Research Article Open Access
Phase Equilibria Calculation and Investigation of Hardness and Electrical Conductivity for Alloys in Selected Sections of Bi-Cu-Ni System
Branislav R. Markovic1*, Dragana T. Živkovic2, Dragan M. Manasijevic2, Nadežda M. Talijan3, Miroslav D. Sokic1 and Vladan R. Cosovic3
1Institute for Technology of Nuclear and Other Mineral Raw Materials, Serbia
2Technical Faculty, University of Belgrade, Serbia
3Institute of Chemistry, Technology and Metallurgy, Serbia
- *Corresponding Author:
- Branislav R. Markovic
Institute for Technology of Nuclear
and Other Mineral Raw Materials
86 Franchet d’Esperey Street
Belgrade 11000, Serbia
E-mail: b.markovic@itnms.ac.rs
Received Date: November 24, 2012; Accepted Date: December 17, 2012; Published Date: December 23, 2012
Citation: Markovic BR, Živkovic DT, Manasijevic DM, Talijan NM, Sokic MD, et al. (2012) Phase Equilibria Calculation and Investigation of Hardness and Electrical Conductivity for Alloys in Selected Sections of Bi-Cu-Ni System. J Powder Metall Min 2:104. doi: 10.4172/2168-9806.1000104
Copyright: © 2012 Markovic BR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Powder Metallurgy & Mining
Abstract
The results of phase equilibria calculation and the experimental investigation of alloys in selected sections of Bi-Cu-Ni system with bismuth constant molar content of 0.6; 0.7; 0.8; and 0.9 are presented in this paper. Thermodynamic calculation was done according to the CALPHAD method using PANDAT software, while chosen alloys in the selected sections were experimentally investigated using hardness and electrical conductivity measurements.
Keywords
Lead-free solders; Bi-Cu-Ni system; Phase equilibria calculation; Alloys characterization
Introduction
Alloys of Pb-Sn system are commonly used solder materials in electrical and electronics industry due to their low cost and unique combination of physical, chemical and mechanical properties, as well as their reliability. According to recent EU legislation - DIRECTIVE WEEE (Waste from Electrical and Electronic Equipment) and DIRECTIVE 2002/95/EC (Restriction of the use of certain Hazardous Substances (RoHS) in electrical and electronic equipment), the use of lead containing solders is prohibited in many industries from July 1st, 2006. Therefore, a great effort has been made on the development of new Pb-free soldering and brazing materials [1-6].
The Bi-Cu-Ni system belongs to the group of potential candidates for lead-free solder materials in the frame of Cu-Ni-based lead-free systems for high temperature application. This system represents the environmental friendly alternative to Sn-based solders which has been examined recently in the frame of COST MP0602 “HISOLD” project [7] and has been described in two recent references [8-10]. Gao et al. [8], investigated the phase equilibria of the Bi–Cu–Ni system at 300, 400, and 500°C using metallography and electron probe microanalysis on equilibrated alloys and diffusion couples, while Markovic et al. [9], performed a thermodynamic modeling of the Bi–Cu–Ni system using the CALPHAD method and the investigation of different alloys characteristics in [10].
In order to contribute to the knowledge on the Bi–Cu–Ni system and a better understanding of the processes occurring during soldering and during operation of the soldered devices, the results of phase equilibria calculation and the experimental investigation of alloys in the selected sections of the Bi-Cu-Ni system are presented in this paper.
Experimental
The investigated samples taken from four sections of the Bi-Cu- Ni system with constant bismuth mole fraction of 0.6; 0.7; 0.8; and 0.9 were prepared in an induction furnace using metals of p.a. purity in protected atmosphere and homogenized at 800°C for 2 h under an argon atmosphere and then furnace cooled to room temperature using cooling rate of 2°C /min.
The composition of the investigated samples is presented in table 1.
| Sample | xBi | xCu | xNi |
| F section - with constant xBi=0.6 | |||
| F1 | 0.6 | 0.2 | 0.2 |
| F2 | 0.6 | 0.3 | 0.1 |
| F3 | 0.6 | 0.1 | 0.3 |
| G section - with constant xBi=0.7 | |||
| G1 | 0.7 | 0.15 | 0.15 |
| G2 | 0.7 | 0.225 | 0.075 |
| G3 | 0.7 | 0.075 | 0.225 |
| H section - with constant xBi=0.8 | |||
| H1 | 0.8 | 0.1 | 0,1 |
| H2 | 0.8 | 0.15 | 0.05 |
| H3 | 0.8 | 0.05 | 0.15 |
| J section - with constant xBi=0.9 | |||
| J1 | 0.9 | 0.05 | 0.05 |
| J2 | 0.9 | 0.075 | 0.025 |
| J3 | 0.9 | 0.025 | 0.075 |
Table 1: Composition of the investigated samples
The hardness and electrical conductivity measurements of the samples were investigated. The electrical conductivity of investigated samples was measured using the standard apparatus SIGMATEST 2.069 (Foerster) eddy current instrument for measurements of electrical conductivity of non-ferromagnetic metals, based on complex impedance of the measuring probe with diameter of 8mm.
The hardness measurements were done using standard procedure according to Vickers.
Results and Discussion
The phase equilibria in chosen Bi-Cu-Ni sections were calculated according to the well known CALPHAD methodology [11], using PANDAT thermodynamic software Vs. 8.0 [12,13]. The calculation was based on known thermodynamic data for the pure elements, socalled SGTE (Scientific Group Thermodata Europe) values [14], and optimized binary and ternary thermodynamic parameters given in [9].
For experimental confirmation of the calculation results, the thermal analysis data of the samples in the selected sections of the Bi- Cu-Ni system, obtained for heating measurements, including liquidus temperatures and other phase transition temperatures, were used from our previous work [9].
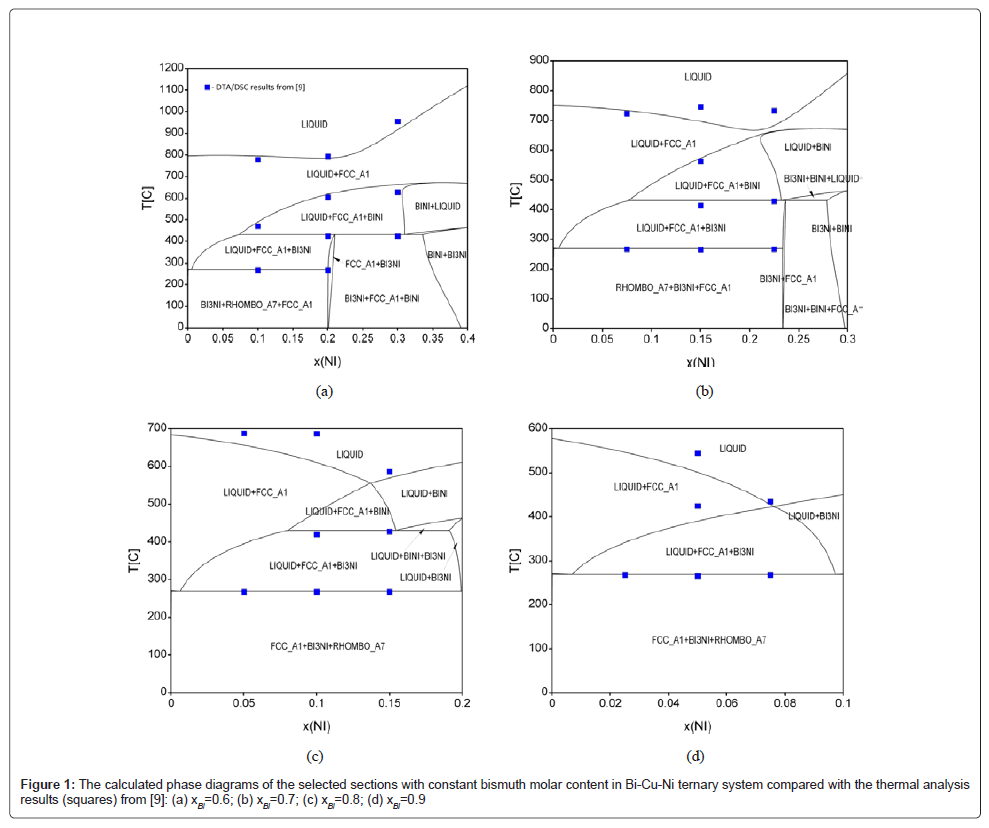
The calculated phase diagrams of the investigated sections are presented in figure 1, together with experimentally determined DTA/ DSC points [9].
As can be noticed, there is a reasonable agreement between the thermodynamic prediction and the experiment, while the only slight deviation between calculation and experiments can be seen at liquidus temperature lines. More, there are two invariant reactions in this system - the ternary quasi-peritectic reaction and the ternary eutectic reaction [9]:
(U1): LIQUID+BINI ↔ FCC_A1+Bi3Ni (at 430°C)
(E1): LIQUID ↔ FCC_A1+BI3NI+RHOMBO_A7 (at 269°C)
The both existing invariant reactions were proven by calculation in this paper to be in excellent agreement with literature [8,9].
The obtained calculation results are also in agreement with the experimental structural analysis from [9], confirming that the Bi–Cu– Ni system consists of five phases (liquid, RHOMBO_A7 (Bi), FCC_A1 (Cu,Ni), BiNi and Bi3Ni), which is in accordance to its crystallographic data [15].
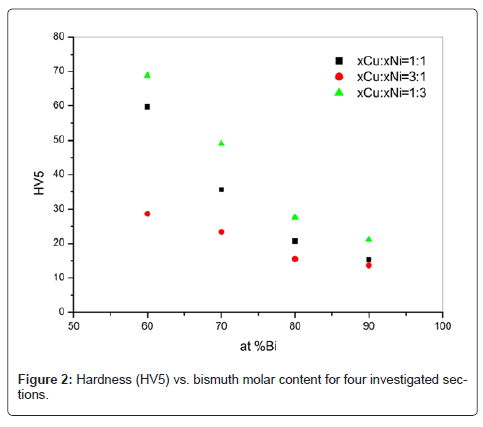
In the second part of the paper, the results of experimental investigation of some Bi-Cu-Ni alloys are given. So, the results obtained by hardness measurements are shown in table 2 and figure 2. There were three sets of hardness measurements at the cross-section of each sample. As can be seen, the hardness decreases rapidly with bismuth concentration increase for selected alloys in the investigated sections. Noticed behaviour can be explained as the consequence of dominant phases present in the alloys structure, mostly Bi-based phases - BiNi, Bi3Ni and RHOMBO_A7, due to the fact that bismuth posess the lowest hardness value among the three constitutive metals in ternary system Bi-Cu-Ni.
| Sample | Mean value (HV5) | Standard deviation |
|---|---|---|
| F1 | 59.7 | 0.43589 |
| F2 | 28.6 | 0.62450 |
| F3 | 68.8 | 0.75498 |
| G1 | 35.6 | 0.45826 |
| G2 | 23.4 | 0.43589 |
| G3 | 49 | 1.34536 |
| H1 | 20.7 | 0.72111 |
| H2 | 15.5 | 0.45826 |
| H3 | 27.6 | 0.70000 |
| J1 | 15.3 | 0.43589 |
| J2 | 13.7 | 0.52915 |
| J3 | 21.2 | 0.79373 |
Table 2: The results of hardness measurements.
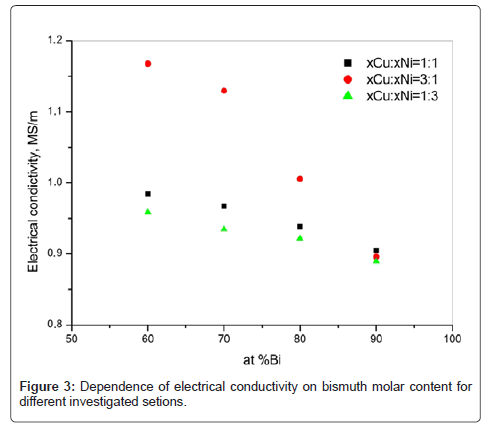
The results of electrical conductivity measurements are given in table 3 (three measuring series), where the electrical conductivity dependence on the composition is showed in figure 3. It can be noticed that increase of bismuth content influences the decrease in electrical conductivity for all samples in the investigated section, which is in accordance with the electrical conductivity of pure bismuth (≈ 0.867 MS/m [16,17]). The electrical conductivity decrease with bismuth content increase is the consequence of prevailing phases presence in the structure, i.e. the bismuth based phases BiNi, Bi3Ni and RHOMBO_A7 influencing the electrical conductivity decrease.
| Sample | Mean value (MS/m) | Standard deviation |
|---|---|---|
| F1 | 0.9843 | 0.00040 |
| F2 | 1.1677 | 0.00153 |
| F3 | 0.9587 | 0.00020 |
| G1 | 0.9673 | 0.00141 |
| G2 | 1.1296 | 0.00061 |
| G3 | 0.9351 | 0.00070 |
| H1 | 0.9385 | 0.00182 |
| H2* | 1.0057 | 0.00058 |
| H3 | 0.9217 | 0.00055 |
| J1* | 0.9045 | 0.00236 |
| J2 | 0.8958 | 0.00095 |
| J3* | 0.8891 | 0.00046 |
* The elecrical conductivity values for H2, J1 and J3 samples were reported in our previous paper [10]
Table 3: The results of electrical conductivity measurements.
Conclusions
The selected sections in the Bi-Cu-Ni system with constant bismuth molar content of 60; 70; 80; and 90 at% have been investigated in this paper. The phase diagrams of these sections were calculated by CALPHAD method using PANDAT thermodynamic software, while some alloys in the mentioned sections were subjected to hardness and electrical conductivity measurements. The obtained phase diagrams were proven by literature experimental results and the main invariant reactions and phases were confirmed. The measured values of hardness and electrical conductivity decreased with bismuth concentration increase for all the investigated alloys. Here presented results are further contribution to the better knowledge of phase equilibria, structural, mechanical and electrical properties of Bi-Cu-Ni alloys as a new potential lead-free solder material for the high temperature application.
Acknowledgment
This work presents the part of realization in the frame of projects N°172037 and N°34023, supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, and in the frame of European COST MP0602 Action.
References
- Li Y, Wong CP (2006) Recent advances of conductive adhesives as a lead-free alternative in electronic packaging: Materials, processing, reliability and applications. Mater Sci Eng R 51: 1-35.
- Laurila T, Vuorinen V, Kivilahti JK (2005) Interfacial reactions between lead-free solders and common base materials. Mater Sci Eng 49: 1-60.
- Takaku Y, Ohnuma I, Kainuma R, Yamada Y, Yagi Y, et al. (2006) Development of Bi-base high temperature Pb-free solders with second-phase dispersion: Thermodynamic calculation microstructure, and interfacial reaction. J Electron Mater 35: 1926-1932.
- Lalena JN, Dean NF, Weiser MW (2002) Experimental investigation of Ge-doped Bi-11Ag as a new Pb-free solder alloy for power die attachment. J Electron Mater 31: 1244-1249.
- Rettenmayr M, Lambracht P, Kempf B, Graff M (2005) High melting Pb-free solder alloys for die-attach applications. Adv Eng Mater 7: 965-969.
- Dinsdale AT, Kroupa A, Vizdal J, Vrestal J, Watson A, Zemanova A (2006) COST 531 Database for Lead-free Solders, Ver. 2.0, 2006, unpublished research, COST531 Homepage.
- http://cost602.ipm.cz/
- Gao F, Liu X, Takaku Y, Ohnuma I, Ishida K (2009) Experimental investigation and thermodynamic calculation in the Ag–Bi–Ni and Cu–Bi–Ni systems. J Mater Res 24: 2644-2653.
- Markovic B, Živkovic D, Vreštál J, Manasijevic D, Minic D, et al. (2010) Experimental study and thermodynamic remodeling of the Bi-Cu-Ni system. Calphad 34: 294-300.
- Markovic B, Živkovic D, Manasijevic D, Sokic M, Minic D, Talijan N, Stajic-Trošic J (2013) Thermal, structural and electrical properties of some Bi-Cu-Ni alloys. Archives of Materials and Metallurgy 58: 2.
- Saunders N, Miodownik AP (1998) CALPHAD Calculation of Phase Diagrams: A Comprehensive Guide. Elsevier, London.
- http://www.computherm.com
- Cao W, Chen SL, Zhang F, Wu K, Yang Y, et al. (2009) PANDAT software with PanEngine, PanOptimizer and PanPrecipitation for multi-component phase diagram calculation and materials property simulation. Calphad, 33: 328-342.
- http://www.sgte.org
- Dinsdale A, Watson A, Kroupa A, Vrestal J, Zemanova A, Vizdal J (2008) COST Action 531 - Atlas of Lead free soldering. COST office, Brussels 124.
- http://www.chemicool.com
- Web Elements: the periodic table on the web.
Relevant Topics
- Additive Manufacturing
- Coal Mining
- Colloid Chemistry
- Composite Materials Fabrication
- Compressive Strength
- Extractive Metallurgy
- Fracture Toughness
- Geological Materials
- Hydrometallurgy
- Industrial Engineering
- Materials Chemistry
- Materials Processing and Manufacturing
- Metal Casting Technology
- Metallic Materials
- Metallurgical Engineering
- Metallurgy
- Mineral Processing
- Nanomaterial
- Resource Extraction
- Rock Mechanics
- Surface Mining
Recommended Journals
Article Tools
Article Usage
- Total views: 14297
- [From(publication date):
March-2013 - Dec 19, 2024] - Breakdown by view type
- HTML page views : 9873
- PDF downloads : 4424