ng/ mL) were extracted. For the pure solution and blank matrix samples, there was no significant interference in the chromatographic regions of interest for docetaxel (<20.0% of the mean lower limit of quantitation response for docetaxel). For the LQC, at least two out of three replicates of the docetaxel quality control samples spiked with ramucirumab were within ± 15% bias. Therefore, the method demonstrated acceptable selectivity in the presence of ramucirumab.
Ramucirumab: Blood samples for ramucirumab analysis were drawn immediately before the ramucirumab infusion (-1 h) and after the ramucirumab infusion/pre-docetaxel infusion (0 h) and 1.5, 2, 3, 5, 7, 24, 48, 72, 168, 264, and 336 h after the start of the ramucirumab infusion in cycle 2 (coadministered with docetaxel). Ramucirumab concentration in serum was determined using a validated enzymelinked immunosorbent assay method (Intertek Pharmaceutical Services, San Diego, California, USA). The lower limit of quantification was 2500.00 ng/mL. The inter-assay accuracy (% relative error) during validation ranged from -24.9-3.9%. The inter-assay precision (% relative standard deviation) during validation ranged from 4.9-17.4%. Interference with docetaxel was also assessed and was determined to have no impact on the quantitation of ramucirumab.
Pharmacokinetic analysis
Pharmacokinetic (PK) parameters for ramucirumab and docetaxel were computed by standard noncompartmental methods of analysis using Phoenix® WinNonlin® Professional 6.2 (Pharsight Corporation, St. Louis, MO, USA).
PK parameters determined for ramucirumab and docetaxel were maximum plasma/serum drug concentration (Cmax), the area under the plasma/serum concentration versus time curve from time zero extrapolated to infinity [AUC(0-∞)], clearance (CL), volume of distribution at steady state (Vss), and terminal half-life (t1/2). AUC(0-∞) and Cmax were dose normalized for the drug-drug interaction (DDI) comparison because patients received different absolute doses.
Statistical analysis
A mixed-effect model was used to analyze the log-transformed PK parameters of AUC(0-∞) and Cmax for docetaxel with or without coadministration with ramucirumab. The model contained cycle (docetaxel, ramucirumab + docetaxel) as fixed effect and patient as a random effect. From the model, least squares mean (LS mean) and 90% CI for the differences of AUC(0-∞) and Cmax of docetaxel in log scale between cycle 1 and cycle 2 were estimated, then transformed back to the original scale to estimate the ratio of geometric LS means and 90% CIs for the comparison (ramucirumab+docetaxel vs. docetaxel). Between-patient and within-patient coefficients of variability were also calculated. All calculations were performed using SAS® version 9.2.
Safety
All patients exposed to any dose level of the study treatments during the trial were considered for the analysis of safety.
Results
Patient demographics and disease characteristics
A total of 22 patients received at least one dose of ramucirumab or docetaxel. A summary of patient baseline characteristics is shown in Table 1. Of these 22 treated patients, there were 13 male (59.1%) and nine female (40.9%). The median age of participants was 61.5 years (range 26-74 years). The majority of patients were white (17 patients, 77.3%) and had an ECOG PS of 0 or 1 (21 patients, 95.5%). The median duration of disease was 25.5 months (range 10 to 80 months). The most commonly reported site of origin for the primary tumor was nonsmall cell lung cancer (five patients, 22.7%), prostate carcinoma and urothelial carcinoma (each in three patients, 13.6%), breast carcinoma and soft tissue sarcoma (each in two patients, 9.1%), and other cancers comprised 7 (31.8%) patients. There were no patients with previous anticancer treatments within 21 days or prior radiotherapy within 14 days of the start of the study.
| Gender, n (%) | |
| Male | 13 (59.1) |
| Female | 9 (40.9) |
| Age, years | |
| Median (range) | 61.5 (26-74) |
| <65, n (%) | 14 (63.6) |
| ≥65, n (%) | 8 (36.4) |
| Race, n (%) | |
| White | 17 (77.3) |
| Black or African American | 4 (18.2) |
| Native Hawaiian or Other Pacific Islander | 1 (4.5) |
| Ethnicity, n (%) | |
| Non-Hispanic or Latino | 22 (100) |
| ECOG PS, n (%) | |
| 0 | 10 (45.5) |
| 1 | 11 (50.0) |
| 2 | 1 (4.5) |
| Duration of disease, months | |
| Median (range) | 25.5 (10 – 80) |
| Prior Taxane, n (%) | 2 (0.09) |
| Type of cancera, n (%) | |
| Non-small cell lung | 5 (22.7) |
| Prostate | 3 (13.6) |
| Urothelial | 3 (13.6) |
| Breast | 2 (9.1) |
| Sarcoma, soft tissue | 2 (9.1) |
| Other | 7 (31.8%) |
Table 1: Patient characteristics.
Pharmacokinetics
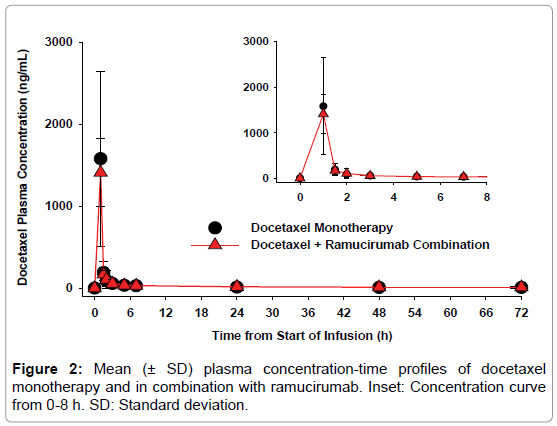
Docetaxel: Mean plasma concentration-time profiles of docetaxel as monotherapy and in combination with ramucirumab are presented in Figure 2. The two curves were superimposable. Maximum docetaxel plasma concentrations were achieved at the end of infusion. Table 2 summarizes docetaxel mean PK parameters obtained following docetaxel administration as monotherapy or as combination therapy following ramucirumab administration. Exposure parameters are similar between cycle 1 and cycle 2, showing that docetaxel exposure was not affected by the presence of ramucirumab.
| Parameter | Geometric Mean (CV%) | |
|---|---|---|
| Docetaxel Alone (cycle 1) n=21 |
Docetaxel+Ramucirumab (cycle 2) n=17 |
|
| Cmax | 1210.51 | 1294.77 |
| (ng/mL) | -88 | -35 |
| Dose-normalised Cmax | 8.18 | 8.78 |
| (ng/mL/mg) | -90 | -37 |
| t1/2a | 25.2b | 30.2c |
| (h) | (7.62-66.9) | (16.5-61.8) |
| AUC(0-∞) | 1970b | 1920c |
| (ng × h/mL) | -47 | -32 |
| Dose-normalised AUC(0-∞) | 13.3b | 12.8c |
| (ng × h/mL/mg) | -54 | -30 |
| CL | 75.0b | 78.1c |
| (L/h) | -54 | -30 |
| Vss | 1280b | 1660c |
| (L) | -111 | -44 |
Table 2: Mean pharmacokinetic parameters of docetaxel administered as monotherapy or as combination therapy with ramucirumab.
Of the total 22 patients who received at least one dose of docetaxel, four patients did not complete day 1, cycle 2. These four patients were not included in the DDI analysis. Of the 18 patients included in the analysis, docetaxel AUC(0-∞) or Cmax could not be calculated in one patient because of incorrect infusion time in cycle 1. In cycle 2, docetaxel AUC(0-∞) or Cmax could not be calculated in one patient because of nonavailability of docetaxel concentrations due to a nonfrozen sample (N=17), and AUC(0-∞) for one additional patient was not included in summary statistics or included in statistical analysis of PK parameters because the area under the plasma/serum concentration versus time curve from time zero until the time of last measurable concentration to infinity (AUCtlast-∞) was >30% (N=16).
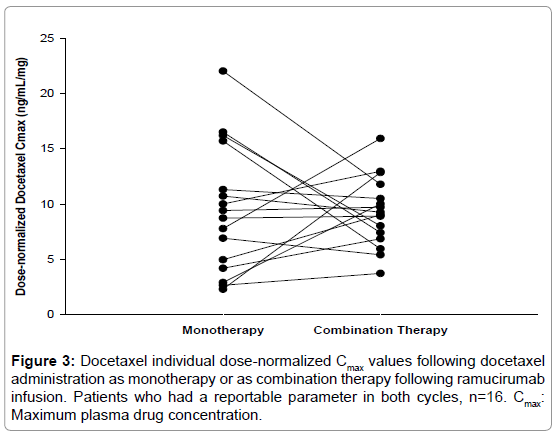
The effect of coadministration of ramucirumab on the pharmacokinetics of docetaxel assessed by statistical analysis is shown in Table 3. Dose-normalized AUCc and Cmax of docetaxel as combination therapy in cycle 2 were similar to those when docetaxel was administered alone in cycle 1, with ratios of geometric LS means (90% CI) at 0.97 (90% CI: 0.84, 1.10) for AUC(0-∞) and 1.14 (90% CI: 0.84, 1.55) for Cmax. Although the Cmax of docetaxel showed a 14% increase when administered in combination with ramucirumab, there was no consistent pattern of increase observed for each patient (Figure 3), indicating a lack of consistency in the increase observed for the mean Cmax.
| Analyses and PK Parameters | N | Geometric LS Means (90% CI) | N | Geometric LS Means (90% CI) | Ratio of Geometric LS Means | 90% CI for the Ratio |
|---|---|---|---|---|---|---|
| The effect of co-administration of ramucirumab on docetaxela | Docetaxel alone cycle 1 | Ramucirumab +Docetaxelcycle 2 | Ramucirumab +Docetaxel: Docetaxel | Lower, upper | ||
| AUC(0-∞) (ng×h/mL/mg)b | 17 | 13.65 | 16 | 13.18 | 0.97 | 0.84, 1.10 |
| (11.78, 15.83) | (11.34, 15.32) | |||||
| Cmax(ng/mL/mg)b | 17 | 7.66 | 17 | 8.76 | 1.14 | 0.84, 1.55 |
| (6.10, 9.64) | (6.97, 11.02) |
Table 3: Drug-drug interaction assessment.
The 90% CI of Cmax includes 1.00, and the 90% CI for AUC(0-∞) is within the interval of 0.80-1.25, indicating that coadministration of docetaxel with ramucirumab had no effect on the pharmacokinetics of docetaxel.
Ramucirumab: Summary statistics for mean PK parameters of ramucirumab when coadministered with docetaxel are shown in Table 4. The exposure parameters for ramucirumab plus docetaxel were similar to those found for ramucirumab monotherapy [8]. The geometric mean dose-normalized Cmax of ramucirumab plus docetaxel was similar to ramucirumab alone (0.365 μg/mL/mg vs. 0.358 μg/mL/ mg, respectively). The geometric mean dose-normalized AUC(0-∞) for ramucirumab plus docetaxel was 54.4 μg × h/mL/mg compared to 55.3 μg × h/mL/mg for ramucirumab monotherapy.
| Geometric Mean (CV%) Docetaxel+Ramucirumab (cycle 2) | |
| Parameter | n=18 |
| Cmax | 303.6 |
| (μg/mL) | -28 |
| Dose-normalized Cmax | 0.365 |
| (μg/mL/mg) | -34 |
| t1/2a | 137b |
| (h) | (95.2-180) |
| AUC(0-∞) | 42400b |
| (μg×h/mL) | -32 |
| Dose-normalized AUC(0-∞) | 54.4b |
| (μg×h/mL/mg) | -29 |
| CL | 0.0184b |
| (L/h) | -29 |
| Vss | 3.47b |
| (L) | -43 |
Table 4: Mean pharmacokinetic parameters of ramucirumab administered as combination therapy with docetaxel.
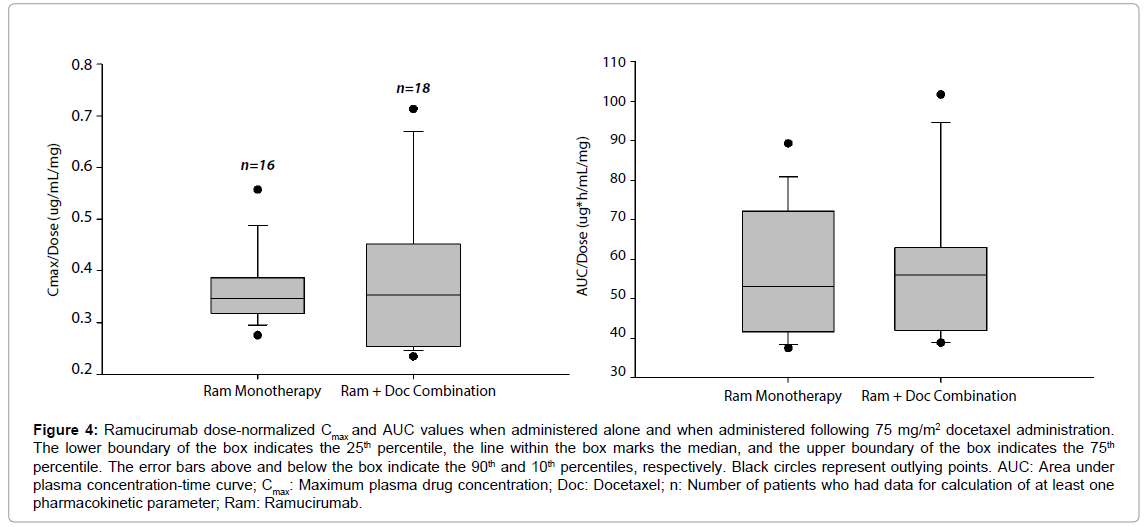
Figure 4 compares the distribution of dose-normalized ramucirumab exposure parameters, Cmax and area under plasma concentration-time curve (AUC) with and without concomitant docetaxel. The results show the distribution of dose-normalized ramucirumab exposure parameters, Cmax and AUC, in two groups of patients where one group was administered ramucirumab alone [8] and the other group was administered ramucirumab in combination with docetaxel. Median values are similar between ramucirumab monotherapy and ramucirumab administered with docetaxel for each parameter, showing that coadministration of ramucirumab and docetaxel is unlikely to impact the pharmacokinetics of ramucirumab.
Figure 4: Ramucirumab dose-normalized Cmax and AUC values when administered alone and when administered following 75 mg/m2 docetaxel administration. The lower boundary of the box indicates the 25th percentile, he line within the box marks the median, and the upper boundary of the box indicates the 75th percentile. The error bars above and below the box indicate the 90th and 10th percentiles, respectively. Black circles represent outlying points. AUC: Area under plasma concentration-time curve; Cmax: Maximum plasma drug concentration; Doc: Docetaxel; n: Number of patients who had data for calculation of at least one pharmacokinetic parameter; Ram: Ramucirumab.
Safety
Of the 22 patients in the safety population, 21 patients experienced a treatment-emergent adverse event (TEAE) regardless of relationship, 14 of whom experienced a grade ≥ 3 TEAE. The most commonly reported grade ≥ 3 TEAEs were leukopenia and neutropenia (each in seven patients, 31.8%) (Table 5). No TEAEs led to discontinuations of either study drug, and no deaths occurred during the study by the cutoff date of December 31, 2012. Four patients had dose modifications or dose interruptions due to TEAEs. These TEAEs were grade 1 chest discomfort; grade 2 fatigue, anorexia, and infusion-related reaction; and grade 4 neutropenia.
The most commonly reported TEAEs were nausea (12 patients, 54.5%); leukopenia, fatigue, and neutropenia (each in nine patients, 40.9%); dyspnea (seven patients, 31.8%); and anemia, hyperglycemia, hypertension, and hyponatremia (each in six patients, 27.3%). Reported bleeding events were epistaxis (three patients, 13.6%), vaginal hemorrhage (two patients, 9.1%), and hemoptysis (one patient, 4.5%); none of these events were grade 3.
Nine patients (40.9%) experienced a serious adverse event (SAE), seven of which were grade ≥ 3 (31.8%). Grade ≥ 3 SAEs included febrile neutropenia and dyspnea (each in two patients, 9.1%) and neutropenia, diarrhoea, fatigue, and dehydration (each in one patient, 4.5%).
Discussion
This study investigated the effect of concomitant ramucirumab on the pharmacokinetics of docetaxel in patients with advanced malignant solid tumors resistant to standard therapy or for whom no standard therapy was available. In patients who completed both cycle 1 day 1 and cycle 2 day 1, dose-normalized AUC(0-∞) and Cmax for docetaxel when administrated with ramucirumab in cycle 2 were consistent with those when docetaxel was administered alone in cycle 1.
Because docetaxel is mainly metabolized by hepatic cytochrome P450 enzymes and monoclonal antibodies (mAbs) are eliminated through nonspecific, Fc receptor-mediated IgG clearance mechanisms and specific target-mediated drug disposition pathways, this finding was not unexpected [6,9]. The results of the present study are consistent with pharmacokinetic data from other clinical studies of docetaxel coadministered with mAbs showing the absence of an interaction between the agents [10-12].
The distribution into tissue of mAbs is known to be slow because of the molecular size of mAbs, and volumes of distribution are generally low [9]. Antibodies are protected from degradation by binding to protective receptors and therefore tend to have long elimination halflives, up to 4 weeks [9]. The pharmacokinetics of ramucirumab were similar to that reported of other mAbs, with a long t1/2 (geometric mean value 137 h), slow CL (geometric mean value 0.0184 L/h), and small Vss following intravenous administration (geometric mean value 3.47 L).
Notably, greater patient variability was observed for docetaxel Cmax relative to AUC, which may contribute to the wider 90% CI for Cmax (90% CI: 0.84, 1.55) relative to AUC (90% CI: 0.84, 1.10). Nevertheless, the 90% CI of Cmax includes 1.00 and the 90% CI for AUC(0-∞) is within the interval of 0.80-1.25, which supports the conclusion that coadministration of docetaxel with ramucirumab had no effect on the pharmacokinetics of docetaxel. Although not an objective of the study, results from a previous study that evaluated the pharmacokinetics of ramucirumab monotherapy [8] were compared to the pharmacokinetics of ramucirumab plus docetaxel in this study (Figure 4). The median values are similar, showing that coadministration of ramucirumab and docetaxel is unlikely to impact the pharmacokinetics of ramucirumab.
The combination of docetaxel (75 mg/m2) once every 3 weeks with ramucirumab (10 mg/kg) was generally well tolerated. No deaths occurred during this study, and the most commonly reported TEAEs were nausea, fatigue, leukopenia, neutropenia, dyspnea, anemia, hyperglycemia, hypertension, and hyponatremia. The most commonly reported grade ≥ 3 TEAEs were leukopenia and neutropenia.
The docetaxel-related neutropenia events reported in this study are within the range cited in the literature [5,13,14]. In the REVEL trial, there was a greater incidence of any grade neutropenia in the ramucirumab plus docetaxel arm (345 patients, 55%) compared to the placebo plus docetaxel arm (284 patients, 45%) [5]; however, this study was not designed to detect such differences. No unexpected TEAEs or SAEs were observed with docetaxel in combination with ramucirumab or with docetaxel monotherapy in this study. The overall safety profile is consistent with what has been observed previously in ramucirumab clinical trials.
The results of this study supported the concomitant use of ramucirumab with docetaxel in the phase 3 REVEL trial for patients with advanced NSCLC [5]. This study demonstrated that ramucirumab plus docetaxel improves survival as second-line treatment compared to placebo plus docetaxel without significant increase in toxicity. The current clinical study confirms the absence of PK DDI between docetaxel and ramucirumab; therefore, no dosage adjustment is required due to DDI concerns when these agents are used concomitantly for the treatment of advanced cancers.
Acknowledgements
This study was funded by Eli Lilly and Company. The authors gratefully acknowledge Dr. Minori Koshiji for her contributions to this study. Eli Lilly and Company funded the medical writing support provided by Stacey E. Shehin, PhD, and Lucy Rutherford, the editorial support provided by Angela C. Lorio, ELS, and Jeanne Claypoole, all of whom are full-time employees of in Ventiv Health Clinical. This work was presented in part as an abstract at the American Society of Clinical Oncology Annual Meeting, 30 May 2014, Chicago, IL, and at the American Association of Pharmaceutical Scientists National Biotechnology Conference; San Diego, CA; 19 May 2014.
References
- Folkman J (2002) Role of angiogenesis in tumor growth and metastasis. SeminOncol 29: 15-18.
- Zhu Z, Bohlen P, Witte L (2002) Clinical development of angiogenesis inhibitors to vascular endothelial growth factor and its receptors as cancer therapeutics. Curr Cancer Drug Targets 2: 135-156.
- Zhu Z, Witte L (1999) Inhibition of tumor growth and metastasis by targeting tumor-associated angiogenesis with antagonists to the receptors of vascular endothelial growth factor. Invest New Drugs 17: 195-212.
- Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, et al. (2010) Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J ClinOncol 28: 780-787.
- Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, et al. (2014) Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 384: 665-673.
- Baker SD, Sparreboom A, Verweij J (2006) Clinical pharmacokinetics of docetaxel: recent developments. ClinPharmacokinet 45: 235-252.
- US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) (2012) Guidance for Industry. Drug Interaction Studies-Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations.
- Chow LQ, Smith DC, Tan AR, Denlinger CS, Wang D, et al. (2016) Lack of pharmacokinetic drug-drug interaction between ramucirumab and paclitaxel in a phase II study of patients with advanced malignant solid tumors. Cancer ChemotherPharmacol 78: 433-441.
- Keizer RJ, Huitema AD, Schellens JH, Beijnen JH (2010) Clinical pharmacokinetics of therapeutic monoclonal antibodies. ClinPharmacokinet 49: 493-507
- Cortés J, Swain SM, Kudaba I, Hauschild M, Patel T, et al. (2013) Absence of pharmacokinetic drug-drug interaction of pertuzumab with trastuzumab and docetaxel. Anticancer Drugs 24: 1084-1092.
- Attard G, Kitzen J, Blagden SP, Fong PC, Pronk LC, et al. (2007) A phase Ib study of pertuzumab, a recombinant humanised antibody to HER2, and docetaxel in patients with advanced solid tumours. Br J Cancer 97: 1338-1343.
- Jatoi A, Jett JR, Sloan J, Novotny P, Ford J, et al. (2004) A pilot study on safety and pharmacokinetics of infliximab for the cancer anorexia/weight loss syndrome in non-small-cell lung cancer patients. Support Care Cancer 12: 859-863.
- Baker SD, Li J, ten Tije AJ, Figg WD, Graveland W, et al. (2005) Relationship of systemic exposure to unbound docetaxel and neutropenia. ClinPharmacolTher 77: 43-53.
- Harvey V, Mouridsen H, Semiglazov V, Jakobsen E, Voznyi E, et al. (2006) Phase III trial comparing three doses of docetaxel for second-line treatment of advanced breast cancer. J ClinOncol 24: 4963-4970.
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 12053
- [From(publication date):
November-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 11109
- PDF downloads : 944