Research Article Open Access
Perineural versus Intravenous Dexamethasone for Prolongation of Multiple Nerve Blocks for Pain Relief after Total Knee Arthroplasty
Anatoli Stav1,2*, Leonid Reytman2,3, Michael-Yohay Stav4, Aksana Machluf5, Roger Sevi2,6 and Mohammed Tallas7
1Postanesthesia Care Unit, Hillel Yaffe Medical Center, Hadera, Israel
2Department of Medicine, Technion-Israel Institute of Technology, Haifa, Israel
3Department of Anesthesiology, Hillel Yaffe Medical Center, Hadera, Israel
4Assaf Harofeh Medical Center, Zerifin, Israel
5Hillel Yaffe Medical Center, Hadera, Israel
6Department of Orthopedics A, Hillel Yaffe Medical Center, Hadera, Israel
7Department of Orthopedics B, Hillel Yaffe Medical Center, Hadera, Israel
- *Corresponding Author:
- Anatoli Stav
Postanesthesia Care Unit, Hillel Yaffe Medical Center
Hadera, 38100, Israel
Tel: +972-522696680
Fax: +972-46334539
E-mail: anatoli99@bezeqint.net
Received date: June 03, 2017; Accepted date: June 20, 2017; Published date: June 26, 2017
Citation: Stav A, Reytman L, Stav MY, Machluf A, Sevi R, et al. (2017) Perineural versus Intravenous Dexamethasone for Prolongation of Multiple Nerve Blocks for Pain Relief after Total Knee Arthroplasty. J Pain Relief 6:293. doi:10.4172/2167-0846.1000293
Copyright: © 2017 Stav A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Pain & Relief
Abstract
Background: Multiple nerve blocks (MNB) provide excellent time-limited perioperative analgesia following total knee arthroplasty. Both perineural and systemic use of dexamethasone (DXM) as an adjuvant to local anesthetic prolong the duration of single-shot MNB. We hypothesized that preoperative perineural injection of DXM prolongs analgesia after MNB more than the same dose of intravenous (IV) DXM injection due to direct action on the nerves and not only by a systemic action mechanism.
Methods: This is a prospective, randomized, controlled and observer-blinded study. One hundred and nine patients were randomly assigned to one of three groups: Group (Gr) 1-perineural DXM+MNB, Gr 2-systemic IV DXM +MNB, Gr 3-control group, MNB without DXM. Postoperative variables including intensity of pain at rest and during motion, grade of sensory and motor block, opioid consumption, comfort time (the first analgesic request) were the primary end-points of investigation.
Results: Ninety patients completed the study protocol. Very low parameters of intensity of pain at rest and during motion, high grade of sensory and motor block were observed up to 12 hours after MNB performance in all three groups. Patients who received MNB with DXM perineurally or systemically, experienced superior pain relief and had reduced opioid consumption 24 hours post-block compared to the control group without differences between the two "dexamethasone" groups. There were no differences between groups at 36 and 48 hours post-block. Patients in the control group suffered from pain at rest and started treatment by any analgesics significantly earlier than patients from the two “dexamethasone” groups. No difference of comfort time was observed between Gr 1 and Gr 2. In the period between 24 and up to 36 hours the block’s effect (i.e. the effect of local anesthetic with adjuvant dexamethasone) gradually weakened and somewhere at 48 hours post-block, it passed almost completely.
Conclusions: Intravenous 8 mg dexamethasone is equivalent to perineural dexamethasone in prolonging the pain relief duration of an ultrasound guided single-shot multiple nerve block with bupivacaine and adrenaline following total knee arthroplasty.
Keywords
Arthroplasty; Postoperative pain; Comfort time; Opioid consumption; Dexamethasone perineural; Systemic; Intravenous; Ultrasound-guided multiple nerve blocks
Abbreviations:
MNB: Multiple Nerve Blocks-block of femoral, popliteal sciatic, obturator (both branches), and lateral femoral cutaneous nerves; DXM: Dexamethasone; IV: Intravenous; PNB: Peripheral Nerve Block; TKA: Total Knee Arthroplasty; LA: Local Anesthetic (bupivacaine 5 mg/mL with adrenaline 5 μg/mL); NRS: Numerical Rating Scale (for intensity of pain measurement); NP scale: Nurses Assessment of Postoperative Pain; DM: Diabetes Mellitus; Gr: Group; PACU: Postanesthesia Care Unit; μg: Microgram; Kg: Kilogram; m: Meter; mg: Milligram; mL: Milliliter; dL: Deciliter; hr: Hours; min: Minutes; Postoperative: Post-op; SE: Standard Error; PONV: Postoperative Nausea and Vomiting; A1C: Hemoglobin A1C, HbA1c, or glycohemoglobin test; h: Height in meters; BW: Body Weight in kg; BMI: Body Mass Index; GA: General Anesthesia; NSAID: Non-Steroidal Anti-Inflammatory Drugs
Introduction
Preoperative peripheral nerve block (PNB) is part of modern technique of anesthesia and postoperative (post-op) pain control for total knee arthroplasty (TKA) [1-3]. Preoperative single injection of MNB, i.e. of femoral, popliteal sciatic, both branches of obturator nerve and lateral femoral cutaneous nerve produce excellent perioperative analgesia and opioids spare effect during the day of surgery and the first post-op day [4]. A significant limitation of this method of analgesia following TKA is its relatively short effect, even after use of long acting local anesthetic as bupivacaine with adrenaline (LA) [4]. The use of perineural catheter for continuous MNB use is unpractical because of blocking three nerves (femoral, sciatic and obturator) the three catheters should be inserted (lateral femoral cutaneous nerve block play role during the operation, but not in the post-op period [5-7]). Another limitation of this method is technical challenge with placement and removal of catheters, secondary failure is dislodgment or infection, nerve injury or prolonged motor weakness resulting in falls may occur during the recovery period [2,8-12]. The desire of prolonging the action of LA is avoiding use of continuous PNB by inserting the perineural catheter, which can lead to the search of various additives to the solution of LA with followed perineural injection [13]. A systemic review with meta-analysis summarized the data of the affectivity and safety of perineural DXM, which is used as an adjuvant to LA for prolongation the PNB [14-16], A significant improvement and prolongation of the post-op analgesia was demonstrated [14,15]. Without difference between 4 mg and 8 mg of perineural DXM use. A negative result was published either [17-19].
The mechanism of action of DXM on peripheral nerve during perineural injection is unknown [14,15]. Scattered experimental data do not allow combining them into an integral theory explaining this phenomenon [20-24].
Desmet et al. concluded that systemic (intravenous) injection of DXM is equivalent to perineural DXM injection about the prolonging the analgesic duration of single-shot interscalene block with ropivacaine [25]. Kawanishi et al. have come to an opposite conclusion: perineural but not systemic DXM prolongs the duration of interscalene block [26].
We hypothesized that preoperative (before TKA) perineural injection of DXM prolongs analgesia after MNB more than the same dose of IV DXM injection due to direct action on the nerves and not only by systemic mechanism of action.
Methods
This is a prospective, randomized, controlled and observer-blinded clinical trial; enrollment began after receiving Hillel Yaffe Review Boards approval and written informed patient consent. Trial eligibility was as follows: over the age of 18; physical status of I-III based on American Society of Anesthesiologists criteria [27]; and scheduled to undergo elective TKA due to osteoarthritis. In total 121 patients were eligible for the trial.
Exclusion criteria were previous TKA, TKA revision, TKA due to trauma or etiology other than osteoarthritis, systemic glucocorticoid use, skin infection near the block injection site, allergy to local anesthetics, pre-existing peripheral neuropathy of the involved limb, proven opioid dependency [28], coagulopathy (INR>1.4), thrombocythopenia (platelets count<100000), chronic pain syndrome (pain in rest more than six months, anxiety, and/or depression, hyperalgesia, allodynia, chronic treatment with analgesics and more), dementia, lack of orientation to person, place and time, inability to comprehend the Numerical Rating Scale (NRS) [29], and the language barrier (the patient does not speak, understand and/or writes on Hebrew, Russian, English, French or Arabian), as well as patients suffered from type I insulin dependent diabetes mellitus (DM), poorly controlled type II DM (hemoglobin A1C ≥ 7%, plasma glucose level of ≥ 131 mg/dL [7.28 mmol/L], peak plasma glucose measurement 1-2 hours after beginning the meal ≥ 181 mg/dL [10.06 mmol/L] [30]. The patients suffered from well controlled type II DM without complications (for example, skin and eyes complications, neuropathy, foot complications, nephropathy, ketoacidosis, stroke, hyperosmolar hyperglycemic nonketonic syndrome and more), which were treated exclusively by oral anti-diabetic drugs were not excluded from the trial. The cause of inclusion the patients with well controlled type II DM into our research is the study of Hans et al. [31] The authors concluded that after preoperative injection of 10 mg dexamethasone IV, blood glucose level increased in non-diabetic, and type II diabetic patients by the same degree (the computation was performed with respect to baseline). A maximum blood level elevation expressed in percent of baseline in non-diabetic group was 35 ± 19%, and in well controlled type II DM group was 29 ± 19% [30] without statistically significant difference between groups.
Patients were instructed regarding the use of a 0-10 Numerical Rating Scale (NRS) [29]: zero represents ‘no pain at all’ whereas the upper limit represents ‘the worst pain ever possible.
The author states that the report includes every item in the Consolidated Standards of Reporting Clinical Trials (CONSORT) checklist for a prospective randomized clinical trial. The study was registered prior to patient enrollment. Data collection: February, 2015 to January, 2017. Registry Url: Clinical trial registration number (NIH)-NCT02253784.
Based on the exclusion criteria 12 patients were excluded from the trial. The inclusion group (109 patients) was randomized into three groups (Figure 1): Group (Gr) 1: MNB with perineural injection of DXM; Gr 2: MNB with systemic (i.e. IV) injection of DXM; Gr 3: (Control group) MNB without DXM. Randomization was done using a computer-generated table of random numbers, placing them in a sealed envelope, and then opening the envelope on the morning of surgery.
The patients and the investigators who collected data during the postoperative period (the nursing staff of the Postanesthesia Care Unit (PACU), A.M. after discharge from PACU, the residents of the Department of Anesthesiology, the nursing staff of both Orthopedic departments) were blinded to group assignments throughout the research.
Patients were premedicated with IV fentanyl (0.5 μg/kg) and midazolam (0.03 mg/kg) with option to titrate the fentanyl up to 1.0 μg/kg, midazolam up to 0.05 mg/kg during the procedure. Local anesthesia was used with lidocaine 10 mg/mL before inserting “block” needle. The maximum 15 mL of lidocaine was used for MNB.
The same anesthesiologists (A.S or L.R.) performed all preoperative US-guided MNBs using an ultrasound system (SonoSite S-Nerve, SonoSite, Bothell, WA, USA). S-Nerve Ultrasound system, Sono Site Corp., USA with 6-13 MHz linear array transducer (L25X). A 22-gauge 80 mm Pajunk Sono Tap cannula (Pajunk® Medical Produkte GmbH, Geisingen, Germany) was used for visualization of the target nerves (femoral, popliteal sciatic, obturator) and to inject the local anesthetic around the nerves. A 50 mm 22-gauge needle was used for the lateral femoral cutaneous nerve block.
Standard non-invasive monitoring was used; oxygen was administered via facemask, 5 L per minute. Bupivacaine 5 mg/mL with adrenaline 5 μg/mL was used as a LA for MNBs performing in Gr 2 and Gr 3. For patients in Gr 1 LA/DXM admixture was used, the dose of the DXM in the admixture was 0.2 mg/mL. The total dose of DXM was 8 mg for perineural use, 8 mg DXM IV was injected to all the patients in Gr 2 immediately before start of the procedure. The volume of LA and/or LA/DXM admixture (for patients in GR 1) was as follows: for popliteal sciatic nerve block-15 mL, for femoral nerve block-15 mL, for obturator nerve block-10 mL (5 mL for each branch, i.e. anterior and posterior branches). Lateral femoral cutaneous nerve in all patients was blocked by injection of 5 mL of lidocaine 10 mg/mL. Block of the lateral femoral cutaneous nerve is important during the surgery, but less than in the postoperative period, because it does not innervate the knee joint [5-7]. The same volume of LA was used in G2 and 3.
All the US-guided blocks were conducted using previously published techniques [32-38]. The success of the sensory and motor blockade was examined 30 min. after completion of the block as described in our previous trial [4]. The sensory function of each of the blocked nerve was evaluated using pinprick as described by Marhofer et al. [39] The tip of a 22-G short beveled needle was applied with force adequate to indent the skin but not enough to puncture it. This action produced a painful sensation on the unblocked side and was compared with the similar test in the contralateral (blocked) side.
Gradation of the sensory block was scored as follows: Grade 1- Intact sensation. i.e. absence of sensory block; Grade 2-Dull sensation (analgesia); Grade 3- No sensation (anesthesia). Lateral femoral cutaneous is a sensory nerve only, and the effectivity of the block was examined by evaluation of the sensation in the appropriate area (lateral side of the thigh).
The power of the motor block was examined about each blocked nerve separately:
• Both branches of the sciatic nerve were examined separately 30 min. after finishing the popliteal sciatic nerve block performing. The presence of the tibial nerve block was examined using loss in flexion of the toes and foot. The availability of the common peroneal nerve was observed using presence of the foot that drops, and unable to hold the foot up.
• Inability to extend affected leg with hip passively flexed is a sign of motor block of femoral nerve.
• Adduction ability of the previous abducted lower limb characterizes the obturator nerve block.
Gradation of the motor block was scored as follow: Grade 1-Normal motor function, i.e. absence of motor block; Grade 2-Reduced possibility of flexion/extension movement in the knee joint (paresis); Grade 3-Loss of possibility of flexion/extension movement in the knee joint (paralysis).
Patients were additionally excluded from the study if a failed sensory or motor block was diagnosed (i.e. if normal sensation and/or normal motor function were observed) of one or more nerves.
If a different grade of sensory or motor block was determined about different nerves-for example, Grade 2 sensory block of the femoral nerve and Grade 3 sensory block of the sciatic nerve were determined during evaluation of patient 24 hours after surgery-than the higher grade of block was considered and inserted in protocol chart (i.e. Grade 3 sensory block in our example case). 8 mg IV ondansetron was injected before general anesthesia induction as prophylaxis for postoperative nausea and vomiting [40].
A standardized general anesthesia (GA) protocol was used for all patients: for induction to GA midazolam 0.015 mg/kg, fentanyl 1.5 μg/kg, propofol titration up to effect and atracurium 0.6 mg/kg was injected IV with following tracheal intubation and mechanical ventilation. Maintenance of anesthesia was left to the discretion of the attending anesthesiologist (N2O/O2 and isoflurane admixture, fentanyl IV). The anesthetic regiment was changed according to patient hemodynamic status. At the beginning of the surgery finger stick blood glucose was measured, as well as two hours after DXM injection (the peak effect of DXM about the elevation of the blood glucose concentration [30,31]) perineurally or systemically in Gr 1 and 2 respectively. All operations were performed by or under the supervision of the same experienced orthopedic surgeons (R.S. and M.T.).
Variables assessed and compared statistically between groups before and during surgery included
• Patients characteristics (age, gender, height (h), body weight (BW). Body mass index (BMI) was calculated using the following formula: BMI=BW (kg)/h2 (m2); Preoperative level of glucose concentration in blood (mg/dL); Preoperative pain at rest and during motion (flexion, extension) was measured using 0 to 10 NRS. Zero represents “no pain at all” whereas the upper limit represents “the worst pain ever possible” [29].
• Time of blocks performing (min); Correlation between BMI and time of block performing; Doses of induction agents: fentanyl (μg/ kg), midazolam (mg/kg), propofol (mg/kg); Duration of surgery; Registration of intraoperative events.
• If oxygen saturation decreased during surgery, or bradycardia, low blood pressure, or other events persisted for more than 2-3 min., the patient should be excluded from the study. The aim of this exclusion is to remove potential temporary intraoperative brain tissue oxygen desaturation which possible influence mental state [41] and pain intensity estimation during the postoperative period when assessed by NRS.
Variables assessed in PACU included
• Intensity of pain at rest in the PACU was measured using the modified nurses’ assessment of postoperative pain scale (NP): 0-no pain or patient asleep; 1-mild pain or discomfort; 2-moderate pain; 3-severe pain; 4-intolerable pain [42].
• Sensory block. Calculation of ratio Nint. sens: (Nanalg + Nanesth) with following comparison between groups.
• Nint sens-number of patients with intact sensation in group (Grade 1 sensory block); Nanalg-number of patients with analgesia (Grade 2 sensory block); Nanesth-number of patients with anesthesia (Grade 3 sensory block).
• Motor block. Calculation of ratio Nnmf: (Nparesis + Nparalysis) with following comparison between groups.
• Nnmf-number of patients with normal motor function in the group (Grade 1 motor block); Nparesis-number of patients with paresis (Grade 2 motor block); Nparalysis-number of patients with anesthesia (Grade 3 motor block).
• Opioids consumption (in mg of morphine IV or IM after conversion). The doses conversion (calculation) of opioids, used in PACU and during hospitalization of the patients in the orthopedic department following 48 hours after surgery was performed by calculator on internet [43]. This equianalgesic conversion were based on American Society guidelines and critical review papers regarding equianalgesic dosing [43-47].
• Time of stay in PACU (min). The discharge criteria from the PACU were based on the revised Aldrete Scoring System [48].
• The glucose concentration in blood (mg/dL).
Variables assessed 12, 24, 36, 48 hours postoperatively included
• Intensity of pain at rest and during motion (flexion, extension) (NRS); The grades of sensory and motor block were evaluated and compared by the same methods that were used in PACU (see above evaluation of that parameters in PACU).
• Opioids consumption (in mg of morphine IV or IM after conversion).
• Glucose concentration in blood (mg/dL).
• Satisfaction with pain relief during postoperative period (this parameter was registered 48 hours after surgery according NRS principle).
Additional variable calculated and compared statistically among groups is ‘Comfort time’, i.e. time in hours after block performing and first time use of rescue any analgesics, like dipyrone (metamizole), paracetamol (acetaminophen), non-steroidal anti-inflammatory drugs (ketorolac, diclofenac), tramadol, opioids (morphine, meperidine, oxycontin).
Postoperative variables as intensity of pain at rest and during motion, grade of sensory and motor block, opioid consumption, as well as comfort time are primary end-points of our investigation. A priori power analysis was calculated for these variables.
Secondary end-points are correlated between blocks performing time and BMI, nausea and vomiting in PACU, PACU time of stay, immediately diagnosed complications of MNB, neurological deficit a month after surgery and MNB (if present, repeated examination should be performed three months after surgery), satisfaction with pain relief during the postoperative period.
Statistical analysis
A priori power analysis of primary end-points was performed using G* Power 3.0.10 (Heinrich Heine Universität Düsseldorf, Dusseldorf, Germany) with a fixed effect, omnibus, one-way ANOVA for all groups. A total sample size of 66 was considered adequate to achieve an effect size of 0.5 with an α error probability of 0.05 and a power (1-β error probability) of 0.95. After all exclusions, our study included 90 patients. Statistical analysis was performed using IBM® SPSS® Statistics 20 (IBM Corporation, Armonk, NY).
Continuous numerical parameters were analyzed using Shapiro- Wilk test for normality distribution, followed by the Levene test for homogeneity of variances (if a normal distribution was determined). Parameters with a normal distribution and homogeneous variances were compared by one-way ANOVA followed by Tukey’s post-hoc test, if necessary. The Kruskal-Wallis test was used when an abnormal distribution of the continuous variables was detected and/or when variances were not homogenic. The Mann-Whitney post-hoc test was used following the Kruskal-Wallis test, if necessary. Frequency tables and chi-square tests (Pearson or Likelihood ration) were used to compare the proportions between the categorical variables among the groups.
The interval “Comfort time” was evaluated by construction of Kaplan-Meier curves, followed by the Log Rank (Mantel-Cox) test for comparison the Kaplan-Meier curves between groups. A value of P<0.05 was considered statistically significant.
Results
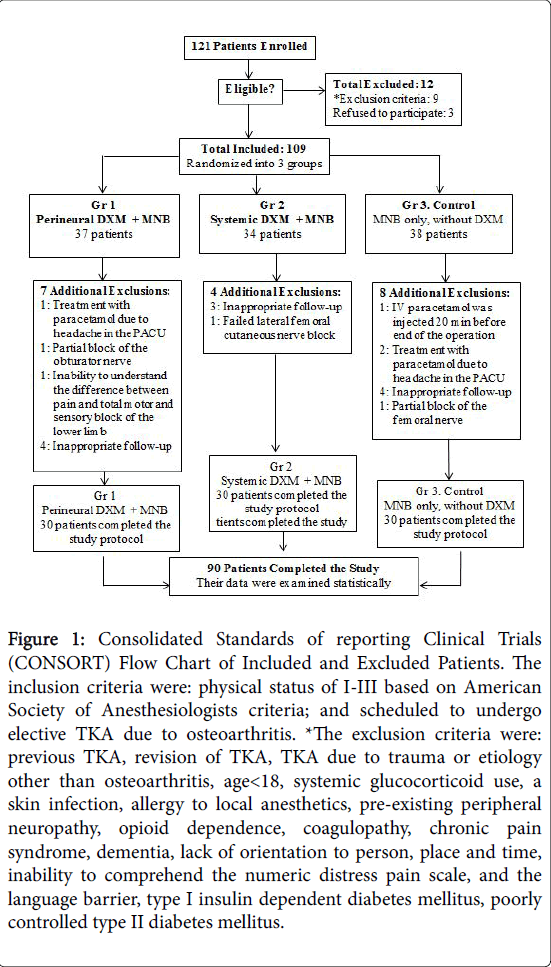
From February 2015 to October 2016, 121 patients were eligible for the trial. Twelve were excluded according to exclusion criteria or refusal to participate. Figure 1 presents the allocation process according to Consolidated Standards of reporting Clinical Trials (CONSORT) statement. A total of 109 patients were included and randomized into three groups, 90 patients completed the study protocol, and that data were analyzed statistically (Figure 1).
Figure 1: Consolidated Standards of reporting Clinical Trials (CONSORT) Flow Chart of Included and Excluded Patients. The inclusion criteria were: physical status of I-III based on American Society of Anesthesiologists criteria; and scheduled to undergo elective TKA due to osteoarthritis. *The exclusion criteria were: previous TKA, revision of TKA, TKA due to trauma or etiology other than osteoarthritis, age< 18, systemic glucocorticoid use, a skin infection, allergy to local anesthetics, pre-existing peripheral neuropathy, opioid dependence, coagulopathy, chronic pain syndrome, dementia, lack of orientation to person, place and time, inability to comprehend the numeric distress pain scale, and the language barrier, type I insulin dependent diabetes mellitus, poorly controlled type II diabetes mellitus.
Pre and intraoperative variables were comparable between groups (Table 1). Correlation between BMI and block performing time was not detected. No patients were suffered from nausea and vomiting in PACU.
| Gr 1 Perineural DXM | Gr 2 Systemic DXM | Gr 3 Control Without DXM | P ANOVA | P Kruskal Wallis | P Chi-Square | |
|---|---|---|---|---|---|---|
| *Age (years) | 67.93 ± 1.76 | 70.00 ± 1.46 | 66.00 ± 1.24 | 0.18 | ||
| *Height (m) | 1.64 ± 0.012 | 1.64 ± 0.01 | 1.62 ± 0.02 | 0.52 | ||
| *BW (kg) | 83.95 ± 2.60 | 83.37 ± 3.47 | 83.47 ± 2.55 | 0.99 | ||
| *BMI kg/m2 | 31.16 ± 0.81 | 31.00 ± 1.22 | 33.00 ± 1.07 | 0.59 | ||
| §Gender M:F | 8:22 | 10:20 | 6:24 | 0.51 | ||
| +Glucose (mg/dL) | 112.54 ± 5.8 | 106.32 ± 6.52 | 128.46 ± 7.19 | 0.052 | ||
| +Pain at Rest (NRS) | 1.67 ± 0.34 | 1.50 ± 0.31 | 1.13 ± 0.24 | 0.47 | ||
| +Pain at motion (NRS) | 5.23 ± 0.32 | 5.2 ± 0.40 | 4.8 ± 0.33 | 0.86 | ||
| +Block Performing time (min) | 23.5 ± 1.29 | 25.33 ± 1.51 | 23.83 ± 1.60 | 0.65 | ||
| +Ondansetron (mg/kg) | 0.1 ± 0.003 | 0.1 ± 0.004 | 0.1 ± 0.003 | 0.80 | ||
| *Time of GA (min) | 120.4 ± 3.97 | 131.1 ± 3.57 | 126.2 ± 3.74 | 0.14 | ||
| +Time of surgery | 93.57 ± 3.90 | 100.6 ± 3.93 | 102.67 ± 3.13 | 0.13 | ||
| +Fentanyl (µg/kg) | 1.64 ± 0.13 | 5.05 ± 3.28 | 1.51 ± 0.90 | 0.14 | ||
| +Propofol (mg/kg) | 1.85 ± 0.09 | 2.23 ± 0.45 | 1.60 ± 0.15 | 0.82 | ||
| All values are presented as mean ± SE (Standard Error). *Age, height, BW, and BMI were of normal distribution (Shapiro-Wilk test) and homogenous of variances (Levene test). These variables were analyzed using ANOVA test for comparison of means between three groups. P>0.05 means no difference between the groups. P>0.05 means no difference between the groups. +These variables were of abnormal distribution (Shapiro-Wilk test). Therefore, the Kruskal-Wallis test was used for comparison of continuous variables between three independent groups. The Mann-Whitney post-hoc test was used following the Kruskal-Wallis test, if necessary. §Chi-Square test was used to compare the proportion of Male: Female between groups. P>0.05 means no difference between groups. NRS - Numeric Rating Scale 0-10. |
||||||
Table 1: Pre and intraoperative data.
No suspected unexpected serious adverse reactions and/or complications were reported and judged as definitely related to trial treatment. Five patients in Gr 1 and seven in Gr 2 with well treated type 2 DM [30] were randomized for injection of DXM perineurally or systemically. The differences between preoperative levels of glucose were not statistically significant among groups. No patients in either arm developed new onset diabetes.
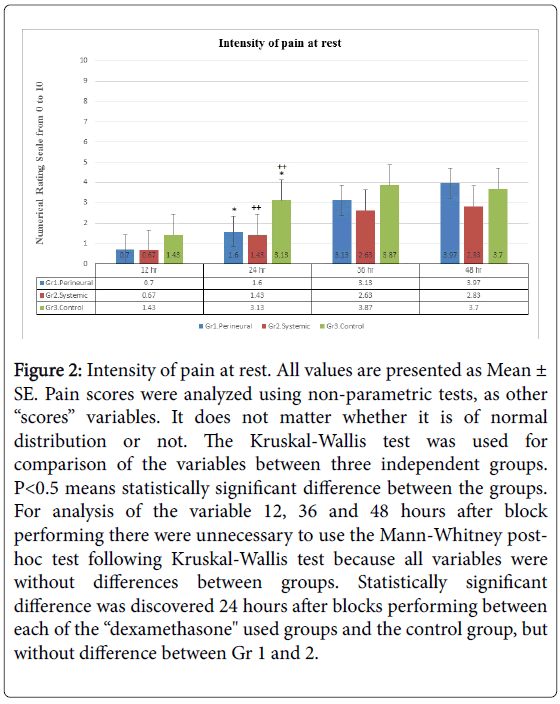
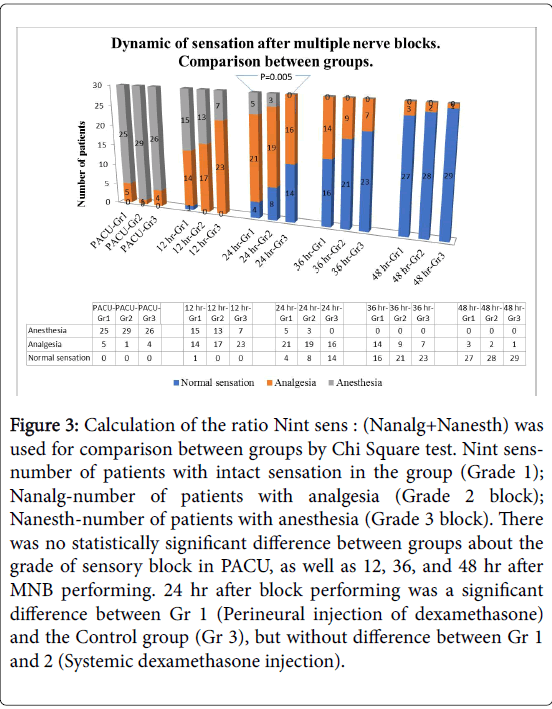
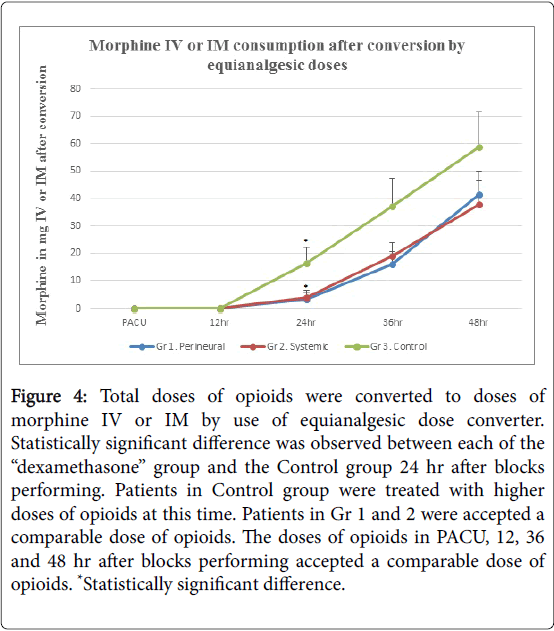
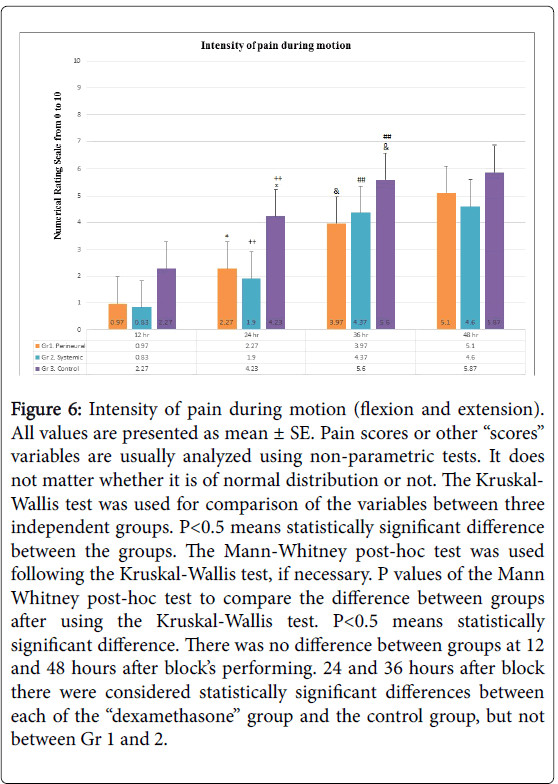
Intensity of pain at rest (Figure 2), the grade of sensory block (Figure 3) and opioid consumption (Figure 4): statistically significant differences between each of the “dexamethasone” group (Gr 1 and 2) versus Control group (Gr 3) was observed 24 hours after block performing, but no difference between Gr 1 and 2. There were no differences between groups 36 and 48 hours post-block.
Figure 2: Intensity of pain at rest. All values are presented as Mean ± SE. Pain scores were analyzed using non-parametric tests, as other “scores” variables. It does not matter whether it is of normal distribution or not. The Kruskal-Wallis test was used for comparison of the variables between three independent groups. P< 0.5 means statistically significant difference between the groups. For analysis of the variable 12, 36 and 48 hours after block performing there were unnecessary to use the Mann-Whitney posthoc test following Kruskal-Wallis test because all variables were without differences between groups. Statistically significant difference was discovered 24 hours after blocks performing between each of the “dexamethasone" used groups and the control group, but without difference between Gr 1 and 2.
Figure 3: Calculation of the ratio Nint sens : (Nanalg+Nanesth) was used for comparison between groups by Chi Square test. Nint sensnumber of patients with intact sensation in the group (Grade 1); Nanalg-number of patients with analgesia (Grade 2 block); Nanesth-number of patients with anesthesia (Grade 3 block). There was no statistically significant difference between groups about the grade of sensory block in PACU, as well as 12, 36, and 48 hr after MNB performing. 24 hr after block performing was a significant difference between Gr 1 (Perineural injection of dexamethasone) and the Control group (Gr 3), but without difference between Gr 1 and 2 (Systemic dexamethasone injection).
Figure 4: Total doses of opioids were converted to doses of morphine IV or IM by use of equianalgesic dose converter. Statistically significant difference was observed between each of the “dexamethasone” group and the Control group 24 hr after blocks performing. Patients in Control group were treated with higher doses of opioids at this time. Patients in Gr 1 and 2 were accepted a comparable dose of opioids. The doses of opioids in PACU, 12, 36 and 48 hr after blocks performing accepted a comparable dose of opioids. *Statistically significant difference.
Pain intensity at rest in PACU (NP scale) [42] was very low (Table 2) in patients of all three groups. Patients in Gr 1 were free from pain. Statistical difference of this parameter was calculated between groups, but it was of low clinical significance (Table 2). The patients in PACU were not treated with any analgesics. Lack of sensation (i.e. analgesia or anesthesia) and lack of normal motor function (i.e. paresis or paralysis) of the blocked lower limb was observed in all patients in all three groups in PACU (Figures 3 and 5). There were no patients in the PACU that suffered from nausea and or vomiting.
| Gr 1 Perineural DXM | Gr 2 Systemic DXM | Gr 3 Control Without DXM | P Shapiro-Wilk test | P Kruskal Wallis test | P Mann-Whitney | |||
|---|---|---|---|---|---|---|---|---|
| 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | ||||||
| Pain intensity at rest (NP scale) in PACU | 0.00 ± 0.00 | 0.14 ± 0.07 | 0.53 ± 0.18 | 0.01 | 0.01 | 0.04 | 0.03 | 0.15 |
| Time of stay in PACU (min) | 55.9 ± 4.24 | 54.9 ± 5.01 | 59.4 ± 4.79 | Gr 1-0.01 | 0.75 | |||
| Gr 2-0.00 | ||||||||
| Gr 3-0.01 | ||||||||
| All values are presented as mean ± SE. Pain intensity in PACU was measured by modified NP scale: 0-no pain or the patient is asleep; 1-mild pain or discomfort; 2-moderate pain; 3-severe pain; 4-intolerable pain. Time of stay in PACU was a parameter of abnormal distribution (Shapiro-Wilk test) in each group (P>0.05 means normal distribution). | ||||||||
Table 2: PACU data.
Figure 5: Calculation of the ratio Nnmf : (Nparesis+Nparalysis) was used for comparison between groups by Chi-Square test. Nnmfnumber of patients with normal motor function in the group (Grade 1 motor block); Nparesis-number of patients with paresis (Grade 2 motor block); Nparalysis-number of patients with anesthesia (Grade 3 motor block). There was no statistically significant difference between groups about the grade of motor block in PACU, as well as 12, 24, 36, and 48 hr after MNB performing.
Blood glucose concentration in PACU was elevated in all 12 patients with type 2 DM from Gr 1 and 2 in comparison with preoperative values, as well as in all non-diabetic patients. An elevated level of glucose in PACU (2-3 hours after DXM use before or during MNB performing) expressed in per-cent of preoperative values was 16-39%. Additional medicamentous treatment for decrease the blood glucose concentration was not needed.
Very low parameters of the intensity of pain at rest and during motion (Figures 2 and 6), as well as high grade of sensory and motor block (Figures 3 and 5) were observed after discharge from PACU and up to 12 hours after performing MNB in all three groups. Patients in Gr 1 and 2 were not treated with opioids, three patients in control group accepted opioids: two patients accepted morphine 10 mg IM total dose, and one was treated with oxycodone 10 mg (4 mg of morphine IV or IM is an equianalgesic dose).
Figure 6: Intensity of pain during motion (flexion and extension). All values are presented as mean ± SE. Pain scores or other “scores” variables are usually analyzed using non-parametric tests. It does not matter whether it is of normal distribution or not. The Kruskal- Wallis test was used for comparison of the variables between three independent groups. P< 0.5 means statistically significant difference between the groups. The Mann-Whitney post-hoc test was used following the Kruskal-Wallis test, if necessary. P values of the Mann Whitney post-hoc test to compare the difference between groups after using the Kruskal-Wallis test. P< 0.5 means statistically significant difference. There was no difference between groups at 12 and 48 hours after block’s performing. 24 and 36 hours after block there were considered statistically significant differences between each of the “dexamethasone” group and the control group, but not between Gr 1 and 2.
In the 24 and 36 hours post-block, the same manner of differences was observed about the variable “intensity of pain during motion” (Figure 6). The same spread of restoration of motor function was observed in all patients in all three groups after block (Figure 5). A significant increase of the doses of opioids use was observed in all patients in all three groups between 24 to 36 hours post-block (Figure 4).
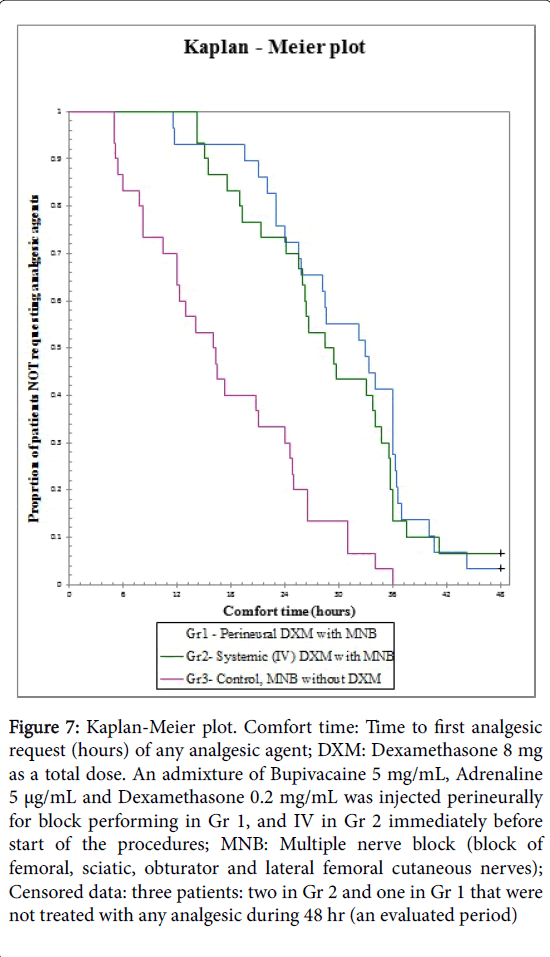
Kaplan-Meier curves [25,49] were constructed for comfort time presentation (time from end of the blocks performing and up to first analgesic request), with following compare between groups, with patients not receiving any analgesics after 48 hours censored to the right (Figure 7).
Figure 7: Kaplan-Meier plot. Comfort time: Time to first analgesic request (hours) of any analgesic agent; DXM: Dexamethasone 8 mg as a total dose. An admixture of Bupivacaine 5 mg/mL, Adrenaline 5 μg/mL and Dexamethasone 0.2 mg/mL was injected perineurally for block performing in Gr 1, and IV in Gr 2 immediately before start of the procedures; MNB: Multiple nerve block (block of femoral, sciatic, obturator and lateral femoral cutaneous nerves); Censored data: three patients: two in Gr 2 and one in Gr 1 that were not treated with any analgesic during 48 hr (an evaluated period)
Log Rank (Mantel-Cox) test was used for comparison of the Kaplan-Meier curves between groups (Table 3). P<0.05 was considered statistically significant.
| P | |
| Gr1 (Perineural DXM) vs. Gr 2 (Systemic DXM) | 0.64 |
| Gr1 (Perineural DXM) vs. Gr 3 (Control) | < 0.001 |
| Gr 2 (Systemic DXM) vs. (Control) | <0.001 |
| Statistically significant difference was observed between each of the "dexamethasone" group and the Control group, but without difference between Gr 1 and 2. | |
Table 3: Log Rank (Mantel-Cox) test.
Patients in Control group suffered from pain at rest and started treatment by any analgesics earlier than patients from two “dexamethasone” groups: significant differences (Log Rrank, i.e. Mantel-Cox test, that is a special form of Chi-Square test) were obtained between Gr 3 (Control group) and each of the “dexamethasone” groups, i.e. Gr 1 and 2 (P<0.0001). No difference was observed between Gr 1 and 2 (P=0.64) as seen in the graph (Figure 7).
There were no observed neurological complications 48 hour postblocks and surgery in all patients in all three groups. No differences were observed between the groups about a satisfaction with pain relief during first 48 hours of the postoperative period.
Our results of the secondary end-points are as follow:
• The correlation between BMI in the range of 24-37 kg/m2 and time of block performing was absent.
• Time of stay in PACU was similar in all three groups.
• Five patients from Gr 1 and seven from Gr 2 that suffered from well controlled type 2 DM were included in the study. The cause of this inclusion is the study of Hans et al. [25]. In those 12 patients the blood glucose elevation was picked two hours post-block at the level of 16-39%, the additional treatment was unnecessary.
Discussion
We used preoperative single-shot MNB as part of surgical anesthesia and management of postoperative pain following TKA [4]. One of significant limitation of this excellent method of anesthesia and analgesia “is its relatively short effect” [4], usually lasting less than 24 hours, even when applied a long-acting local anesthetic bupivacaine with adjuvant as adrenaline [4]. The use of additional adjuvants were approbated in clinical practice with the aim of prolongation duration of the single shot PNB, for example clonidine [50], opioids [51], midazolam [52], ketamine [53] with little effect. DXM was evaluated and appears as effective agent for prolongation of the duration of PNB in preclinical [23,24], and clinical investigations [13-19,25,26]. Most of the previous studies were focused on clarifying the effects of DXM as a local anesthetic adjuvant for brachial plexus block [14,16], but even after a systemic review and meta-analysis the authors were concluded, that “Perineural administration of dexamethasone with LA prolongs brachial plexus block effects with no observed adverse events. The effects of systemic administration of dexamethasone on brachial plexus block must be investigated” [14]. Desmet et al. [25] did not find difference between IV or perineural effect of DXM about the increasing the analgesic duration of a single-shot interscalene block with ropivacaine, but opposite result was accepted by Kawanishi et al. [26] The results of Kawanishi et al. should be accepted carefully, because this study is underpower. From the previous trial, it is known that DXM prolongs the duration of bupivacaine used interscalene nerve block up to 22 hours of analgesia [54].
We compared the effect of IV and perineural DXM as an adjuvant to bupivacaine/adrenaline admixture on six variables (primary end points of the study) that represent a duration of MNB: sensation, motor function, intensity of pain at rest and during motion (flexion and extension), comfort time and opioid consumption. No one has investigated this specific issue previously. No statistically significant differences were observed between Gr 1 (perineural DXM) and Gr 2 (systemic DXM) of all primary end-points variables, not immediately after operation in the PACU, and not during 48 hours of the follow up after surgery. The differences were observed between each of the “dexamethasone” groups and Gr 3 (Control group) 24 hours after blocks about the intensity of pain at rest and during motion and opioid consumption. The difference of the comfort time between Gr 3 and two “dexamethasone” groups was observed early, already 6 hours postblock (Figure 7). This is a main point of the study, because it proves that adjuvant DXM independently of route of administration prolong an analgesic property of MNB, that was used by bupivacaine/ adrenaline admixture during more than 24 hours, but usually no more than 36 hours (Figures 2-7). The same block without DXM produces analgesia usually less than 24 hours, and only single patients do not need the appointment of analgesics within 36 hours (Figures 2-7). The differences between “dexamethasone” groups and the Control group were present 36 hours after blocks performing about the intensity of pain during motion (Figure 6). 36 hours after blocks performing all the patients in Control group was treated with any analgesics (Figure 7, Kaplan-Meier plot). All primary end-points parameters were nominally low in patients from Gr 1 and 2 versus Gr 3, but statistically significant difference was observed usually 24 hours post-blocks (Figures 2,4 and 6), except the comfort time (Figure 7).
DXM that used perineurally or IV as an adjuvant to MNB slows recovery speed of sensory and motor function of the blocked peripheral nerves. The significant “leap” was observed between 24 and 36 hours post-block (Figures 3 and 5). In the same period of time an increase of intensity of pain at rest and during motion, and opioid consumption was registered (Figures 2,4 and 6). Thus, the desired effect of DXM (independently from the route of administration) was observed in the period of 12-24 hours and went flat-out from 24 to 36 hours post-block.
The mechanism of action of DXM on peripheral nerve during perineural injection is unknown [13-15]. The scattered experimental data do not allow to combine them into an integral theory explaining this phenomenon [20-24]. Systemic anti-inflammatory action of DXM is well known, and this agent is recommended as one of drugs that is commonly used in multimodal pain control method in knee arthroplasty [55]. Systemic DXM decreases pain during motion after total hip arthroplasty [56], reduce pain and emesis after TKA [57]. Consequently, the administration of 8 mg DXM IV immediately before the use of MNB can be recommended in several reasons: one of the agents of multimodal pain control method, prolongation of MNB, which is the best method of preemptive regional analgesia in comparison with FNB alone [4], as an antiemetic drug [57] etc.
As in our previous studies [4,58,59], US-guided technique makes it possible to perform PNB fairly quickly in patients with moderate obesity. Appropriate pain relief after MNB immediately after surgery reduces time of stay in PACU up to minimum.
Persistent nerve palsy during two weeks as a complication associated with brachial plexus blocks with local anesthetics and dexamethasone as an adjuvant, was registered in the studies of Cummings et al. [54] and Parrington et al. [60] Desmet et al. only reported about one patient from the seria of 144 ones that suffered from hypoesthesia in the deltoid region during four months after surgery, and cervical disc herniation was diagnosed at the level of C4- C5 and this factor may be an etiology of this long neurologic deficit, but not the interscalene block [25]. In our small seria persistent neurological deficit were not diagnosed after MNB.
Limitations of the Study
Measurement bias
Part of data on the PNB (sensory and motor function, intensity of pain at rest and during motion) were obtained once every 12 hours post-block and surgery. More frequent registration of these parameters (for example every few hours) can allow more accurately determine the dynamic of their (parameters) changed.
We used two scales for pain intensity measurement, the modified NP scale [42] in the PACU and NRS after discharge from PACU [29]. Readers should note this difference in the presented results.
We used a moderate dose of DXM as an adjuvant to LA, 8 mg (approximately 0.1 mg/kg). In the literature, different recommendations are presented about the doses of DXM for perineural use with ropivacaine or bupivacaine for PNB [13-19,25,26,54,55,60]. Our results and conclusions should be considered only with reference to 8 mg of DXM. The duration of action of MNB depending on the dose of DXM used systemically or perineurally makes sense to investigate in the future.
There are no unambiguous recommendations in literature about safety use of DXM (systemically or perineurally) for prolongation the duration of MNB or other PNB with bupivacaine or ropivacaine in patients with uncontrolled type 2 DM, insulin dependent DM (type 2 and type 1). While the safety of the use of DXM in these patients has not been investigated and generally accepted recommendations in this regard are absent, one should refrain from applying to use this method in those patients.
General anesthesia was used in all patients in our trial. Fentanyl before induction was used to blunt the hemodynamic response to intubation. We think than this short-acting agent did not influence pain sensation immediately and 12 hours post-op.
Even a single dose of 8 mg DXM can lead to a number of side effects:
• Perioperative hyperglycemia due to whole body insulin resistance with following alters of cardiac fatty acid and carbohydrate metabolism [61]. Monitoring of electrocardiography, heart rate, blood pressure, arterial blood gases, blood glucose concentration, urine analysis (glucose, ketones) is recommended in patients with impaired cardiac function, obesity [62] and DM.
• Gastric ulceration [63].
• Tumor lysis syndrome. Case report of perioperative death after single dose of DXM causing this syndrome in a three-year-old during tonsillectomy was published [64]. Rapid lysis of lymphocytes with following rapid acute hyperpotassemia, hyperphosphatemia, lactic acidosis and acute renal failure may occur [65].
• Psychiatric effects: mania, psychosis, depression and more [66].
Bartlett and Hartle recommend in Editorial article: "all patients receiving dexamethasone should be warned of its potential side-effects. Steroids are powerful agents and should be used with caution [67]."
Conclusion
Eight milligrams intravenous dexamethasone is equivalent to the same dose of perineural dexamethasone in prolonging the pain relief duration of a single injection multiple nerve blocks with bupivacaine 5 mg/mL and adrenaline 5 μ/mL after total knee arthroplasty. The comfort time was prolonged, intensity of pain at rest and during motion, as well as opioids consumption were low during 24 hours after block production in comparison with patients that accepted multiple nerve block without adjuvant dexamethasone. In the period between 24 and up to 36 hours the effect of blocks (i.e. the effect of local anesthetic with adjuvant dexamethasone) is gradually weakening and somewhere in the period of 48 hours it passes almost completely. The same blocks that were performed without adjuvant dexamethasone start to be weak significantly early, in the period of 6 to 12 hours. Not only the dynamics of purely subjective parameters (intensity of pain at rest and during motion, opioid consumption and comfort time), but also more objective variables, such as time of recovery of sensory and motor function after multiple nerve blocks confirm this conclusion. We recommend to take into account our results when prescribing an analgesic agent after total knee arthroplasty, preoperative use of multiple nerve blocks with or without adjuvant dexamethasone must be taken into account. In moderately obese patients the time of USguided multiple nerve blocks performing is similar compared to not obese. As adjuvant to local anesthetic bupivacaine with adrenaline, dexamethasone does not affect the time of stay in the PACU. During 48 hour post-op there was not diagnosed any neurological complications in our seria. We agree with Bartlett and Hartle. If the patient is going to accept multiple nerve blocks with adjuvant dexamethasone systemically or perineurally, it should be informed about potential side effects of dexamethasone.
Acknowledgement
The authors would like to acknowledge the nursing staff of the PACU of Hillel Yaffe Medical Center for practical assistance when performing blocks; the residents of the Department of Anesthesiology for practical assistance in data collection, especially intraoperative data; the nursing staff of the Department of Orthopedics for their assistance in data collection during the first two postoperative days.
Registration: ClinicalTrials.gov PRS.NCT02253784. Protocol registration receipt 10/01/2014
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- Slover J, Riesgo A, Payne A, Umeh U (2014) Modern anesthesia for total joint arthroplasty. Ann OrthopRheumatol 2: 1026.
- Danninger T, Opperer M, Memtsoudis SG (2014) Perioperative pain control after total knee arthroplasty: An evidence based review of the role of peripheral nerve blocks. World J Orthop 5: 225-232.
- American Society of Anesthesiologists Task Force on Acute Pain Management (2012) Practice guidelines for acute pain management in the perioperative setting: An updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 116: 248-273.
- Stav A, Reytman L, Sevi R, Stav MY, Powell D, et al. (2017) Femoral versus multiple nerve blocks for analgesia after total knee arthroplasty. Rambam Maimonides Med J 8: e0006.
- Horner G, Dellon AL (1994) Innervation of the human knee joint and implications for surgery. ClinOrthopRelat Res 301: 221-226.
- Moore DC (1981) Block of the lateral femoral cutaneous nerve. In: Moore DC, editors. Regional block. (4thedn.) Illinois: Charles C Thomas; pp: 294-299.
- Kim JH, Cho MR, Kim SO, Kim JE, Lee DK, et al. (2012) A comparison of femoral/sciatic nerve block with lateral femoral cutaneous nerve block and combined spinal epidural anesthesia for total knee replacement arthroplasty. Korean J Anesthesiol 62: 448-453.
- Ilfeld BM, Duke KB, Donohue MC (2010) The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. AnesthAnalg 111: 1552-1554.
- Widmer B, Lustig S, Scholes CJ, Molloy A, Leo SP, et al. (2013) Incidence and severity of complications due to femoral nerve blocks performed for knee surgery. Knee 20: 181-185.
- Ng FY, Chiu KY, Yan CH, Ng KF (2012) Continuous femoral nerve block versus patient-controlled analgesia following total knee arthroplasty. J OrthopSurg (Hong Kong) 20: 23-26.
- Capdevila X, Bringuier S, Borgeat A (2009) Infectious risk of continuous peripheral nerve blocks. Anesthesiology 110: 182-188.
- Cuvillon P, Ripart J, Lalourcey L, Veyrat E, L'Hermite J, et al. (2001) The continuous femoral nerve block catheter for postoperative analgesia: Bacterial colonization, infectious rate and adverse effects. AnesthAnalg 93: 1045-1049.
- Kirksey MA, Haskins SC, Cheng J, Liu SS (2015) Local anesthetic peripheral nerve block adjuvant for prolongation of analgesia: A systemic qualitative review. PLoS One 10: e0137312.
- Choi S, Rodseth R, McCartney CJL (2014) Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: A systematic review and meta-analysis of randomized trials. Br J Anaesth 112: 427-439.
- De Oliveira GS Jr, Alves LJC, Nader A, Kendall MC, Rahangdale R, et al. (2014) Perineural dexamethasone to improve postoperative analgesia with peripheral nerve blocks: A meta-analysis of randomized controlled trials. Pain Res Treat 2014:179029.
- Albrecht E, Kern C, Kirkham R (2015) A systemic review and meta-analysis of perineural dexamethasone for peripheral nerve blocks. Anaesthesia 70: 71-83.
- Knezevic NN, Anantamongkol U, Candido KD (2015) Perineural dexamethasone added to local anesthesia for brachial plexus block improves pain but delays block onset and motor blockade recovery. Pain Physician 18: 1-14.
- Fredrickson FMJ, Danesh-Clough TK, White R (2013) Adjuvant dexamethasone for bupivacaine sciatic and ankle blocks: Results from 2 randomized placebo-controlled trials. RegAnesth Pain Med 2013; 38: 300-307.
- YaDeau JT, Paroli L, Fields KG, Kahn RL, LaSala VR, et al. (2015) Addition of dexamethasone and buprenorphine to bupivacaine sciatic nerve block: A randomized controlled trial. RegAnesth Pain Med 40(4): 321-329.
- Devor M, Govrin-Lippmann R, Raber P (1985) Corticosteroids suppress ectopic neural discharge originating in experimental neuromas. Pain 22: 127-137.
- Attardi B, Takimoto K, Gealy R, Severns C, Levitan ES (1993) Glucocorticoid induced up-regulation of a pituitary K+ channel mRNA in vitro and in vivo. Receptors Channels 1: 287-293.
- Johansson A, Hao J, Sjolund B (1990) Local corticosteroid application blocks transmission in normal nociceptive C-fibres. ActaAnaesthesiolScand 34: 335-338.
- An K, Elkassabany NM, Liu J (2015) Dexamethasone as adjuvant to bupivacaine prolongs the duration of thermal antinociception and prevents bupivacaine-induced rebound hyperalgesia via regional mechanism in mouse sciatic block model. PLoS One 10: e0123459.
- Colombo G, Padera R, Langer R, Kohane DS (2005) Prolonged duration local anesthesia with lipid-protein-sugar particles containing bupivacaine and dexamethasone. J Biomed Mater Res A 75: 458-464.
- Desmet M, Braems H, Reynvoet M, Plasschaert S, Van Cauwelaert J, et al. (2013) I.V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: A prospective, randomized, placebo-controlled study. Br J Anaesth 111: 445-452.
- Kawanishi R, Yamamoto K, Tobetto Y, Nomura K, Kato M, et al. (2014) Perineural but not systemic low dose dexamethasone prolongs the duration of interscalene block with ropivacaine: A prospective randomized trial. Local RegAnesth 7: 5-9.
- American Society of Anesthesiologists (1963) New classification of physical status. Anesthesiology 24: 111.
- Malenka RC, Nestler EJ, Hyman SE (2009) Reinforcement and Addictive Disorders. In: Sydor A, Brown RY, editors. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2ndedn.) New York: McGraw-Hill Medical; pp: 364-368.
- Haefeli M, Elfering A (2006) Pain assessment. Eur Spine J 15: S17-S24.
- American Diabetes Association (2016) Standards of medical care in diabetes-2016: Summary of revisions. Diabetes Care. 39: S4-S5.
- Hans P, Vanthuyne P, Dewandre J, Brichant JF, Bonhomme V (2006) Blood glucose concentration profile after 10 mg dexamethasone in non-diabetic and type 2 diabetic patients undergoing abdominal surgery. Br J Anaesth 97: 164-170.
- Beek JV (2009) The Femoral Block. In:Beek JV, editors. The Neuraxiom Playbook of 9 Essential Blocks. A Handbook of Ultrasound Guided regional Nerve Blocks, 1st ed. Olympia, WA: Neuraxiom LLC; pp: 108-117.
- Fujiwara Y, Sato Y, Kitayama M, Shibata Y, Komatsu T, et al. (2007) Obturator nerve block using ultrasound guidance. AnesthAnalg 105: 888-889.
- Anagnostopoulou S, Kostopanagiotou G, Paraskeuopoulos T, Alevizou A, Saranteas T. Obturator nerve block: From anatomy to ultrasound guidance. AnesthAnalg 106: 350-351.
- Tsui BCH (2007) Popliteal Sciatic Nerve Block (Lateral Approach). In: Tsui BCH, editors. Atlas of Ultrasound and Nerve Stimulation Guided regional Anesthesia. New York: Springer Science & Business Media, LLC; pp: 200-203.
- Beek JV (2009) The Popliteal Sciatic Block. In: Beek JV, editors. The Neuraxiom Playbook of 9 Essential Blocks. A Handbook of Ultrasound Guided regional Nerve Blocks (1stedn.) Olympia, WA: Neuraxiom LLC; pp: 164-173.
- Hurdle MF, Weingarten TN, Crisostomo RA, Psimos C, Smith J (2007) Ultrasound-guided blockade of the lateral femoral cutaneous nerve: Technical description and review of 10 cases. Arch Phys Med Rehabil 88: 1362-1364.
- Tumber PS, Bhatia A, Chan VW (2008) Ultrasound-guided lateral femoral cutaneous nerve block for meralgiaparesthetica. AnesthAnalg 106: 1021-1022.
- Marhofer D, Karmakar MK, Marhofer P, Kettner SC, Weber M, et al. (2014) Does circumferential spread of local anaesthetic improve the success of peripheral nerve block? Br J Anaesth 113: 177-185.
- Tramer MR, Reynolds DJ, Moore RA, McQuay HJ (1997) Efficacy, dose-response, and safety of ondansetron in prevention of postoperative nausea and vomiting: A quantitative systemic review of randomized placebo-controlled trials. Anesthesiology 87: 1277-1289.
- Suehiro K, Okutai R (2011) Duration of cerebral desaturation time during single-lung ventilation correlates with mini mental state examination score. J Anesth 25:345-349.
- Chung IS, Sim WS, Kim GS, Park SH, Park YS, et al. (2001) Nurses’ assessment of postoperative pain: Can it be alternative to patients self-report? J Korean Med Sci 16: 784-788.
- Equivalent opioid calculator.
- Principles of analgesic use in the treatment of acute pain and cancer pain. (6thedn.) Glenview, IL: American Pain Society; 2008.
- Anderson R, Saiers JH, Abram S, Schlicht C (2001) Accuracy in equianalgesic dosing. conversion dilemmas. J Pain Symptom Manage 21: 397-406.
- Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E (2001) Equianalgesic dose ratios for opioids. a critical review and proposals for long-term dosing. J Pain Symptom Manage 22: 672-687.
- Patanwala AE, Duby J, Waters D, Erstad BL (2007) Opioid conversions in acute care. Ann Pharmacother 41: 255-266.
- Aldrete JA (1995) The post-anesthesia recovery score revisited. J ClinAnesth 7: 89-91.
- Gross A, Ziepert M, Scholz M (2012) KMWin -A convenient tool for graphical presentation of results from Kaplan-Meir survival time analysis. PLoS One 7: e38960.
- Duma A, Urbanek B, Sitzwohl C, Zimpfer M, Kapral S (2005) Clonidine as an adjuvant to local anaesthetic axillary brachial plexus block: A randomized, controlled study. Br J Anaesth 94: 112-116.
- Karakaya D, Buyukgoz F, Baris S, Guldogus F, Tur A (2001) Addition of fentanyl to bupivacaine prolongs anesthesia and analgesia in axillary brachial plexus block. RegAnesth Pain Med 26: 434-438.
- Jarbo K, Batra YK, Panda NB (2005) Brachial plexus block with midazolam and bupivacaine improves analgesia. Can J Anaesth 52: 822-826.
- Noyan A (2002) On effects of ketamine to axillary block in hand surgery. J ReconstrMicrosurg 18: 197.
- Cummings KC III, Napierkowski DE, Parra-Sanchez I, Kurz A, Dalton JE , et al. (2011) Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth 107: 446-453.
- Azam MQ, Sadat-Ali M, Bader A (2015) Pain management in knee arthroplasty: An overview. Int J Surg Res Pract 2: 035.
- Kardash KJ, Sarrazin F, Tessler MJ, Velly AM (2008) Single-dose dexamethasone reduces dynamic pain after total hip arthroplasty. AnesthAnalg 106: 1253-1257.
- Koh IJ, Chang CB, Lee JH, Jeon YT, Kim TK (2013) Preemptive low-dose dexamethasone reduces postoperative emesis and pain after TKA: A randomized controlled study. ClinOrthopRelat Res 471: 3010-3020.
- Stav A, Reytman L, Stav MY, Troitsa A, Kirshon M, et al. (2016) Transversusabdominis plane versus ilioinguinal and iliohypogastric nerve blocks for analgesia following open inguinal herniorrhaphy. Rambam Maimonides Med J 7: e0021.
- Stav A, Reytman L, Stav MY, Portnoy I, Kantarovsky A, et al. (2016) Comparison of the supraclavicular, infraclavicular and axillary approaches for ultrasound-guided brachial plexus block for surgical anesthesia. Rambam Maimonides Med J 7: e0013.
- Parrington SJ, O’Donnell D, ChanWW, Brown-Shreves D, Subramanyam R, et al. (2010) Dexamethsone added to mepivacaine prolongs the duration of analgesia after supraclavicular brachial plexus blockade. RegAnesth Pain Med 35: 422-426.
- Qi D, Pulinikunnil T, An D, Ghosh S, Abrahani A, et al. (2004) Single-dose dexamethasone induces whole-body insulin resistance and alters both cardiac fatty acid and carbohydrate metabolism. Diabetes 53: 1790-1797.
- Waldron NH, Jones CA, Gan TJ, Allen TK, Habib AS (2013) Impact of perioperative dexamethasone on postoperative analgesia and side-effects: Systemic review and meta-analysis. Br J Anaesth 110: 191-200.
- Bandyopadhyay U, Biswas K, Bandyopadhyay D, Ganguly CK, Banerjee RK (1999) Dexamethasone makes the gastric mucosa susceptible to ulceration by inhibiting prostaglandin synthetase and peroxidase-two important gastroprotective enzymes. Mol Cell Biochem 202: 31-36.
- McDonnell C, Barlow R, Campisi P, Grant P, Malkin D (2008) Fatal peri-operative acute tumourlysis syndrome precipitated by dexamethasone. Anaesthesia 63: 652-655.
- Khan F, Ayub S, Mehmood Q, Hussain SF (2017) Steroid-induced tumourlysis syndrome in small-cell lung cancer. Oxf Med Case Reports 2017: omx018.
- Warrington TP, Bostwick JM (2006) Psychiatric adverse effects of corticosteroids. Mayo ClinProc 81: 1361-1367.
- Bartlett R, Hartle AJ (2013) Routine use of dexamethasone for postoperative nausea and vomiting: the case against. Anaesthesia 68: 892-896.
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 4777
- [From(publication date):
July-2017 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 3891
- PDF downloads : 886