Research Article Open Access
Pesticides Detection Using Acetylcholinesterase Nanobiosensor
Vimala V*, Samantha Katherine Clarke and Urvinder Kaur S
Department of Biotechnology, School of Bioscience and Technology, VIT University, Vellore Tamilnadu, India
- *Corresponding Author:
- Vimala V
Department of Biotechnology
School of Bioscience and Technology
VIT University, Vellore, Tamilnadu, India
Tel: 8464942298
E-mail: vimala094@gmail.com
Received date: October 05, 2015; Accepted date: February 18, 2016; Published date: February 28, 2016
Citation: Vimala V, Clarke SK, Urvinder Kaur S (2016) Pesticides Detection Using Acetylcholinesterase Nanobiosensor. Biosens J 5:133. doi:10.4172/2090-4967.1000133
Copyright: © 2016 Vimala V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Biosensors Journal
Abstract
Pesticides are chemical substances used for crop improvement and these substances when degraded leave behind certain toxic residues which can be detected using nano biosensors which are relatively inexpensive, does not require sample pre-treatment and provides sensitive screening. The application of nanobiosensors for detection of organophosphates using acetylcholinesterase is introduced in detail. Future prospects toward the development of selective, sensitive biosensing systems are discussed.
Keywords
Pesticides; Crop improvement; Biosensors
Introduction
Pesticides are the chemical compounds used for preventing, killing or controlling any pest. They are generally used for crop protection. They are classified as inorganic, synthetic or biologicals. Most commonly used pesticides are organophosphates, carbamates, triazine, organochlorines etc., Organochlorine hydrocarbons (e.g. DDT) disrupts the sodium/potassium balance of the nerve fibre; thereby impulses are transmitted continuously in the pest. They have been banned because of their persistance and potential bioaccumulation. So they are replaced by organophosphates (e.g. malathion) and carbamates. Both inhibit an enzyme acetylcholinesterase, thereby allowing acetylcholine to transmit continuously and causing weakness or paralysis. Others act by inhibiting photosynthesis, crushing plant nutrient transport system in case of herbs. As most of the pesticides are toxic to human and environment, they are banned but still used in developing countries. The toxicity of pesticides is due to the production of compounds during their degradation which are even more toxic than the parent compounds. These organic toxins enter animal and human bodies directly or indirectly through the food chain or drinking water, and threat human health by inhibiting acetylcholinesterase which is essential for the function of the central nervous system in humans. So it is of great significance to develop a fast, reliable, economical and analytical method for determination of trace amounts of these pesticides [1].
Conventional Methods
Mass spectrometry, GC, HPLC, UV-Vis spectrometry are common conventional methodologies for the detection of pesticides. These methods are highly efficient and can discriminate between various organophosphates but require sample pre-treatment which is a tedious work, skilled technicians, time consuming and not suitable for field analysis of multiple samples so to overcome this several rapid, relatively inexpensive, sensitive screening analytical technique for which no sample pre-treatment is needed were designed for identifying and quantifying such pesticides. Nano biosensorsfit the above criteria, have the following advantages:
• They have high surface-to-volume ratios due to extremely small size, leading to loading of more enzyme as well as high sensitivity.
• They have excellent optical or electrical properties, which can be used as the highly-sensitive transducer signals in biosensors.
• Enzyme engineering at the Nano scale is the most promising techniques because of its high sensitivity and selectivity AChE and OPH variants [2].
Acetylcholine Esterase Biosensor
Enzymes can be used for molecular recognition in biosensors because they amplify the sensing signals. The signal amplification that occurs due to enzymatic reaction helps in designing biosensors that are highly sensitive. Owing to their good selectivity, sensitivity, rapid response, miniature size and reproducible results, Amperometric enzyme biosensors is one of the most widely used biosensor for biochemical analysis.
Enzyme based electrochemical biosensors can be developed by combining enzymatic reactions with electrochemical methods. Amperometric Acetylcholinesterase (AChE) biosensors function on the basis of inhibition of AChE can be used for the detection of pesticides [3] which is depicted as follows [4].
Hydrolysis of acetylcholine:

Oxidation of choline:

Electrolysis of H2O2:
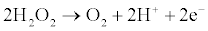
In-situ synthesis of gold nano particles (GNPs) was done by mixing tetrachloroauric III acid with chitosan and immobilizing it in the chitosan hydrogel during the electrodeposition. This interface thus deposited consists of chitosan and GNPs and displayed high biocompatibility and stability thereby enabling immobilization of the enzyme. The current produced showed the viability of AChE thereby giving a quantitative measure of AChE. To optimize and characterize the deposition conditions, methods like ATR, FTIR, UV-Vis spectra, AFM and electrochemical scan were used. This method was easy, rapid and green [3]. Assembly of AuNPs on a sol gel derived silicate network was used for the immobilization of AChE thereby establishing a stable AChE biosensor for pesticide detection. Pesticides like moncrotophos, carbaryl and methyl parathione was used to verify this method. The results obtained were similar to that got from UV- a traditional method of pesticide detection [5].
Shu Ping Zhanget et al., [6] described a controllable modification technique of glassy carbon electrode (GCE) with MWNTs and introduced a controllable direct immobilization of acetylcholinesterase (AChE) on the modified electrode. MWNTs was Electro-deposited on GCE by magnetic stirring, then the modified electrode was dipped into borate buffer solution so that the functional groups becomes negatively charged and this is followed by immersing the GCE in 1% PDDA poly (diallyldimethylammonium chloride) solution and in MWNTs solution of borate buffer solution alternately, generated a five-PDDA/ MWNTs-bilayer. After preparing {PDDA/MWNTs} 5/ED/GCE, AChE can be immobilized directly by LBL self-assembly, and is important that a PDDA membrane was fabricated at the last surface in order to avoid the fall-off of AChE. The activity value of AChE was detected by using i-t technique based on the modified Ellman method. The carbaryl residue were detected by the enzyme biosensor with 0.01 U enzyme activity, with the detection limit of 10−12 g L−1 and the biosensor proved to be good in pesticides detection and monitoring [6].
Subramanian Viswanathan et al., described the fabrication of a electrochemical biosensor by integrating the special features of ssDNA-SWCNT, PANI and AChE for pesticides determination. They immobilized AChE on the pH sensitive redox polymer; PANI coated on vertically aliened thiol terminated ssDNA-SWCNTs which were used as a transducer layer that is responsible for high sensitivity of the biosensor. This biosensor has superior charge transport properties than the ssDNA SAMs. The incorporation of SWCNTs provided the conductive pathways to promote the electron transfer, increased the surface area of flexible three-dimensional conductive supports for acetylcholinesterase enzyme. Thin PANI film on SWCNT acts as good sensor for enzyme byproduct acetic acid. The changes of local pH in the vicinity of an electrode surface by enzymatic reaction increases the redox activity of PANI thin film on SWCNTs. The biosensor was successful in testing the determination of two common pesticides such as methyl parathion and chlorpyrifos, which belongs to the organophosphorous family, which was based on enzyme inhibition mechanism. The detection limit is 1 × 10−12 M for methyl parathion and chlorpyrifos which is lower than the maximum contaminant level recommended by the European Environmental Bureau and other environmental protection agencies. The proposed transducer layer can be used as a suitable base for biosensors based on enzymes which can release acidic or basic byproducts [7]. Commonly employed immobilization methods include direct physical adsorption onto a solid support, encapsulation into a hydrogel, cross-linking, and covalent binding. The conducting polymers are used due to their interesting electrical and electrochemical properties. Among them, polyaniline (PAn) and polypyrrole (PPy) are most commonly employed because of high conductivity, easy preparation, and excellent environmental stability due to which they are used in electrochemical biosensing, as they can provide suitable environment for immobilization of biomolecules and act as mediator. It is synthesized either by electrolyzing the solution including two monomers or by two-step electropolymerization. In the second method, the first monomer is electrochemically polymerized on the electrode surface and used as an electrode for the polymerization of second monomer. Choi and Park [8] showed that these copolymer composites will not only display better properties than that of homopolymers but also overcome the limitation of the rareness of new conjugated-bond-containing monomers. Star et al., Chen et al., Arduini et al., Li et al., proved that the electrical ability as well as mechanical properties of heteropolymers are enhanced compared with pure conducting polymer or nanomaterials [8]. Dan Dua et al., proposed a simple method to immobilize acetylcholinesterase (AChE) on polypyrrole (PPy) and polyaniline (PANI) copolymer doped with multi-walled carbon nanotubes (MWCNTs). The synthesized PAn- PPy-MWCNTs copolymer has a porous and homogeneous morphology that provided an ideal size to entrap enzyme molecules. Due to the biocompatible microenvironment provided by the copolymer network, the obtained composite was devised for AChE attachment which resulted in a stable AChE biosensor for screening of organophosphates. MWCNTs promoted electron-transfer reactions at a lower potential and catalyzed the electro-oxidation of thiocholine, thus increasing detection sensitivity and based on the inhibition of Organophosphates on the AChE activity, using malathion as a model compound, the inhibition of malathion was proportional to its concentration ranging from 0.01 to 0.5 g/mL and from 1 to 25 g/ mL, with a detection limit of 1.0 ng/mL. This biosensor exhibited good reproducibility and acceptable stability, thus providing a new promising tool for analysis of enzyme inhibitors [8]. Dan Du et al., [9] used β Cyclodextrin (CD) to improve solubility of MWCNT in water because conventional methods with concentrated sulphuric acid, nitric acid, or other strong oxidizing agents cause the problems of corrosion and environmental pollution. It is the novel eco-friendly method to functionalize CNT. β-CD are used to attach other functional groups by two rims of hydroxyl groups i.e., a primary (tail) and a secondary (head). In this work, β-CD had been assembled onto the surface of MWCNTs through polymer wrapping strategy. The synthesized MWCNTs-β- CD composite displayed good dispersibility in water media. Due to the excellent biocompatibility of β-CD and fast electron transfer of MWCNTs, the resulting AChE biosensor exhibited high affinity to its substrate and produced a detectable and fast. Thus this nanobiosensor possesses good reproducibility, stability and also low detection limit of organophosphate and other toxic compounds against to AChE [9]. Tao Liu, Haichao Su et al., developed a novel AChE biosensor based on immobilizing AChE on CPBA (3-carboxyphenylboronicacid), RGO (reduced gragine oxide) and AuNPs modified electrode, and used to determine organophosphorus and carbamate pesticides. The immobilized enzyme retained high activity by specific binding between the boronic acid group of 3-carboxyphenylboronic and the glycosyl of acetylcholinesterase. It has a disadvantage of low selectivity of AChE because both organophosphate and carbamate pesticides can inhibit AChE activity. But it also shows high potentiality in total amount of pesticide analysis. The biosensor has good sensitivity due to the gold nanoparticles and reduced graphene oxide, which promoted electron transfer reaction and enhanced the electrochemical response. Parameters affecting the biosensor response such as pH, applied potential, enzyme loading and inhibition time were optimized. Moreover, this biosensor had good repeatability and high stability [10].
Zhaozhu Zheng et al., reported a highly sensitive optical biosensor for the detection of organophosphates (OPs) in foods and water. This biosensor is composed of nanostructured multilayers of the enzyme AChE and photoluminescent (PL) CdTe QDs, and fabricated using the layer-by-layer (LbL) assembly technique which is an environmentfriendly method and ideal for cost efficient mass production (Decher, 1997; Podsiadlo et al., 2007), being a very attractive and powerful technology for development of biosensors (Li et al., 2009; Yu et al., 2005). This biosensor can be applied to determine low concentrations of OPs in real vegetable and fruit samples, and the demonstrated detection limits of the developed biosensors are significantly better than those of reported biosensor so far. It is highly-sensitive and detects paraoxon and parathion present in picomolar concentrations. This is the first report on employing the biosensors to detect OPs in the real samples of vegetables and fruits with satisfactory reproducibility and accuracy. This biosensor could also be used for visual monitoring of OPs.
The possible disadvantages of this biosensor are
• The quenching of QD multilayers is not fully reversed and its fluorescence partly recovers (about 60% of the original fluorescence intensity is recovered by keeping it overnight under ambient condition, and it is designed for one-time use.
• It shows different response characteristics in presence of different types of OPs, so the measurement may be not accurate in the mixture of different OPs.
• The detection limit is low as 1.05 × 10-11 M for paraoxon and 4.47 × 10−12 M for parathion, which are significantly better than those of the conventional GC/MS methods or amperometric biosensors (0.5 nM).
There are many advantages provide by the sensors, such as easy sample pretreatment, high sensitivity, and mass production with low cost, fluorescent change recognized by naked eyes and mass production with low cost, could facilitate future development of rapid, high-throughput screening of OP residues. LbL enables stabilizing environment for the enzyme in the nanostructures which increases the stock stability [11].
Various nanoparticle based enzyme biosensors have been used for the determination of pesticides such as ZrO2 NPs-modified screen printed electrode, multiwalled carbon nanotube modified GCE, AuNPs-polypyrrole nanowires composite film modified GCE, goldplatinum bimetallic nanoparticles onto 3-aminopropyltriethoxy silane modified GC electrode, Au-MWNTs-modified GC electrode, onedimensional gold nanoparticles onto GC electrode and prussian blue modified GC electrode. But the use of these electrodes has a major drawback of the enzyme leaking into the electrode and thereby several tedious pretreatment processes are required.
To overcome these problem
Carbon nanotubes (CNT) can be used for chemical and biological sensing applications. They are hollow graphite tubes and have a fast electron transfer rate and also possess electrocatalytic effect. Fe3O4 are unique because of their magnetic and electric property and can therefore be used in pesticide detection. Nano-sized materials which have been magnetically bioconjugated have also been used electrochemical biosensor devices owing to their large surface area, high bioactivity, high conformational stability and high levels of contact between the biocatalyst i.e. the enzyme and the substrate [12].
Others
Tyrosine-based nanobiosensor is used for the detection of pesticides, phenolic compounds like catechol (a pesticide precursor). Many scientist developed tyrosine based biosensor by immobilizing tyrosine enzyme on different materials like polyaniline-ionic liquidcarbon nanofiber composites, cellulose based carriers, etc., Lijun Yang et al., exploited immobilisation of tyrosine on chitosan-carbon coated nickel nanoparticles for monitoring catechol because of their increased stability as the entrapped metals would not be hydrolysed and oxidized and decreased agglomeration of nanoparticles [13].
Self-assembled Monolayers in Nano Biosensor Design
Enzymes are most commonly used in biosensing, as the signal can be amplified millions of times due to enzymatic reactions and due to this they are highly sensitive. Amperometric enzymatic biosensors are most suitable for biochemical analysis due to their good selectivity, sensitivity, rapid response, miniature size, and reproducible results. A combination of enzymatic reactions with the electrochemical increases the sensitivity and thus facilitates rapid determination of environmental analysis. Among these, amperometric acetylcholinesterase (AChE) biosensors has been shown satisfactory results for pesticides analysis based on their inhibition on AChE , in which the enzymatic activity is employed as an indicator of quantitative measurement of pesticides. Sensitive and stable biosensors can be developed by the effective immobilization of enzyme to solid electrode surface. Self-assembled monolayers (SAMs) of alkanethiols on gold substrate prevent denaturation and loss of bioactivity of enzyme.
This interface can anchor different functional groups in biosensors. The SAMs serves partly as a barrier to prevent proteins and other ligands from coming into contact with the metal. The stable, closepacked, and well-ordered SAMs on gold electrodes provides a very low fraction of defects. They are strongly resistant to ion penetration, and thus impede the electron transfer between the electrode surface and the electroactive species in solution.
Among several nano-materials, gold nanoparticles (AuNPs) are widely used for preparation of electrochemical biosensors. Compared with traditional process, electrochemical deposition is simple, the performs under moderate conditions, and suitable for selective deposition of films with controllable thickness, which provides an easy and rapid alternative for the preparation of AuNPs modified electrodes in a short time. Dan Du et.al synthesized AuNP in situ and electrodeposited onto gold electrode surface. SAMs of 11-mercaptoundecanoic acids were then formed onto the resulting surface. After activation of surface carboxyl groups with 1-ethyl-3-(3- dimethylaminopropyl) carbodiimide and N-hydroxysuccinimide, the interface possessing good stability is suitable for facile immobilization of enzyme, leading to a stable AChE biosensor for quantitative measure of organophosphate pesticide and it has excellent activity to its substrate. The presence of AuNPs provided a conductive pathway to promote electron transfer reactions at a lower potential, as well as increased the surface hydrophilicity for immobilization of enzyme. The electrodeposited AuNPs combining with the unique properties of SAMs circumvented the drawbacks of electron transfer and offered a simple method for further functionalization by biomolecules [14].
Another method to overcome the leaking process is the use of a single layered enzyme membrane. This was done by pore filling of an asymmetric porous bromomethylated poly (2,6-dimethyl-1,4- phenyleneoxide) film with a cross-linked polyvinyl alcohol (PVA) containing TYR. This would act as both the external and the internal membrane. Because pore-filling was not done completely in the upper part, large molecules were not allowed to enter and since the inner part of the membrane was completely filled, chemicals and other substances were not allowed to leak in. thereby stability was obtained on a long term basis due to the PVA which is a hroydgel that provides proper environment for the enzyme [15].
Membrane Based
Label-free diagnostics devices such as carbon nanotubes, silicon nanowires, nanoporous alumina and a wide range of polymer, metal/ metal oxide nanoparticles have been widely used toward designing ultra-sensitive and rapid biosensors.
A number of label free sensor, polymer based nanotextured active sensing surfaces techniques using a combination EIS and nanomaterials, using impedemetric methods such as impedance spectroscopy and cyclic voltammetry were developed for ultrasensitive detection of atrazine (a ground water contaminant). Electro generated polyquinone films functionalized by a hydroxyatrazine moiety which is a label-free electrochemical detection of atrazine with a limit of detection of 0.2 pg/mL in phosphate buffered saline (Tran et al., 2011). Similarly polypyrrole films using N-substituted by nitrilotriacetic acid (NTA) electro generated on a gold electrodes was demonstrated for detecting atrazine with sensitivity of 10 pg/mL (Javier et al., 2008). These sensors show ultra-low sensitivity with relatively low cross reactivity, but the performance is dependent on the activity as well as the stability of the polymer films in addition to the stability of the antibodies when immobilized onto the polymer films. Impedemetric sensors on interdigitated metal electrodes with electro active polymer active sensing surfaces have sensitivity of 0.04 ng/mL from wine and phosphate buffer saline without the use of labels (Ionescu et al., 2010)
Pie Pichetsurnthorn et al., used nanoporous alumina membranes and interfaced them with printed circuit boards for generating a high density array of nanoscale confined spaces. They designed an assay similar to ELISA which do not use enzymes to achieve non-faradic detection, i.e. the transfer of charge between the biomolecule and the electrode is mediated without the use of a redox probe. Sensitivity is achieved through nano confinement of the small molecules in size matched spaces resulting in amplification of the impedance signals measured due to the modulation of the electrical double layer. This sensor does not use electro active polymers/polymer composites to achieve amplification of the detected signal.
Detection of atrazine from three types of water sources
• Phosphate buffered saline
• River and
• Drinking water
With the sensitivity of femtogram/mL and with cross-reactivity less than a third of the specific binding signal from malathion, with detection time in the order of minutes. They also observed that localization of biomolecules in size matched nanoscale confined spaces enhances the sensitivity of the molecular detection [16].
References
- Liu T, HaichaoS, Xiangjin Q, Peng J, Lin C, et al. (2011) Acetylcholinesterase biosensor based on 3-carboxyphenylboronic acid/reduced graphene oxide-gold nanocomposites modified electrode for amperometric detection of organophosphorus and carbamate pesticides.Sensors and Actuators B160: 1255-1261.
- Liu S, Yuan L, XiuliYue, ZhaozhuZheng,Zhiyong Tang (2008) Recent Advances in Nanosensors for Organophosphate Pesticide Detection. Advanced Powder Technology 19: 419-441.
- Du D, Ding J, Cai J, Zhang A (2007) One-step electrochemically deposited interface of chitosan-gold nanoparticles for acetylcholinesterase biosensor design. Electroanalytical Chemistry 605: 53-60.
- Pundir S, Chauhan N, Narang J, Pundir CS (2012) Amperometric choline biosensor based on multiwalled carbon nanotubes/zirconium oxide nanoparticles electrodeposited on glassy carbon electrode. Analytical Biochemistry 427: 26-32.
- Du D, Chen S, Cai J, Zhang A (2008) Electrochemical pesticide sensitivity test using acetylcholinesterase biosensor based on colloidal gold nanoparticle modified sol-gel interface. Talanta 74: 766-772.
- Zhang S, Shan L, Tian Z,Zheng Y, Shi L, et al. (2008) Study of enzyme biosensor based on carbon nanotubes modified electrode for detection of pesticides residue. Chinese Chemical Letters 19: 592-594.
- Viswanathan S, Radecka H, Radecki J (2009) Electrochemical biosensor for pesticides based on acetylcholinesterase immobilized on polyaniline deposited on vertically assembled carbon nanotubes wrapped with ssDNA. Biosensors and Bioelectronics 24: 2772-2777.
- Dua D, Yea X, Caib J, Liua J, Zhanga A(2010) Acetylcholinesterase biosensor design based on carbon nanotube-encapsulated polypyrrole and polyaniline copolymer for amperometric detection of organophosphates. Biosensors and Bioelectronics 25: 2503-2508.
- Dua D,Wanga M, Caib J, Zhanga A(2010)Sensitive acetylcholinesterase biosensor based on assembly ofß-cyclodextrins onto multiwall carbon nanotubes for detection of organophosphates pesticide, Sensors and Actuators B 146: 337-341.
- Liu T, Su H, Qu X,Ju P, Cui L, et al.(2011)Acetylcholinesterase biosensor based on 3-carboxyphenylboronic acid/reduced graphene oxide-gold nanocomposites modified electrode for amperometric detection of organophosphorus and carbamate pesticides, Sensors and Actuators B 160: 1255-1261.
- Zhenga Z, Zhoub Y, Li X, Liua S, Tangb Z (2011)Highly-sensitive organophosphorous pesticide biosensors based on nanostructured films of acetylcholinesterase and CdTe quantum dots, Biosensors and Bioelectronics 26: 3081-3085.
- Chauhan N, Chandra P (2011) Anamperometric biosensor based on acetylcholinesterase immobilized onto iron oxide nanoparticles/multi-walled carbon nanotubes modified gold electrode formeasurement of organophosphorus insecticides. AnalyticaChimicaActa 701: 66-74.
- Yang L, Xiong H, Zhang X, Wang S (2012) A novel tyrosinase biosensor based on chitosan-carbon-coated nickel nanocomposite film. Bioelectrochemistry 84: 44-48.
- Dua D,Ding J, Cai J, Zhang J, Liua L (2008) In situ electrodeposited nanoparticles for facilitating electron transfer across self-assembled monolayers in biosensor design. Talanta 74: 1337-1343.
- Shim J, Woo J, Moon S, Kim G(2009) A preparation of a single-layered enzyme-membrane using asymmetric pBPPO base film for development of pesticide detecting biosensor. Journal of Membrane Science 330: 341-348.
- Pichetsurnthorn P, Vattipalli K, Prasad S(2012) Nanoporousimpedemetric biosensor for detection of trace atrazine from water samples. Biosensors and Bioelectronics 32: 155- 162.
--
Relevant Topics
- Amperometric Biosensors
- Biomedical Sensor
- Bioreceptors
- Biosensors Application
- Biosensors Companies and Market Analysis
- Biotransducer
- Chemical Sensors
- Colorimetric Biosensors
- DNA Biosensors
- Electrochemical Biosensors
- Glucose Biosensors
- Graphene Biosensors
- Imaging Sensors
- Microbial Biosensors
- Nucleic Acid Interactions
- Optical Biosensor
- Piezo Electric Sensor
- Potentiometric Biosensors
- Surface Attachment of the Biological Elements
- Surface Plasmon Resonance
- Transducers
Recommended Journals
Article Tools
Article Usage
- Total views: 14473
- [From(publication date):
June-2016 - Apr 16, 2025] - Breakdown by view type
- HTML page views : 12870
- PDF downloads : 1603
