Research Article Open Access
Periodontal Pathogens and Clinical Periodontal Status of School Children: A Cross-Sectional Study
Sheila Cavalca Cortelli1*, Davi R Aquino1, Jose Roberto Cortelli1, Suzane A Raslan1, Caio VG Roman-Torres3, Rodrigo DP Balejo2 and Fernando O Costa4
1Department of Periodontology, Nucleus of Periodontal Research, University of Taubate, SP, Brazil
2Department of Periodontics, University of Taubate, SP, Brazil
3Department of Dentistry, University of Santo Amaro, SP, Brazil
4Department of Periodontology, Federal University of Minas Gerais, MG, Brazil
- *Corresponding Author:
- Sheila Cavalca Cortelli
Department of Periodontology
Nucleus of Periodontal Research
University of Taubate, SP, Brazil
Tel: +55 12 3625-4149
Fax: +55 12 3632-4968
E-mail: cavalcacortelli@uol.com.br
Received Date: March 11, 2014; Accepted Date: April 25, 2014; Published Date: May 02, 2014
Citation: Cortelli SC, Aquino DR, Cortelli JR, Raslan SA, Roman-Torres CVG, et al. (2014) Periodontal Pathogens and Clinical Periodontal Status of School Children: A Cross-Sectional Study. J Oral Hyg Health 2:131. doi:10.4172/2332-0702.1000131
Copyright: © 2014 Cortelli SC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Oral Hygiene & Health
Abstract
Although gingivitis affects dentate people in all ages it reaches high prevalence levels in children and adolescents. Purpose: This cross-sectional study compared the frequency of target bacterial species and its relation to periodontal status in children. Methods: 254 systemically healthy children, between 6 and 12 years of age, with mixed dentition, having a healthy periodontium or gingivitis were selected. Whole-mouth dichotomous plaque and gingival indices were evaluated and microbial samples were collected from tongue dorsum, first molars, right maxillary and left mandibular incisors. Results: P. gingivalis was the most frequent pathogen in the sulci of periodontally healthy children; T. forsythia and A. actinomycetemcomitans were the less detected species in tongue samples. P. gingivalis was the most frequent pathogen in both teeth and tongue samples among gingivitis children. C. rectus was more frequent in the sulci of healthy children while frequency of P. gingivalis was higher in gingivitis. Conclusions: It can be concluded that P. gingivalis was highly frequent and that C. rectus was more frequent in heathy children. At this range of age clinical status was not always directly related to the presence of the searched pathogens.
Keywords
Gingivitis; Child Development; Periodontal diseases; Bacteria
Introduction
Periodontal disease (PD) has a multifactorial etiology and initiates by the accumulation of dental biofilm that affects tissues surrounding. Its pattern, severity and progression are determined by social, systemic, genetic and microbial composition among other risk factors [1]. The most prevalent type of periodontal disease worldwide is plaque-induced gingivitis which shows reversibility as a key characteristic [2].
Epidemiological data have shown that plaque-induced gingivitis affects, in different rates, dentate populations in all ages [3], but mainly children and adolescents [4,5]. Jalaleddin and Ramezani [6] found a prevalence of 97-98% of gingivitis in children aged from 6 to 9. According to the United Nations Organization in 2010 more than 1.8 billion people were aged between 10 and 25. In Brazil it is estimated that in 2030 19.6% of population will be up to 14 years old, this makes the country vulnerable to gingivitis occurrence [7].
Bacterial species that can cause gingivitis colonize oral cavity since early in life [8] reaching high numbers at the age of 2 [9]. Independent of age, the complexity of the microbiota is often related to the healthy or diseased status of periodontium [10]. Also, presence of some bacterial species may increase the risk for periodontal diseases [11]. Microbial data is clearer in adults than in children, Sakai et al. [12], for example, observed a high percentage of children harboring at least one periodontal pathogenic species (A. actinomycetemcomitans, P. gingivalis, T. denticola), however, Gafan et al. [13] found a higher frequency of T. forsythia in children without disease. Interestingly, it was suggested that children are more resistant to gingivitis than adults in spite of the fact that they show increased subgingival levels of Leptotrichia sp., Capnocytophaga sp., Selenomonas sp. and Bacteroides sp [14]. Cortelli et al. [15] in a sample population of high plaque index reported and association between P. intermedia and gingivitis and between C. rectus and periodontal health.
Therefore, it has been suggested that colonization by pathogens associated with gingivitis happens earlier than previously believed. According to Tanner et al. [16] this knowledge would be helpful for the understanding of disease development and determination of interceptive measures. Although not related to tooth loss, per se, gingivitis is a chronic inflammatory disease that deserves to be controlled aiming at a healthier status for the entire individual. This cross-sectional study compared the frequency of target bacterial species and its relation to periodontal status in school children.
Materials and Methods
The present study was reviewed and approved by the Ethics Committee of the University of Taubaté (Protocol #0317/07). Legal guardians or parents signed the informed consent form after verbal and written explanations about study design and procedures.
Inclusion criteria and determination of groups
This convenience sample was composed of 254 systemically healthy children, aged between 6 and 12, with mixed dentition who were divided into two groups according to their periodontal status: periodontally healthy (<30% of periodontal sites showing gingival bleeding); gingivitis (>30% of periodontal sites showing gingival bleeding) [17]. Children who had taken antibiotics in the previous 3 months, had chronic systemic diseases, extensive caries lesions, had no molars and incisors or who wore orthodontic appliances were excluded from the study.
Clinical measurements and diagnosis
Two trained and calibrated examiners measured plaque [18] and gingival [19] indices in a single afternoon visit in the dental unit of a public school. Later, original scores were dichotomized according to absence (0) or presence of any amount of plaque (1) and to absence (0) or presence of gingival bleeding (1). Intra and inter-examiners reproducibilityvalues were tested using Kappa test (K). The examiners were considered calibrated when agreement rates of not less than 90% were reached. Also, a bite-wing X-ray examination was conducted to evaluate the presence of periodontal bone resorption [20].
Microbiological assessment
A single microbial sample was taken from the central 1cm2 area of the dorsum of the tongue of each child, using a swab with reduced Ringer’s solution, rotated six times. Each swab was transferred into a microtube also containing reduced Ringer’s solution (1 ml). After removal of supragingival plaque, a pooled subgingival sample was collected from the mesiobuccal aspect of all first molars and mesial aspect of right maxillary and left mandibular incisors using sterile paper points number 30 (Dentsply, York, PA, United State) inserted into the depth of the gingival sulcus. After being placed in the sulcus for 60 seconds [21], paper points were removed and immediately transferred into a microtube containing 1.5 mL of reduced Ringer’s solution (Oxoid Ltd., Basingstoke, Hampshire, United Kingdom) and transported to the laboratory. All samples were kept at -80°C until processing.
The presence of C. rectus, P. gingivalis, A. actinomycetemcomitans, P. intermedia and T. forsythia was determined by polymerase chain reaction (PCR). A total volume of 25 μL of the PCR mixture contained 10 μL of the DNA sample, 2.5 μL of a 10x PCR buffer (Invitrogen, Carlsbad, CA, USA), 1.25 units of Taq DNA polymerase (Invitrogen), 0.2 mM of each deoxyribonucleotide (Invitrogen, Carlsbad, CA, USA), 1.5 mM of MgCl and 1.0 μM of each primer.
The PCR amplification was performed in a Mastercycler Gradient thermal cycler (Eppendorf, Westbury, NY, USA) using specific primers (Table 1) under a standard protocol that includes an initial denaturation step at 95°C for 5 minutes, followed by 35 cycles of denaturation at 95°C for 30 seconds, an annealing step at 55°C for 30 seconds, and an extension step at 72°C for 1 minute, with a final extension period of 72°C for 5 minutes.
| Bacterial species | Primers | Amplicon |
|---|---|---|
| C. rectus | Sense 5’ TTTCGGAGCGTAAACTCCTTTTC-3’ | 598bp |
| Antisense 5’-TTTCTGCAAGCAGACACTCTT-3’ | ||
| A.actinomycetemcomitans | Sense 5’-AAACCCATCTCTGAGTTCTTCTTC-3 | 550bp |
| Antisense 5’-ATGCCAACTTGACGTTAAAT-3’ | ||
| P. intermedia | Sense 5’-TTTGTTGGGGAGTAAAGCGGG-3’ | 575bp |
| Antisense 5’-TCAACATCTCTGTATCCTGCGT-3’ | ||
| P. gingivalis | Sense 5’-AGGCAGCTTGCCATACTGCGG-3’ | 404bp |
| Antisense 5’-ACTGTTAGCAACTACCGATGT-3’ | ||
| T. forsythia | Sense 5’GCGTATGTAACCTGCCCGCA3’ | 641bp |
| Antisense 5’-TGCTTCAGTGTCAGTTATACCT-3’ |
Table 1: Bacteria and specific primer sequences
The final products were separated on 1.5% agarose gel, stained with ethidium bromide (0.5 μg/mL) and photographed under ultraviolet light to confirm the existence of the target oral bacterial species. A 100bp DNA ladder (Invitrogen, Carlsbad, CA, USA) was used as the molecular weight marker. Both positive and negative controls were included for the PCR reaction in order to verify the primer specificity and identify and DNA contamination.
Statistical analysis
Clinical data was compared using Mann-Whitney test. The frequencies of the periodontal pathogens were analyzed using the Chi-squared test (χ2). Statistical analyses were performed using statistical software (Bio Estat 5.0 and SPSS11.0) where the statistical significance was established at alpha 5%.
Results
A total of 254 children of both genders were included in the present study (Table 2). Table 3 shows comparative inter-group analysis regarding plaque and gingival indices.
| Periodontally Healthy | Gingivitis | Total | |
|---|---|---|---|
| Male | 73 | 49 | 122 |
| Female | 72 | 60 | 132 |
| Total (Mean age ± SD) |
145 (8.11 ± 2.59) |
109 (8.54 ± 1.32) |
254 (8.41 ± 1.79) |
Table 2: Distribution of study population according to gender, age and periodontal status. MA: Mean Age; SD: Standard Deviation.
| Plaque Index(mean ± SD) | Gingival Index(mean ± SD) | |
|---|---|---|
| Periodontally Healthy | 0.78 ± 0.14 | 0.09 ± 00.07 |
| Gingivitis | 0.94 ± 0.14 | 0.48 ± 0.17 |
| p Value | 0.0001 | 0.0001 |
Table 3: Comparative analysis between periodontally healthy and gingivitis children regarding clinical indices. SD: Standard Deviation; Mann-Whitney test (p<0.05).
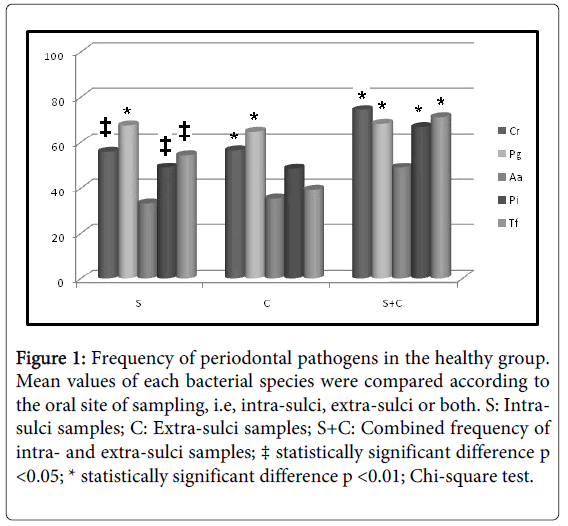
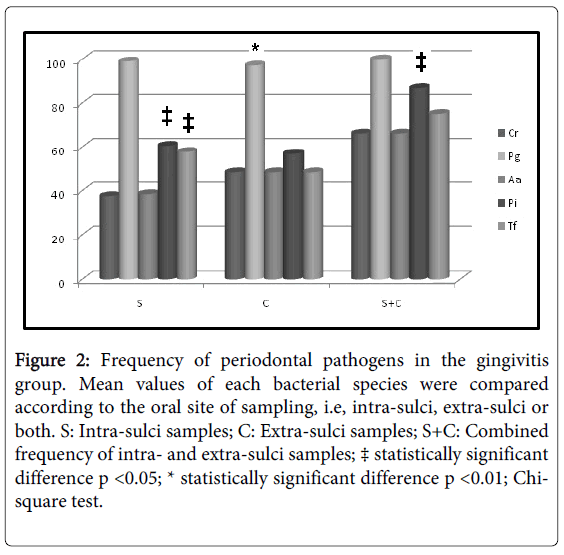
Initially, intra-group analysis of periodontally healthy children showed P. gingivalis as the most frequent pathogen in intra-sulci samples, and T. forsythia and A. actinomycetemcomitans (Figure 1) were the less detected species in tongue samples. P. gingivalis was the most frequent pathogen in both types of sampled sites (Figure 2) among gingivitis children. Table 4 shows in details all frequency and comparative inter-group statistical analysis (periodontally healthy vs. gingivitis). C. rectus was more frequent in the sulci of healthy than in gingivitis children. Frequency of P. gingivalis was higher in gingivitis children in comparison to healthy children in both sulci and tongue samples. Table 4 also shows comparative data among sampling site (intra-sulci vs. extra-sulci vs. combined sites).
Figure 1: Frequency of periodontal pathogens in the healthy group. Mean values of each bacterial species were compared according to the oral site of sampling, i.e, intra-sulci, extra-sulci or both. S: Intrasulci samples; C: Extra-sulci samples; S+C: Combined frequency of intra- and extra-sulci samples; ‡ statistically significant difference p <0.05; * statistically significant difference p <0.01; Chi-square test.
Figure 2:Frequency of periodontal pathogens in the gingivitis group. Mean values of each bacterial species were compared according to the oral site of sampling, i.e, intra-sulci, extra-sulci or both. S: Intra-sulci samples; C: Extra-sulci samples; S+C: Combined frequency of intra- and extra-sulci samples; ‡ statistically significant difference p <0.05; * statistically significant difference p <0.01; Chisquare test.
| C. rectus | P. gingivalis | A. actinomycetemcomitans | P. intermedia | T. forsyhtia | ||||||||||||
| S | C | S+C | S | C | S+C | S | C | S+C | S | C | S+C | S | C | S+C | ||
| Healthy | + | 81 | 82 | 108 | 98 | 94 | 99 | 48 | 51 | 71 | 71 | 70 | 97 | 79 | 57 | 103 |
| - | 64 | 63 | 37 | 47 | 51 | 46 | 97 | 94 | 74 | 74 | 75 | 48 | 66 | 88 | 42 | |
| f(%) | 55.9 *b | 56.5 b | 74.4 a | 67.5 a | 64.8 a | 68.2 a | 33.1 b | 35.2 b | 48.9 a | 49.0 b | 48.3 b | 66.8 a | 54.4 b | 39.3 c | 71.0 a | |
| Gingivitis | + | 41 | 53 | 72 | 108 | 106 | 109 | 42 | 53 | 72 | 66 | 62 | 95 | 63 | 53 | 82 |
| - | 68 | 56 | 37 | 1 | 3 | 0 | 67 | 56 | 37 | 43 | 47 | 14 | 46 | 56 | 27 | |
| f(%) | 37.6 b | 48.6 b | 66.1 a | 99.1 *a | 97.2 *a | 100.0 *a | 38.5 b | 48.6 *b | 66.1 *a | 60.6 b | 56.9 b | 87.1 *a | 57.8 b | 48.6 b | 75.2 a | |
Table 4: Comparative inter-group frequency of bacterial species. * Statistically significant difference when frequencies were compared between periodontally healthy and gingivitis children (Chi-Square test; p < 0.05); a, b, c: different lower-case letters for the same bacterial species indicate different frequencies observed among sites of sampling (Chi-Square test; p < 0.05); S: Intra-sulci samples; C: Extra-sulci samples; S+C: Combined frequency of intra- and extra-sulci samples.
Discussion
Only after caries control, gingival inflammation in children received scientific attention. Also, the perception that colonization by periodontal pathogenic species occurs earlier in life [20] contributed for the interest in microbial investigation of this age group.
Although studies have been carried out to determine the prevalence of periodontal pathogens related to periodontal clinical status of children, due to the applied methodology there are many aspects that still need to be clarified [12,13,16,22-27]. In Brazil, a similar variable methodological pattern among studies could also be observed [15,28-30]. In a previous study our group [20] sampled 33 periodontally healthy children also between 6 and 12 years of age and compared among different age groups frequencies of the same bacterial species searched in the present study. On the other hand, the present cross-sectional study was designed aiming at comparing bacterial frequencies between health and gingivitis status within the same age (6-12 years) group. It is also important to emphasize that the present study investigated a higher number of children in comparison to the one published in 2008. Now, 145 periodontally healthy children and 109 gingivitis children were investigated.
There is a lack of agreement whether gender is related to periodontal disease in children or not. According to López et al. [31] girls less than 12 years of age had a higher risk for periodontal disease in comparison to boys. On the contrary, Cortellazzi et al., [32] and Chambrone et al. [30] observed a higher prevalence of gingivitis among boys. In the present study there was no significant difference between genders regarding gingivitis occurrence (45.45% in girls and 40.16% in boys).
Our intra-group analysis revealed P. gingivalis as the most frequent species in the sulci of both healthy and diseased children. This bacterium was also one of the most frequent in tongue samples. In the gingivitis group P. gingivalis was alone the most prevalent, while in the healthy group both C. rectus and P. gingivalis shared the highest prevalence level.
Periodontal diseases represent a polymicrobialinfection where microorganisms display extensive interactions: (i) competition for bacterial nutrients, (ii) synergistic interaction, (iii) antagonism when one resident inhibits the growth of another, (iv) neutralization of a virulence factor and (v) interference in the growth-dependent signaling mechanisms [33]. P. gingivalis often coexists with other periodontopathic bacteria such as P. intermedia, Fusobacterium nucleatum, T. forsythia and Treponema denticola [14,34] contributing to the higher number of interactive relations observed in periodontal biofilm.
Presence of key pathogens such as P. gingivalis, A. actinomycetemcomitans and T. forsythia in samples of children without gingivitis confirms that biofilm is one of the factors related to disease development and that these species are members of human indigenous microbiota. For instance, our group detected in a previous study the presence of T. forsythia in newborns and babies, from 0 to 4 months of life [8]. However, considering the factor time our data also suggest the need for monitoring this population because there is no guarantee of keeping the healthy status until adult life.
Gafan et al., [13] found higher levels of T. forsythia in healthy children and Riep et al. [35] reported that P. gingivalis, P. intermedia and T. forsythia could even be detected in subjects resistant against periodontitis. In addition, an increase in pathogenic species overtime was suggested by Papapannou et al. [26]. In this context, this increase could overlap immune tolerance level anytime leading to the loss of opportunity for primary preventive measures. In older subjects (14 to 17 years), P. gingivalis showed a positive correlation with gingival index, bleeding index and probing depth [24,27].
Again, besides the presence of these bacteria there are other factors related to the development of periodontal diseases. Especially in the studied age many risk factors will change during life, impacting the overall risk for periodontal diseases.
It can be concluded that P. gingivalis was highly frequent and that C. rectus was more prevalent in healthy children. At this age group clinical status was not always directly related to presence of the searched pathogens.
Conclusion
Evolving shear stress in vessels smaller than 150 microns causes stimulated reshaping oxygen carriers. As a consequence of these changes, the liquid phase is moved by the pressure gradient from the capillary lumen into the erythrocyte. The hematocrit and the blood viscosity in the vessel are reduced. These transformations are reversible. When the erythrocyte leaves capillary, the shear deformations are reduced, cell shape is restored and the water re-enters inside. Using labeled media and fluorescent dyes, as well as experiments with cooking buffers on heavy water and subsequent stress by passing the erythrocyte suspension through a Millipore filters or by syringe hopefully confirms our conclusion.
References
- Nunn ME (2003) Understanding the etiology of periodontitis: an overview of periodontal risk factors. Periodontol 2000 32: 11-23.
- LOE H, Theilade E, Jensen SB (1965) Experimental Gingivitis iN Man. J Periodontol 36: 177-187.
- Bhat M (1991) Periodontal health of 14-17-year-old US schoolchildren. J Public Health Dent 51: 5-11.
- Gjermo P, Bellini HT, Pereira Santos V, Martins JG, Ferracyoli JR (1984) Prevalence of bone loss in a group of Brazilian teenagers assessed on bite-wing radiographs. J Clin Periodontol 11: 104-113.
- Masamatti SS, Kumar A, Virdi MS (2012) Periodontal diseases in children and adolescents: a clinician's perspective part. Dent Update 39: 541-544.
- Jalaleddin H, Ramezani GH (2009) Prevalence of gingivitis among school attendees in Qazvin, Iran. East Afr J Public Health 6: 171-174.
- Chiapinotto FA, Vargas-Ferreira F, Demarco FF, Corrêa FO, Masotti AS (2013) Risk factors for gingivitis in a group of Brazilian schoolchildren. J Public Health Dent 73: 9-17.
- Cortelli JR, Aquino DR, Cortelli SC, Nobre Franco GC, Fernandes CB, et al. (2008) Detection of periodontal pathogens in oral mucous membranes of edentulous individuals. J Periodontol 79: 1962-1965.
- Moore LV, Moore WE, Cato EP, Smibert RM, Burmeister JA, et al. (1987) Bacteriology of human gingivitis. J Dent Res 66: 989-995.
- Ximénez-Fyvie LA, Haffajee AD, Socransky SS (2000) Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. J Clin Periodontol 27: 648-657.
- Potera C (1999) Forging a link between biofilms and disease. Science 283: 1837, 1839.
- Sakai VT, Campos MR, Machado MA, Lauris JR, Greene AS, et al. (2007) Prevalence of four putative periodontopathic bacteria in saliva of a group of Brazilian children with mixed dentition: 1-year longitudinal study. Int J Paediatr Dent 17: 192-199.
- Gafan GP, Lucas VS, Roberts GJ, Petrie A, Wilson M, et al. (2004) Prevalence of periodontal pathogens in dental plaque of children. J Clin Microbiol 42: 4141-4146.
- Moore WE, Holdeman LV, Smibert RM, Cato EP, Burmeister JA, et al. (1984) Bacteriology of experimental gingivitis in children. Infect Immun 46:1-6.
- Cortelli SC, Cortelli JR, Aquino DR, Holzhausen M, Franco GC, et al. (2009) Clinical status and detection of periodontopathogens and Streptococcus mutans in children with high levels of supragingival biofilm. Braz Oral Res 23: 313-318.
- Tanner AC, Milgrom PM, Kent R Jr, Mokeem SA, Page RC, et al. (2002) The microbiota of young children from tooth and tongue samples. J Dent Res 81: 53-57.
- López NJ, Smith PC, Gutierrez J (2002) Periodontal therapy may reduce the risk of preterm low birth weight in women with periodontal disease: a randomized controlled trial. J Periodontol 73: 911-924.
- SILNESS J, LOE H (1964) PERIODONTAL DISEASE IN PREGNANCY. II. CORRELATION BETWEEN ORAL HYGIENE AND PERIODONTAL CONDTION. Acta Odontol Scand 22: 121-135.
- LOE H, SILNESS J (1963) PERIODONTAL DISEASE IN PREGNANCY. I. PREVALENCE AND SEVERITY. Acta Odontol Scand 21: 533-551.
- Cortelli JR1, Aquino DR, Cortelli SC, Fernandes CB, de Carvalho-Filho J, et al. (2008) Etiological analysis of initial colonization of periodontal pathogens in oral cavity. J Clin Microbiol 46: 1322-1329.
- Cortelli JR, Cortelli SC, Jordan S, Haraszthy VI, Zambon JJ (2005) Prevalence of periodontal pathogens in Brazilians with aggressive or chronic periodontitis. J Clin Periodontol 32: 860-866.
- Kamma JJ, Diamanti-Kipioti A, Nakou M, Mitsis FJ (2000) Profile of subgingival microbiota in children with mixed dentition. Oral Microbiol Immunol 15: 103-111.
- Suda R, Kurihara C, Kurihara M, Sato T, Lai CH, et al. (2003) Determination of eight selected periodontal pathogens in the subgingival plaque of maxillary first molars in Japanese school children aged 8-11 years. J Periodontal Res 38: 28-35.
- Chen LL, Wu YM, Yan J, Sun WL, Sun YZ, et al. (2005) Association between coinfection of Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans and Treponema denticola and periodontal tissue destruction in chronic periodontitis. Chin Med J 118: 915-921.
- Kulekci G, Leblebicioglu B, Keskin F, Ciftci S, Badur S (2008) Salivary detection of periodontopathic bacteria in periodontally healthy children. Anaerobe 14: 49-54.
- Papapanou PN, Behle JH, Kebschull M, Celenti R, Wolf DL, et al. (2009) Subgingival bacterial colonization profiles correlate with gingival tissue gene expression. BMC Microbiol 9: 221.
- Hayashi F, Okada M, Oda Y, Kojima T, Kozai K (2012) Prevalence of Porphyromonas gingivalis fimA genotypes in Japanese children. J Oral Sci 54: 77-83.
- Rebelo MA, Lopes MC, Vieira JM, Parente RC (2009) Dental caries and gingivitis among 15 to 19 year-old students in Manaus, AM, Brazil. Braz Oral Res 23: 248-254.
- Bonanato K, Pordeus IA, Moura-Leite FR, Ramos-Jorge ML, Vale MP, et al. (2010) Oral disease and social class in a random sample of five-year-old preschool children in a Brazilian city. Oral Health Prev Dent 8: 125-132.
- Chambrone L, Macedo SB, Ramalho FC, Trevizani Filho E, Chambrone LA (2010) [Prevalence and severity of gingivitis among scholars (7-14 years): local conditions associated to bleeding on probing]. Cien Saude Colet 15: 337-343.
- López R, Fernández O, Jara G, Baelum V (2001) Epidemiology of clinical attachment loss in adolescents. J Periodontol 72: 1666-1674.
- Cortellazzi KL, Pereira SM, Tagliaferro EP, Tengan C, Ambrosano GM, et al. (2008) Risk indicators of dental caries in 5-year-old Brazilian children. Community Dent Health 25: 253-256.
- Kuramitsu HK, He X, Lux R, Anderson MH, Shi W (2007) Interspecies interactions within oral microbial communities. Microbiol Mol Biol Rev 71: 653-670.
- Moore WE, Holdeman LV, Cato EP, Smibert RM, Burmeister JA, et al. (1985) Comparative bacteriology of juvenile periodontitis. Infect Immun 48: 507-519.
- Riep B, Edesi-Neuss L, Claessen F, Skarabis H, Ehmke B, et al. (2009) Are putative periodontal pathogens reliable diagnostic markers? J Clin Microbiol 47: 1705-1711.
Relevant Topics
- Advanced Bleeding Gums
- Advanced Receeding Gums
- Bleeding Gums
- Children’s Oral Health
- Coronal Fracture
- Dental Anestheia and Sedation
- Dental Plaque
- Dental Radiology
- Dentistry and Diabetes
- Fluoride Treatments
- Gum Cancer
- Gum Infection
- Occlusal Splint
- Oral and Maxillofacial Pathology
- Oral Hygiene
- Oral Hygiene Blogs
- Oral Hygiene Case Reports
- Oral Hygiene Practice
- Oral Leukoplakia
- Oral Microbiome
- Oral Rehydration
- Oral Surgery Special Issue
- Orthodontistry
- Periodontal Disease Management
- Periodontistry
- Root Canal Treatment
- Tele-Dentistry
Recommended Journals
Article Tools
Article Usage
- Total views: 14744
- [From(publication date):
May-2014 - Jul 02, 2025] - Breakdown by view type
- HTML page views : 10070
- PDF downloads : 4674