Research Article Open Access
Pharmacokinetics of Recombinant Soluble Human Thrombomodulin in Subjects with Normal and Various Impaired Renal Function
Shinichiro Shirae1*, Yutaka Osawa1, Thomas C Marbury2 and Kazuhisa Tsuruta1
1Asahi Kasei Pharma America Corporation, 200 Fifth Avenue, Waltham 02451, USA
2Orlando Clinical Research Center, Orlando, FL, USA
- *Corresponding Author:
- Shinichiro Shirae
Asahi Kasei Pharma America Corporation
200 Fifth Avenue, Waltham 02451, USA
Tel: 781419-1919
Fax:781890-0660
E-mail: shirae.sb@om.asahi-kasei.co.jp
Received date: July 19, 2016; Accepted date: August 08, 2016; Published date: August 15, 2016
Citation: Shirae S, Osawa Y, Marbury TC, Tsuruta K (2016) Pharmacokinetics of Recombinant Soluble Human Thrombomodulin in Subjects with Normal and Various Impaired Renal Function. Clin Pharmacol Biopharm 5:159. doi: 10.4172/2167-065X.1000159
Copyright: © 2016 Shirae S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Background: Non-clinical and clinical studies showed efficacy of thrombomodulin alfa for disseminated intravascular coagulation, and its potential efficacy for severe sepsis and coagulopathy. Thrombomodulin alfa is excreted primarily via the kidney and renal function is known to affect the clearance. However the dosing adjustments for patients with renal dysfunction were not warranted, except for patients on hemodialysis, who had not been studied well. This study was conducted to assess the effect of renal impairment on the exposure of thrombomodulin alfa and to support a dosing rationale in patients with renal impairment especially patients on hemodialysis. Material and methods: Forty subjects with varying renal function participated in 5 groups. Each subject received one intravenous bolus injection of 0.06 mg/kg thrombomodulin alfa. The PK parameters were analyzed by a twocompartment model. The plasma concentration following multiple doses was simulated based on the PK parameters in each subject and various renal function groups. The predicted maximum plasma concentration (Cmax) was compared with the maximum non-toxic concentration (5,400 ng/mL). Results: Following multiple dose simulations of six, once-daily intravenous bolus injections of 0.06 mg/kg thrombomodulin alfa, the predicted mean Cmax in subjects with normal renal function, and mild, moderate, or severe renal impairment and on hemodialysis was 2,030, 2,350, 2,410, 3,710, 3,180 ng/mL, respectively. The highest individual simulated Cmax was 4,730 ng/mL, hence no renal function groups or no individually simulated Cmax exceeded the maximum non-toxic concentration. Conclusion: Although renal impairment was associated with increased exposure of thrombomodulin alfa, no dose adjustment is required for patients with renal impairment including patients on hemodialysis.
Keywords
Thrombomodulin alfa; Sepsis; Hemodialysis; Pharmacokinetics; Renal function
Introduction
Thrombomodulin alfa is a soluble recombinant human thrombomodulin that is composed of the active, extracellular domain of thrombomodulin and lacks the cytoplasmic and transmembrane domains [1]. Thrombomodulin alfa acts to inhibit thrombin generation by binding to thrombin and via activating Protein C [1]. Experimental data suggest that thrombomodulin alfa has anti-inflammatory effects through activation of protein C and thrombin-activatable fibrinolysis inhibitor (TAFI), and inhibition of high mobility group box 1 (HMGB1) activity directly [2]. Thrombomodulin alfa was approved for the treatment of disseminated intravascular coagulation (DIC) in Japan in 2008 [3]; and a global Phase 3 study is being conducted to confirm the efficacy and safety of thrombomodulin alfa for the treatment of severe sepsis and coagulopathy [4]. The dosing regimen for the ongoing study is 0.06 mg/kg/day up to a maximum dose of 6.0 mg/day for six days [4].
Due to the anticoagulant properties of thrombomodulin alfa, a possible risk derived from administration of thrombomodulin alfa is serious hemorrhaging due to an abrupt increase in plasma concentrations of thrombomodulin alfa. In a multiple dose study in monkeys, the no observed adverse effect level (NOAEL) was 0.2 mg/kg (maximum plasma concentration [Cmax]: 5,400-7,200 ng/mL) and toxic reactions were observed at the dose of 0.6 mg/kg (Cmax: Equal or more than 13,000 ng/mL). Therefore, the highest concentration at which no bleeding event was observed in the nonclinical toxicology studies was 5,400 ng/mL [5].
A phase 1 study in healthy subjects showed that the maximum plasma concentration (Cmax) and area under the plasma concentrationtime curve (AUC) are linear following intravenous administration of thrombomodulin alfa. Repeated administration did not alter the pharmacokinetics (PK) of thrombomodulin alfa [1]. Thrombomodulin alfa is excreted primarily via the kidney, with approximately 45% to 70% of thrombomodulin alfa recovered in the urine following intravenous administration [1,6,7]. Two population pharmacokinetics (PPK) analyses were performed; one in a Japanese population [8] and the other in a non-Japanese population [5] which included subjects with various levels of renal impairment but excluded the population requiring hemodialysis. The analyses results suggested that renal function affects clearance of thrombomodulin alfa [5,8].
Even in subjects with renal impairment, no additional safety concerns arose in the previous global phase 2b study where subjects with various levels of renal function were enrolled, except subjects requiring hemodialysis [9]. Thrombomodulin alfa dosing adjustments for subjects with renal dysfunction, except those requiring hemodialysis, were not warranted in subsequent clinical trials [5], as the potential to lower the dosage below the effective plasma concentrations could have presented a significant risk to subjects who have sepsis with coagulopathy.
As shown above, pharmacokinetics and safety in a renal impaired population were studied in several studies. However, due to a lack of enough pharmacokinetics and safety information, subjects on hemodialysis had been excluded from clinical study enrollment [5]. It is well known that many patients with severe sepsis and/or septic shock frequently require hemodialysis due to the development of acute renal impairment [10-12]. This study was conducted to assess the effect of renal impairment on the exposure of thrombomodulin alfa and to support a dosing rationale in patients with renal impairment especially patients on hemodialysis.
Material and Methods
This study was conducted in accordance with the International Conference on Harmonisation E6 Good Clinical Practice, Consolidated Guidance, and was designed generally in accordance with the draft FDA guidance for industry [13]. The study was conducted at 3 centers in the United States. All of the centers received approval from an institutional review board, and a signed informed consent was obtained from each subject before any study related procedures were performed.
Subject selection
A total of 40 men and women participated in this open-label, single dose study. At the screening visit, subjects were allocated into 5 groups (8 subjects in each group) on the basis of estimated creatinine clearance (CLcr) values using the Cockcroft Gault equation [13] and on the basis of hemodialysis use. The 5 groups were defined as normal renal function group (Healthy Control Group; CLcr ≥ 80 mL/min), mild renal impairment group (Mild Impairment Group; CLcr 60-79 mL/min), moderate renal impairment group (Moderate Impairment Group; 30-59 mL/min), severe renal impairment group (Severe Impairment Group; 15-29 mL/min), end-stage renal disease (ESRD) group (ESRD Group; requiring hemodialysis or CLcr <15 mL/ min not on hemodialysis). The above definition was based on a draft FDA guidance with a modification. The CLcr cut off value for Healthy Control Group was changed from CLcr ≥ 90 mL/min to CLcr ≥ 80 mL/min and Mild Impairment Group was changed from CLcr 60-89 mL/min to CLcr 60-79 mL/min for subjects matching purpose: To the extent possible, subjects in Healthy Control Group were matched 1:1 to subjects in ESRD Group for similar age (± 10 years), gender and weight (BMI ± 15%). Due to this matching scheme, the Healthy Control Group could be relatively older and have lower calculated Clcr value even with healthy status. Hence, CLcr ranges for Healthy Control Group and Mild Group have been changed from the FDA draft guidance [13].
Thrombomodulin alfa
Thrombomodulin alfa is produced by Asahi Kasei Pharma Corporation (Tokyo, Japan) from Chinese hamster ovary cells. It is synthesized as a 498 amino acid glycoprotein, composed of the D1, D2, and D3 domains, but lacks the transmembrane (D4) and cytoplasmic (D5) domains of human thrombomodulin.
Thrombomodulin alfa administration and sample collection
All subjects received a single dose of 0.06 mg/kg of thrombomodulin alfa by IV bolus injection within 1 min. Subjects with hemodialysis received thrombomodulin alfa between hemodialysis treatments.
Plasma samples for the measurement of thrombomodulin alfa concentration were taken at the following time-points: Pre-dose, and at 5 min, 15 min, 30 min, 1, 2, 4, 8, 12, 24, 48, 72, 96, and 120 h post thrombomodulin alfa administration. For subjects on hemodialysis, the PK samples were collected at least 1 h after the end of the subject’s last hemodialysis treatment.
Measurement of thrombomodulin alfa concentration in plasma.
Thrombomodulin alfa concentrations in plasma were measured by an enzyme-linked immunosorbent (ELISA) assay method. The ELISA method used plates coated with 2 types of mouse monoclonal antibodies against thrombomodulin alfa, which recognize epitopes in the active center of thrombomodulin alfa. The lower limit of qualification of thrombomodulin alfa is 3.125 ng/mL. This assay method was validated based on the FDA Guidance for Industry [14] and the recommendations and best practices for ligand binding assays [15,16].
Pharmacokinetic analysis
Using Phoenix WinNonlin (Pharsight Corporation, St. Louis, MO, USA, version 6.2.1), PK parameters were determined from the plasma concentrations of thrombomodulin alfa using a 2-compartmental procedure. Cmax for the thrombomodulin alfa recommended dosage period of six doses were simulated and calculated based on the determined PK parameters. The predicted Cmax during the recommended dosage period was compared with the maximum nontoxic concentration (5,400 ng/mL) by group and by individual.
Safety assessments
Safety assessments included physical examination, vital signs (blood pressure, heart rate, and oral temperature), 12-lead ECG, laboratory tests (hematology, biochemistry, and urinalysis), and adverse events by 28 days after thrombomodulin alfa administration. In addition, presence of anti-thrombomodulin alfa antibodies was tested for the day of thrombomodulin alfa administration and 28 days after thrombomodulin alfa administration or early termination.
Measurement of anti-thrombomodulin alfa antibody
Anti-thrombomodulin alfa antibodies in serum were measured by an electrochemiluminescence (ECL) assay method. The assay involves; capturing anti-thrombomodulin alfa antibodies from serum samples using biotin labeled thrombomodulin alfa immobilized onto streptavidin coated plate, and detecting the anti-thrombomodulin alfa antibody with SULFO-TAG labeled thrombomodulin alfa.
First, serum samples are subjected to a screening assay. Samples that show signals more than plate cut point are judged as screening positive. Plate cut point is defined as the mean of nonspecific background of negative control in the plate plus normalization factor of 14. Subsequently, samples tested positive in the screening assay are subjected to a confirmatory assay, in which the decrease of signals by spiking samples with excess amount of thrombomodulin alfa is measured. If % inhibition of signal by spiking sample with thrombomodulin alfa exceeds 20%, the sample is confirmed as positive. This assay method was validated based on the FDA Guidance for Industry [17] and the recommendations for immunoassays [18,19].
Results
Disposition and demographics
A total of 70 subjects were screened and consented, 40 of them were enrolled; and 8 subjects were enrolled to 5 groups. In this study, all ESRD subjects were enrolled based on the criteria of “requiring hemodialysis”.
All of the 40 subjects completed the study and were included in the pharmacokinetic and safety analyses. However, plasma concentration data from 2 subjects (1 subject from the Severe Impairment Group and 1 subject from the ESRD Group) were excluded from the summary statistics for PK analyses due to anomalous plasma concentration-time profiles. Additionally, several outlying plasma concentration data were also excluded.
Demographic characteristics at the screening visit are summarized in Table 1. Weight and BMI were similar and ratio of gender was slightly different among the 5 Groups. Age ranged from 26-75 years; the mean age for subjects in the Mild, Moderate, and Severe Renal Impairment Groups (60-68 years) was greater than the mean age for subjects in the Healthy Control and ESRD Groups (42 years). Each subject in the Healthy Control Group was well matched to a respective subject with ESRD with respect to age, gender, and BMI. CLcr values met the eligibility criteria predetermined for each Group, and all ESRD subjects were enrolled based on the criteria of “requiring hemodialysis”.
| Healthy Control Group | Mild Impairment Group | Moderate Impairment Group | Severe Impairment Group | ESRD Group | |||
|---|---|---|---|---|---|---|---|
| Demographic | |||||||
| Age (years) | Mean | 42 | 68 | 66 | 60 | 42 | |
| SD | (11.2) | (6.4) | (8.5) | (8.4) | (11.1) | ||
| Range | (26, 56) | (56, 75) | (52, 75) | (49, 74) | (26, 61) | ||
| Sex | |||||||
| Male | n (%) | 7 (87.5) | 5 (62.5) | 4 (50.0) | 4 (50.0) | 7 (87.5) | |
| Female | n (%) | 1 (12.5) | 3 (37.5) | 4 (50.0) | 4 (50.0) | 1 (12.5) | |
| Weight (kg) | Mean | 86.2 | 75.6 | 72.9 | 78.2 | 85.5 | |
| SD | (10.00) | (13.84) | (9.27) | (8.01) | (13.49) | ||
| Range | (63.2, 95.2) | (55.3, 98.4) | (61.3, 90.0) | (70.0, 94.0) | (63.0, 95.7) | ||
| BMI (kg/m2) | Mean | 27.5 | 27.1 | 28.3 | 29.0 | 28.9 | |
| SD | (2.48) | (3.15) | (3.36) | (2.93) | (4.49) | ||
| Range | (25.1, 31.6) | (21.9, 31.8) | (24.2, 33.4) | (25.7, 33.7) | (22.1, 35.6) | ||
| Creatinine Clearance (mL/min) | Mean | 132 | 67 | 47 | 22 | 16 | |
| SD | 19.2 | 5.1 | 5.9 | 4.2 | 8.4 | ||
| Range | (105, 139) | (62, 77) | (39, 56) | (18, 29) | (8, 31) | ||
BMI: Body mass index; N: Number of subjects studied; ( ): Percentage of subjects; Note: Range (Minimum, Maximum).
Table 1: Demographic data at screening.
Pharmacokinetics
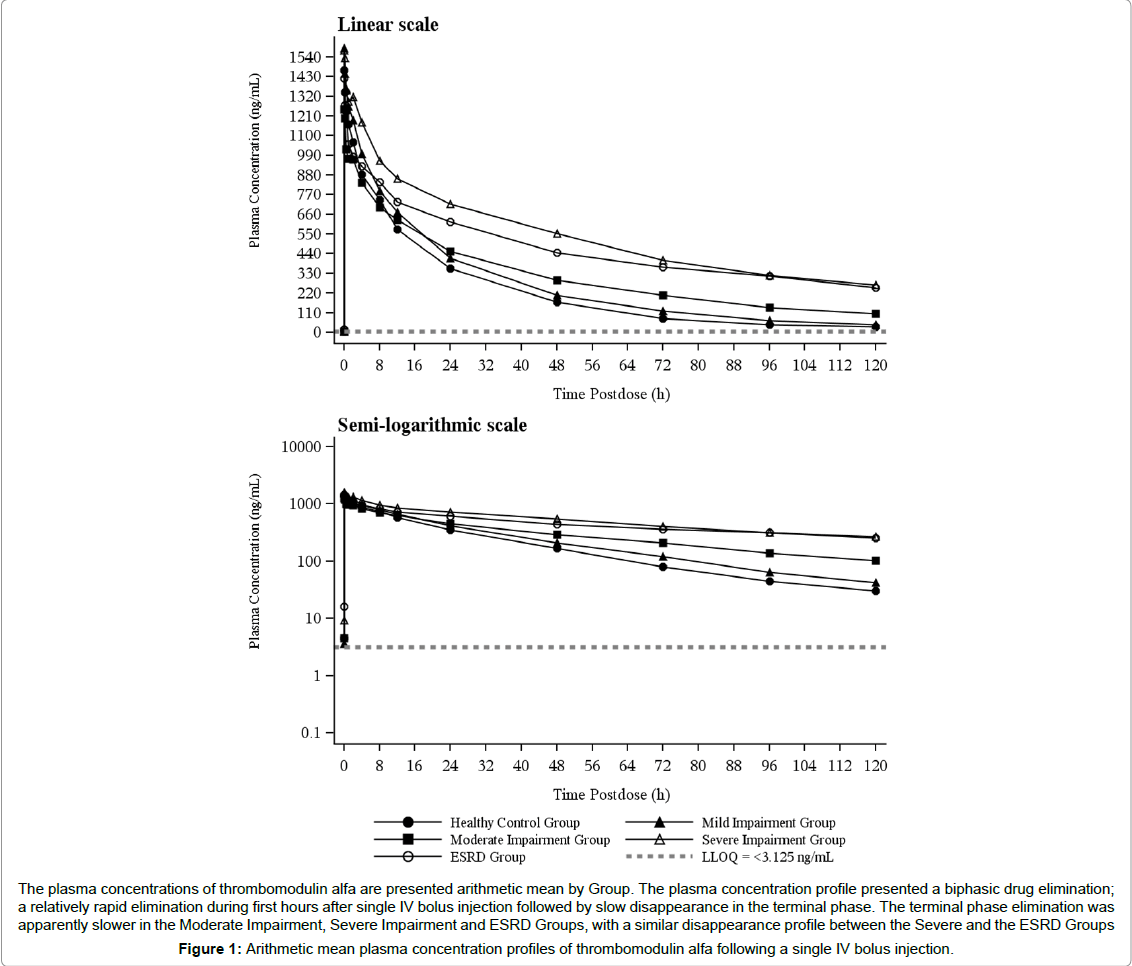
Arithmetic mean plasma concentrations of thrombomodulin alfa for single dose are plotted in Figure 1. The plasma concentration profile presented a biphasic drug elimination; a relatively rapid elimination during first hours after single IV bolus injection followed by slow disappearance in the terminal phase. The terminal phase elimination was apparently slower in the Moderate Impairment, Severe Impairment and ESRD Groups, with a similar disappearance profile between the Severe and the ESRD Groups (Figure 1).
A summary of PK parameters for thrombomodulin alfa is presented in Table 2. Mean Cmax values ranged from 1,240 to 1,600 ng/mL and were generally similar across different groups. Mean AUC ranged from 27,000 to 88,400 ng*hr/mL and generally increased with the increase in renal impairment severity. Mean t1/2,beta was closely associated with AUC, which ranged from 25.2 to 76.5 h. Mean V1 values ranged from 2,930 to 3,900 mL, and mean V2 values ranged from 1,670 to 2,450 mL. For both V1 and V2, mean values were similar across groups. Mean CL values ranged from 52.3 to 190 mL/h and generally decreased with the increase in severity of renal impairment.
| Parameter (units) | Healthy Control Group (N=8) | Mild Renal Impairment Group (N=8) | Moderate Renal Impairment Group (N=8) | Severe Renal Impairment Group (N=7) | ESRD Group (N=7) |
|---|---|---|---|---|---|
| V1 (mL) | 3220 (7.57) | 2930 (22.2) | 3520 (43.9) | 3090 (22.2) | 3900 (49.8) |
| V2 (mL) | 2270 (75.6) | 1750 (19.1) | 1850 (42.2) | 1670 (32.9) | 2450 (32.5) |
| K10 (1/h) | 0.0592 (28.4) | 0.0465 (17.1) | 0.0286 (43.9) | 0.0169 (18.5) | 0.0158 (45.4) |
| K21 (1/h) | 0.301 (841) | 0.202 (198) | 0.633 (146) | 0.138 (57.2) | 0.339 (292) |
| K12 (1/h) | 0.212 (2190) | 0.120 (209) | 0.332 (117) | 0.0745 (43.6) | 0.213 (243) |
| a (1/h) | 0.647 (833) | 0.357 (180) | 0.999 (126) | 0.221 (47.8) | 0.589 (246) |
| ß (1/h) | 0.0275 (36.6) | 0.0263 (12.4) | 0.0181 (35.1) | 0.0106 (21.2) | 0.00906 (34.4) |
| A (ng/mL) | 888 (35.0) | 741 (28.4) | 453 (53.5) | 584 (21.8) | 518 (84.7) |
| B (ng/mL) | 567 (84.0) | 771 (21.4) | 762 (32.6) | 898 (19.1) | 714 (44.4) |
| Cmax (ng/mL) | 1600 (9.97) | 1540 (14.0) | 1240 (34.2) | 1490 (15.4) | 1290 (46.8) |
| AUC (ng*h/mL) | 27000 (22.5) | 33100 (17.4) | 43200 (19.5) | 88400 (17.2) | 81800 (25.1) |
| t1/2,alfa (h) | 1.07 (833) | 1.94 (180) | 0.694 (126) | 3.13 (47.8) | 1.18 (246) |
| t1/2,alfa(h) | 25.2 (36.6) | 26.3 (12.4) | 38.2 (35.1) | 65.6 (21.2) | 76.5 (34.4) |
| CL (mL/h) | 190 (28.7) | 137 (22.1) | 101 (19.3) | 52.3 (20.3) | 61.5 (26.9) |
| Vss (mL) | 5760 (37.5) | 4700 (18.6) | 5410 (40.5) | 4770 (25.0) | 6560 (33.0) |
| Geometric mean (CV%) are presented. | |||||
Geometric mean (CV%) are presented.
Table 2: Plasma pharmacokinetic parameters for thrombomodulin alfa from compartmental analysis following single IV bolus injection of 0.06 mg/kg thrombomodulin alfa.
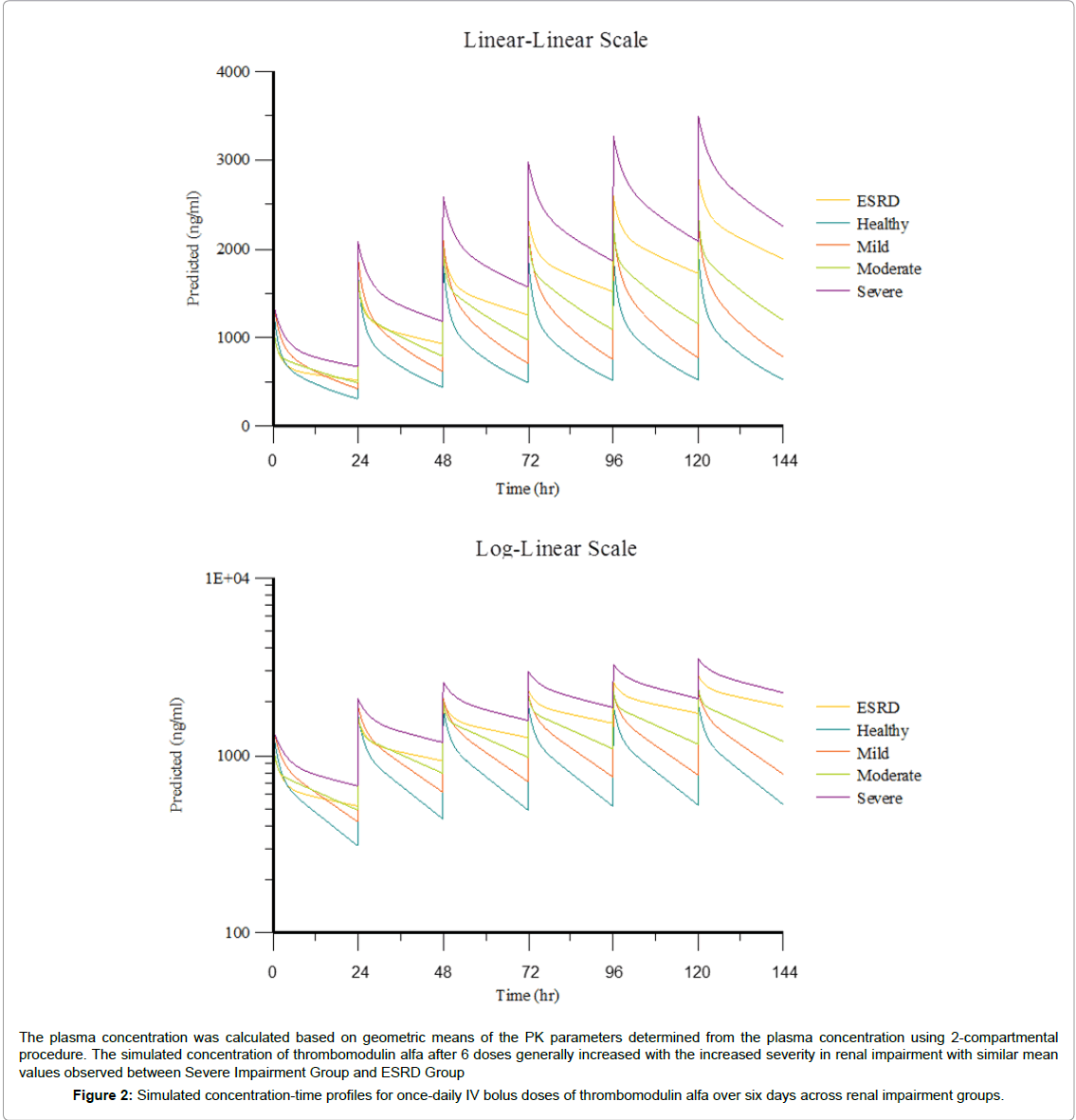
Simulation of thrombomodulin alfa plasma concentration at 0.06 mg/kg/day for six IV bolus doses was conducted using PK parameters in each group. A summary of simulated plasma PK parameters is presented in Table 3. Simulated mean thrombomodulin alfa concentration-time profiles across renal impairment groups for oncedaily IV bolus injections of thrombomodulin alfa over six days are presented in Figure 2.
| Group | Cmax (ng/mL) |
| Healthy Control (N=8) | 2030 (1340 – 2500) |
| Mild Impairment (N=8) | 2350 (1870 - 2740) |
| Moderate Impairment (N=8) | 2410 (1720 - 2870) |
| Severe Impairment (N=7) | 3710 (3210 - 4730) |
| ESRD (N=7) | 3180 (2180 - 4540) |
Minimum to Maximum (Minimum – Maximum) individual value are presented. One each subject from Severe Renal Impairment and ESRD were excluded from analysis since these subjects had anomalous plasma concentration-time profiles.
Table 3: Summary of predicted plasma pharmacokinetic parameters for thrombomodulin alfa following once-daily IV bolus injections of 0.06 mg/kg thrombomodulin alfa for six days.
Following multiple dose simulations of six, once-daily IV bolus injections of 0.06 mg/kg thrombomodulin alfa, the predicted mean Cmax in subjects with normal renal function, and mild, moderate, or severe renal impairment and on hemodialysis was 2,030, 2,350, 2,410, 3,710, 3,180 ng/mL, respectively, with the individual Cmax values ranging from 1,340 to 4,730 ng/mL.
The simulated mean Cmax values across all renal impairment groups were at least 31.3% lower than the maximum concentration deemed safe (5,400 ng/mL) [9]. No individually simulated multiple dose PK profile exceeded a Cmax value of 5,400 ng/mL, with the highest simulated Cmax in the severe renal impairment group at 4,730 ng/mL and in the ESRD group at 4,540 ng/mL (Table 3).
Safety
There were no serious adverse events (SAEs) and no subjects were withdrawn from the study due to treatment emergent adverse events (TEAEs). Overall, 14 (35%) subjects reported a total of 27 TEAEs (Table 4). TEAEs that occurred in ≥ 5% of all study subjects were as follows: vessel puncture site hemorrhage (10.0%), abdominal pain (7.5%), headache (5%), nausea (5%), and somnolence (5%). The number of subjects reporting TEAEs did not differ markedly based on renal function, but drug-related TEAEs were isolated to subjects with renal impairment or ESRD. The number of events was most frequently reported in the ESRD Group with 8 TEAEs reported by 2 subjects (compared to 3 TEAEs reported by 3 Healthy Control subjects). All TEAEs were mild, except for one moderate TEAE which was reported in the ESRD Group.
| Healthy | Mild | Moderate | Severe | |||
| Control | Impairment | Impairment | Impairment | ESRD | ||
| Group | Group | Group | Group | Group | Overall | |
| (N=8) | (N=8) | (N=8) | (N=8) | (N=8) | (N=40) | |
| Subjects with adverse events | 3 (37.5) | 3 (37.5) | 2 (25.0) | 4 (50.0) | 2 (25.0) | 14 (35.0) |
| Number of adverse events | 3 | 5 | 5 | 6 | 8 | 27 |
| Severity (all adverse events) | ||||||
| Grade 1 | 3 (37.5) [3] | 3 (37.5) [5] | 2 (25.0) [5] | 4 (50.0) [6] | 2 (25.0) [7] | 14 (35.0) [26] |
| Grade 2 | --- | --- | --- | --- | 1 (12.5) [1] | 1 (2.5) [1] |
| Total | 3 (37.5) [3] | 3 (37.5) [5] | 2 (25.0) [5] | 4 (50.0) [6] | 2 (25.0) [8] | 14 (35.0) [27] |
| Severity (related to study drug) | ||||||
| Grade 1 | --- | 3 (37.5) [3] | 2 (25.0) [2] | 2 (25.0) [2] | 1 (12.5) [5] | 8 (20.0) [12] |
| Grade 2 | --- | --- | --- | --- | 1 (12.5) [1] | 1 (2.5) [1] |
| Total | --- | 3 (37.5) [3] | 2 (25.0) [2] | 2 (25.0) [2] | 1 (12.5) [6] | 8 (20.0) [13] |
| Relationship to study drug | ||||||
| Not Related | 3 (37.5) [3] | 1 (12.5) [2] | 2 (25.0) [3] | 2 (25.0) [4] | 1 (12.5) [2] | 9 (22.5) [14] |
| Related | --- | 3 (37.5) [3] | 2 (25.0) [2] | 2 (25.0) [2] | 1 (12.5) [6] | 8 (20.0) [13] |
ESRD = end-stage renal disease; N = number of subjects studied; ( ) = percentage of subjects with adverse events; [ ] = number of adverse events.The Severity was graded using the Division of AIDS Toxicity Grading Scale (Version 1.0, Dec 2004; Clarification August 2009).Grade 1 = Mild; Grade 2 = Moderate; Grade 3 = Severe; Grade 4 = Potentially Life-threatening.For Relationship to study drug, AEs coded as 'None' are counted as Not Related. AEs coded as 'Unlikely' or 'Related' are counted as Related.
Table 4: Summary of treatment emergent adverse events.
No clinically significant changes or findings were noted from clinical laboratory evaluations, vital sign measurements, 12-lead ECGs. Any relevant physical examination findings were reported as AEs for this study. No subjects developed anti-thrombomodulin alfa antibodies at Day 28.
Discussion
It was observed, while demographic differences other than renal function were found among the 5 Groups in this study, they were thought not to affect the PK profile. There were slight differences in the ratio of subjects’ gender among the 5 Groups and the mean age for subjects in the Mild, Moderate, and Severe Renal Impairment Groups (60-68 years) was greater than the mean age for subjects in the Healthy Control and ESRD Groups (42 years). However age or gender was not determined a significant covariate for the PK of thrombomodulin alfa in the PPK analysis in the non-Japanese population [5], as such these age or gender differences were unlikely to affect the PK profile.
This study suggests that renal function affects thrombomodulin alfa clearance. By single dose, clearance of thrombomodulin alfa was lowered as renal function decreased: the terminal phase elimination was apparently slower in the Moderate Impairment, Severe Impairment and ESRD Groups. As a result, the simulated mean Cmax after 6 doses generally increased with the increased severity in renal impairment with similar mean values observed between Severe Impairment Group and ESRD Group. However, no individually simulated C exceeded the maximum concentration deemed safe (5,400 ng/mL).
The previous phase 1 studies in healthy subjects showed approximately 45-70% of thrombomodulin alfa administered intravenously was excreted in urine [1,6,7] and the remaining thrombomodulin alfa was cleared via non-renal pathways. CLtot of Severe Impairment and ESRD Groups decreased to 27.5% and 32.4% of CLtot of Healthy Group, hence the renal clearance of thrombomodulin alfa in these renal-impaired population was thought to be very minimal. A pharmacokinetics study of thrombomodulin alfa in rats reported metabolism in liver and lung [3]. In subjects from Severe Impairment and ESRD Groups, thrombomodulin alfa was thought to be cleared by such non-renal metabolisms.
As similar PK profile was found between Severe Impairment Group and ESRD Group, hemodialysis treatment was thought not to affect the clearance of thrombomodulin alfa. Similar results were reported by Hayakawa et al. [20]. In his study, plasma concentration of thrombomodulin alfa in septic DIC patients with more severe renal impairment (N=11, CLcr <30 mL/min) were studied. Of 11 patients with severe renal impairment, 7 patients had undergone continuous renal replacement therapy (CRRT), and no apparent difference was found between PK of the subjects on CRRT and that of subjects not on CRRT.
In summary, clearance of thrombomodulin alfa was lowered as renal function decreased, and the least clearance of thrombomodulin alfa were found in Severe Impairment and ESRD Groups. There was no hemodialysis effect on the PK profile of thrombomodulin alfa. Even though subjects from the Severe Impairment or ESRD Groups could possibly clear thrombomodulin alfa by non-renal pathways, no individually simulated multiple dose PK profile exceeded a Cmax value of 5,400 ng/mL. As such, no dose adjustment is warranted in patients with renal impairment including patients on hemodialysis.
Safety results from this study supported no dose adjustment in patients with renal impairment including patients on hemodialysis. No incidence of a major bleeding event was reported in this study, and the incidence of the TEAEs relating to non-major bleeding was comparable across the Groups. The increased thrombomodulin alfa concentration in patients with renal impairment was not thought to increase the risk of bleeding, which is the most important risk of thrombomodulin alfa administration. No additional safety concern arose in subjects with renal impairment from safety assessment on other safety variables, as such 0.06 mg/kg of thrombomodulin alfa was thought to be safe and tolerable in patients with renal impairment including patients on hemodialysis.
The results of this study would give a clear guide to physicians who treat patients with DIC and/or with severe sepsis and coagulopathy by thrombomodulin alfa, as many those patients frequently require hemodialysis.
Conclusions
Results from single dose and multiple dose PK simulation in this study showed renal impairment was associated with increased exposure of thrombomodulin alfa. However, no renal function groups or no individually simulated Cmax after 6 doses exceed the acceptable safety range even for subjects with hemodialysis, and as such no dose adjustment would be required for patients with hemodialysis.
References
- Nakashima M, Kanamaru M, Umemura K, Tsuruta K (1998) Pharmacokinetics and safety of a novel recombinant soluble human thrombomodulin, ART-123, in healthy male volunteers. J ClinPharmacol 38:40-44.
- Abeyama K, Stern DM, Ito Y, Kawahara K, Yoshimoto Y, et al. (2005) The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest 115: 1267-1274.
- Tsuruta K, Kodama T, Serada M, Hori K, Inaba A, et al. (2009) Pharmacokinetics of recombinant human soluble thrombomodulin, thrombomodulinalfa in the rat. Xenobiotica 39:125-134
- ClinicalTrials.gov NCT01598831
- Mouksassi MS, Marier JF, Bax L, Osawa Y, Tsuruta K (2015) Population Pharmacokinetic Analysis of Thrombomodulin Alfa to Support Dosing Rationale in Patients with Renal Impairment. ClinPharmacol Drug Dev 4: 210-217.
- Moll S, Lindley C, Pescatore S, Morrison D, Tsuruta K, et al. (2004) Phase I study of a novel recombinant human soluble thrombomodulin, ART-123. J ThrombHaemost 2:1745-1751.
- Nakashima M, Uematsu T, Umemura K, Maruyama I, Tsuruta K (1998) A novel recombinant soluble human thrombomodulin, ART-123, activates the protein C pathway in healthy male volunteers. J ClinPharmacol38:540-544.
- Tsuruta K, Yamada Y, Serada M, Tanigawara Y (2011) Model-based analysis of covariate effects on population pharmacokinetics of thrombomodulinalfa in patients with disseminated intravascular coagulation and normal subjects. J ClinPharmacol 51:1276-1285.
- Vincent JL, Ramesh MK, Ernest D, LaRosa SP, Pachl J, et al. (2013) A randomized, double-blind, placebo-controlled, Phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit Care Med 41:2069-2079.
- Levy MM, Dellinger RP, Townsend SR Linde-Zwirble WT, Marshall JC, et al. (2010) The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 38: 222-231
- Sakr Y, Lobo SM, Moreno RP, Gerlach H, Ranieri VM, et al.(2012) Patterns and early evolution of organ failure in the intensive care unit and their relation to outcome. Critical Care 16:R222
- Brochard L, Abroug F, Brenner M, Broccard AF, Danner RL, et al.(2010) An Official ATS/ERS/ESICM/SCCM/SRLF Statement: Prevention and Management of Acute Renal Failure in the ICU Patient, Am JRespirCrit Care Med 181: 1128-1155
- US Food and Drug Administration (2010) Guidance for Industry: Pharmacokinetics in Patients with Impaired Renal Function - Study Design, Data Analysis, and Impact on Dosing and Labeling.
- US Food and Drug Administration (2001) Guidance for Industry: Bioanalytical Method Validation.
- DeSilva B, Smith W, Weiner R, Kelley M, Smolec J, et al. (2003) Recommendations for the Bioanalytical Method Validation of Ligand-binding Assays to Support Pharmacokinetic Assessments of Macromolecules. Pharm Res 20:1885-1900.
- Viswanathan CT, Bansal S, Booth B, DeStefano AJ, Rose MJ, et al. (2007) Quantitative Bioanalytical Methods Validation and Implementation: Best Practices for Chromatographic and Ligand Binding Assays. AAPS J 9: E30-E42.
- US Food and Drug Administration (2009) Guidance for Industry: Assay Development for Immunogenicity Testing of Therapeutic Proteins
- Mire-Sluis A, Barrett YC, Devanarayan V, Koren E, Liu H, et al. (2004) Recommendations for the Design and optimization of Immunoassays Used in the Detection of Host Antibodies against Biotechnology Products. J Immunol Methods 289:1-16.
- Shankar G, Devanarayan V, Amaravadi L, Barrett YC, Bowsher R, et al. (2008) Recommendations for the Validation of Immunoassays used for Detection of Host Antibodies Against Biotechnology Products. J Pharm Biomed Anal 48:1267-1281.
- Hayakawa M, Yamamoto H, Honma T, Mukai N, Higashiyama A, et al. (2012) Pharmacokinetics and Pharmacodynamics of Recombinant Soluble Thrombomodulin in Disseminated Intravascular Coagulation Patients with Renal Impairment. Shock 37:569-573.
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 11850
- [From(publication date):
August-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10915
- PDF downloads : 935