Peripheral T-cell Lymphoma with Epstein-Barr Virus-associated Reed-Sternberg-like B cells Mimicking Hodgkin Lymphoma
Received: 23-Sep-2014 / Accepted Date: 10-Nov-2014 / Published Date: 15-Nov-2014 DOI: 10.4172/2161-0681.1000200
Keywords: Peripheral T-cell lymphoma; Epstein-Barr virus; Reed-Sternberg-like cells; Hodgkin lymphoma
310706Summary
Differentiation of Hodgkin lymphoma (HL) from non-Hodgkin lymphoma is the central dogma on hematopathology. The former is characterized by scattered Hodgkin (H) or Reed-Sternberg (RS) cells against an inflammatory background rich in T cells with Epstein-Barr virus (EBV) association. In contrast, the latter typically shows dense infiltration of relatively monotonous tumor cells without H or RS cells. In rare circumstance when EBV-positive RS-like cells are present in a T-cell-rich infiltrate, the distinction may be challenging [1]. Furthermore, the nature of EBV-positive RS-like cells or large B cells in T-cell lymphoma is also multifaceted, ranging from reactive to neoplastic [2,3] which further complicates the pathogenesis of these diseases. Correct diagnosis relies on extensive immunohistochemical investigation, with or without molecular analysis. Here we describe a case of peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS), with proliferation of EBV-positive RS-like B cells resembling HL. Molecular studies with laser capture micro dissection separating CD30-positive RS-like cells from the remaining background T cells showed positive T-cell clonality in the latter and negative B-cell clonality in the former. Our case expands the histologic spectrum of PTCL with EBV-associated large B cells.
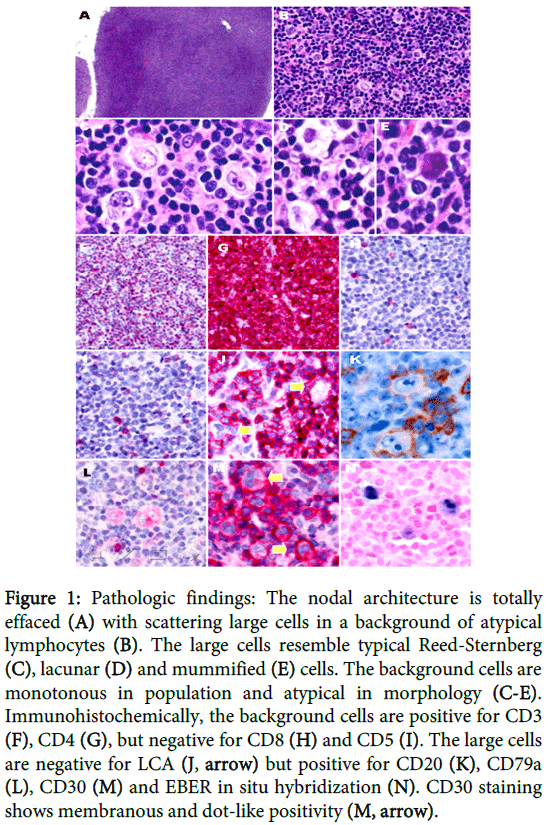
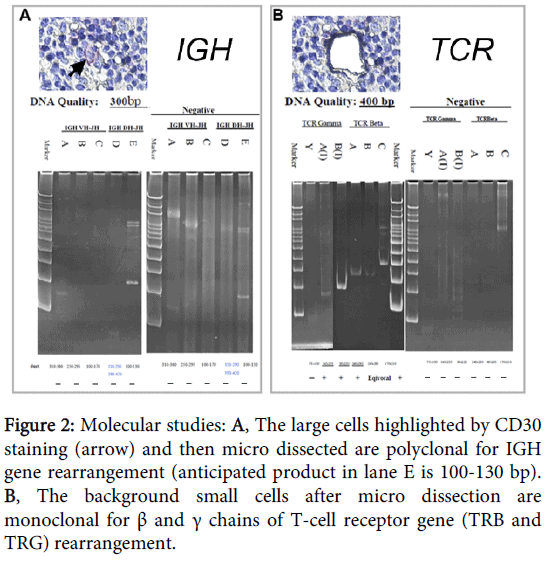
A 50-year-old female presented with multiple lymphadenopathy over bilateral axillary and neck areas for 1 month. She also had facial erythematous papules and bilateral bulging pulmonary hila on chest X-ray. Neck lymph node biopsy showed total effacement of nodal architecture by a diffuse infiltrate (Figure 1A), which was composed predominantly of small to medium-sized lymphocytes with nuclear atypia and scattering large cells (Figure 1B). These large cells showed a morphology spectrum reminiscent of HL cells, including RS (Figure 1C), lacunar (Figure 1D), and mummified cells (Figure 1E). Differential diagnosis included HL, T-cell rich large B-cell lymphoma, small B-cell lymphoma with RS-like cells, and T-cell lymphoma with RS-like cells. Immunohistochemically, the atypical lymphocytes were positive for CD3 (Figure 1F) and CD4 (Figure 1G) but negative for CD8 (Figure 1H) and CD5 (Figure 1I). The RS-like cells were negative for LCA (Figure 1J) and CD15, but positive for CD20 (Figure 1K), CD79a (Figure 1L), PAX5 and CD30 (Figure 1M). In situ hybridization of EBV-encoded early RNA (EBER) was positive in the large cells (Figure 1N). Molecular analysis of immunoglobulin heavy chain (IGH) gene from 2000 micro dissected CD30+ HL-like cells revealed a polyclonal rearrangement (Figure 2A), while the remaining background cells were positive for T-cell receptor (TCR) gene rearrangement (Figure 2B). The clinical survey revealed an Ann-Arbor stage III disease. Unfortunately, the patient died of hemophagocytic syndrome 24 days post-pathologic diagnosis. No chemotherapy was given in time.
Figure 1: Pathologic findings: The nodal architecture is totally effaced (A) with scattering large cells in a background of atypical lymphocytes (B). The large cells resemble typical Reed-Sternberg (C), lacunar (D) and mummified (E) cells. The background cells are monotonous in population and atypical in morphology (C-E). Immunohistochemically, the background cells are positive for CD3 (F), CD4 (G), but negative for CD8 (H) and CD5 (I). The large cells are negative for LCA (J, arrow) but positive for CD20 (K), CD79a (L), CD30 (M) and EBER in situ hybridization (N). CD30 staining shows membranous and dot-like positivity (M, arrow).
Figure 2: Molecular studies: A, The large cells highlighted by CD30 staining (arrow) and then micro dissected are polyclonal for IGH gene rearrangement (anticipated product in lane E is 100-130 bp). B, The background small cells after micro dissection are monoclonal for β and γ chains of T-cell receptor gene (TRB and TRG) rearrangement.
Proliferation of RS-like cells or large B cells is rarely seen in T-cell lymphomas [2]. It is well known in angioimmunoblastic T-cell lymphoma, adult T-cell lymphoma/leukemia, and reported in a few cases of PTCL-NOS. EBV is detected in the transformed cells in 71%-100% cases [2,3]. The relatively immunocompromised nature of hosts with T-cell lymphomas may facilitate EBV reactivation that subsequently leads to transformation of the EBV-infected B cells through a polyclonal-oligoclonal-monoclonal program, as in post-transplant lymphoproliferative disorders.
The transformed B cells vary in morphology, immunophenotype and genotype. Smuk et al. [3] categorize them into three major forms: (1) scattered Immunoblastic/centroblastic morphology with CD20+/CD30+/CD45+/LMP1+/EBNA2+/- phenotype; (2) morphology and immunophenotype identical to those in group 1, but forming homogenous sheets demarcated from the T-cell lymphoma, mimicking diffuse large B-cell lymphoma; and (3) scattered HL-like cells with CD15+/-/CD20-/+/CD30+/CD45-/LMP1+/EBNA2- phenotype, consistent with our case. In the third group, the large cells show lost expression of IgH-associated transcription factors, corresponding to classical HL tumor cells [3]. By analyzing IgH gene rearrangement, the transformed cells could be polyclonal, oligoclonal or monoclonal. In monoclonal cases with overt morphological features of B-cell lymphoma, that is sheets of cells with architectural destruction, a composite lymphoma is more appropriate for designation. Otherwise, it should be called T-cell lymphoma complicated by proliferation of large B cells with the clonality analysis included [4].
The clinical significance of the large B-cell proliferation remains uncertain. It seems that large B-cell proliferation occurring in AITL is not associated with a more aggressive clinical course [5]. There were cases of T-cell lymphomas with transformed B-cells presenting with simultaneous or secondary B-cell lymphomas or HL.6 During follow-up, the survival rate was 38% at 101 months after diagnosis [6].
References
- Hong RL, Su IJ, Chen YC, Hsieh HC, Wang CH, et al. (1992) Hodgkin's disease and non-Hodgkin's lymphoma containing Reed-Sternberg-like giant cells in Taiwan. A clinicopathologic analysis of 50 cases. Cancer 69: 1254-1258.
- Higgins JP, van de Rijn M, Jones CD, Zehnder JL, Warnke RA (2000) Peripheral T-cell lymphoma complicated by a proliferation of large B cells. Am J ClinPathol 114: 236-247.
- Smuk G, Illés A, Keresztes K, Kereskai L, Márton B, et al. (2010) Pheno- and genotypic features of Epstein-Barr virus associated B-cell lymphoproliferations in peripheral T-cell lymphomas. PatholOncol Res 16: 377-383.
- Reichard KK, Schwartz EJ, Higgins JP, Narasimhan B, Warnke RA, et al. (2006) CD10 expression in peripheral T-cell lymphomas complicated by a proliferation of large B-cells. Mod Pathol 19: 337-343.
- Lome-Maldonado C, Canioni D, Hermine O, Delabesse E, Damotte D, et al. (2002) Angio-immunoblastic T cell lymphoma (AILD-TL) rich in large B cells and associated with Epstein-Barr virus infection. A different subtype of AILD-TL? Leukemia 16: 2134-2141.
- Zettl A, Lee SS, Rüdiger T, Starostik P, Marino M, et al. (2002) Epstein-Barr virus-associated B-cell lymphoproliferative disorders in angloimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma, unspecified. Am J ClinPathol 117: 368-379.
Citation: Song HL, Chang KC (2014) Peripheral T-cell Lymphoma with Epstein-Barr Virus-associated Reed-Sternberg-like B cells Mimicking Hodgkin Lymphoma. J Clin Exp Pathol 4:200. DOI: 10.4172/2161-0681.1000200
Copyright: © 2014 Song HL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16336
- [From(publication date): 12-2014 - Jul 02, 2025]
- Breakdown by view type
- HTML page views: 11638
- PDF downloads: 4698