Perineural Invasion of Skin Cancers in the Head and Neck: An Uncommon Phenomenon Revisited
Received: 19-Mar-2014 / Accepted Date: 04-Apr-2014 / Published Date: 11-Apr-2014 DOI: 10.4172/2161-119X.1000169
Abstract
Objective: The purpose of this article is to describe the epidemiology, imaging findings, pathogenesis, and clinical impact of perineural invasion of skin cancers in the head and neck.
Conclusion: Perineural invasion in head and neck skin cancer can be microscopic disease discovered on pathology or gross perineural spread that can be predicted on imaging often accompanied with clinical symptoms. Physicians and radiologists should have high index of suspicion when evaluating with patient with skin cancer that is in close proximity to a cranial nerve. The advancement of imaging techniques has improved pre-operative detection of perineural invasion of skin cancer.
Keywords: Perineural invasion, Head and neck skin cancer, MRI
246355Introduction
Perineural Invasion (PNI) of tumor cells was first discovered by Cruveilhier in 1835, when he reported mammary carcinoma invading the facial nerve. In 1862, Neumann first described lower face skin cancer invading the mental nerve.
PNI describes a microscopic finding of tumor infiltration along a nerve, and is distinguished from Perineural Spread (PNS), which describes the presence of gross tumor growth along a nerve distinct from the main tumor mass on imaging. PNI is defined as cancer cell invasion in, around, and through nerves where tumor cells are seen within any of the layers (epineurium, perineurium, endoneurium) of the nerve sheath [1]. When tumor cells are not seen in the layers of nerve sheath, it can be difficult to differentiate it from tumor abutting on nerve.
PNI of tumor is a well-recognized mechanism of tumor dissemination. PNI from head and neck cancer is well-known disease entity, ranging from 27-82%, depending on histology and type of cancer [2]. Within the head and neck, adenoid cystic cancer has a high propensity for PNI, and squamous cell carcinoma can be often associated with PNI. Presence of PNI has a major negative impact on management and prognosis of patients with Hand N cancer. PNI from cutaneous malignancy is under-recognized and frequently overlooked clinically and radio graphically. Most common type of skin cancers with PNI are Squamous Cell Cancer (SCC), Basal Cell Cancer (BCC) and Neurotrophic Malignant Melanoma (NMM). The reported incidences of PNI in non-melanoma skin cancers are 0.18% to 10% in BCC and 2.5% to 14% in SCC [3,4]. The concept of “skip lesions” in PNI is controversial. One set of authors believe in the presence of so called skip lesions in PNI, although none of them provide a convincing evidence to prove the presence of this entity [5-8]. The other set of authors believe that the concept of skip lesions in PNI does not exist and is a misinterpretation which is being propagated in the medical literature by blind quoting of existing false information in the medical literature [9-11]. PNI should be differentiated from neural clinical manifestations that are due to gross extrinsic compression by a tumor on the nerve or direct tumor extension along the skull base foramina or fissures through different anatomical planes [10]. The cranial nerve most vulnerable for PNI from skin cancer is the trigeminal nerve due to its rich cutaneous innervation in the region of most UV exposed regions of the head and neck [12]. The facial nerve is also commonly involved with PNI, especially in cases of SCC that are metastatic to the parotid gland. Less commonly, greater auricular nerve from cervical plexus and nerves of extra-ocular movement (III, IV, and VI) can be involved.
Relevant Cranial Nerve Anatomy
Trigeminal nerve (V cranial nerve)
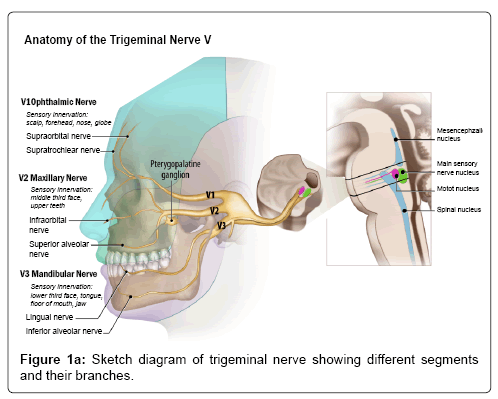
The trigeminal nerve is a mixed nerve with both motor and sensory components and is considered to be the largest of all cranial nerves. There are four cranial nerve V nuclei, mesencephalic nucleus (proprioception), main sensory (tactile sensation), main motor nucleus (motor innervation to the muscles of mastication) and the spinal nucleus (primitive sensations like pain and temperature), which span across the inferior midbrain to the upper cervical cord to the level of C2. The entry point of the cisternal segment of the nerve in the lateral aspect of pons is called ‘root entry zone’ (REZ). The nerve then relays in the gasserian ganglion located in the Meckel cave, which then divides in to V1 (Ophthalmic), V2 (Maxillary) and V3 (Mandibular) divisions. V1 and V2 divisions are pure sensory nerves and transit through the lateral wall of cavernous sinus and exit the cranial cavity through the superior orbital fissure (in to orbit) and foramen rotundum (in to pterygopalatine fossa), respectively. V1 and V2 divisions of trigeminal nerve innervate upper and mid 1/3rd of the face, respectively. The V3 division is a mixed nerve with motor innervation to the muscles of mastication and sensory to the lower 1/3rd of the face. It exits the cranial cavity through the foramen ovale in to the masticator space (Figure 1a).
Facial nerve (VII cranial nerve)
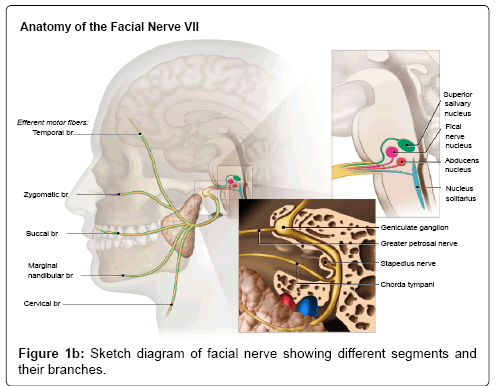
The facial nerve is a mixed cranial nerve with motor, parasympathetic and taste sensory components in it. It has three nuclei (motor nucleus, superior salivatory and solitarius tract nucleus) located in the inferior pons antero-lateral to the abducens nucleus. The motor fibers arch around the ipsilateral abducens nucleus in the brainstem forming a bulge in the floor of IV ventricle called facial colliculus. The nerve then exits in to the cerebellopontine cistern at the pontomedullary junction and travels along the anterior and superior part of Internal Auditory Canal (IAC) to enter the petrous bone. The petrous portion of the nerve is divided into various segments such as labrynthine segment, geniculate ganglion, tympanic segment, and mastoid segments. The nerve then exits the skull base through stylomastoid foramen in to the parotid gland,which divides in to five major branches including temporal, zygomatic, buccal, marginal mandibular, and cervical (Figure 1b).
Pathogenesis
The biologic mechanism of PNI pathogenesis is not well understood, but a recent theory is that it relates to reciprocal signaling interactions and the (acquired) capacity of tumor cells to respond to signals within the peripheral nerve, which promote invasion. A number of neurotrophic agents have been identified as being of possible importance in PNI in other malignancies (prostate, pancreas) [13,14]. Some of these such as Nerve Growth Factor (NGF) have been shown to have increased expression in mucosal head and neck SCC demonstrating PNI [15]. The role of Neural Cell Adhesion Molecule (NCAM) in the pathogenesis of PNI in head and neck SCC is controversial. In 2000, Vural et al. [16] evaluated surgical specimens of 66 patients using monoclonal IgG antibody immuno peroxidase staining for NCAM and concluded that there was significant increase in the expression of NCAM in patients with PNI than in patients without PNI. In 2009, Solares et al. [17] reported that there was no expression of NCAM in all of the 18 patients with head and neck cutaneous SCC (including 14 patients with clinical PNI and 4 control patients without PNI). Previous theories proposing a mechanism of spread along lowresistance planes and endoneural lymphatic channels have now largely been discounted. Lymphatic channels do not penetrate the epineurium (the outer connective tissue layer binding fascicles within a single nerve).
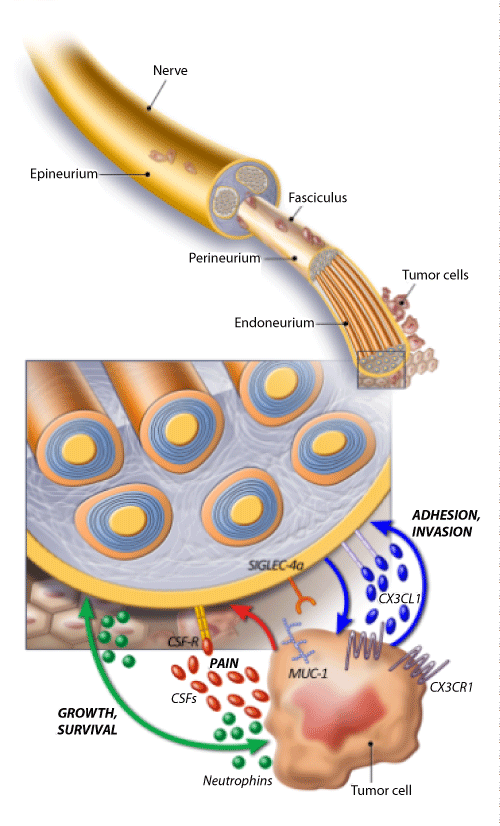
The pathogenesis of PNI is unclear. Recently Liebig et al. [14] described PNI as a process resulting due to an active and reciprocal interaction between the nerve endings and the tumor cells. Tumor microenvironment plays a crucial role in the PNI of tumor cells (Figure 2). Few of the important molecular factors involved in this cross talk between the nerve endings and tumor cells include neurotropic factors like Nerve Growth Factor (NGF), Brain Derived Nerve Growth Factor (BDNF), neurotropins (NT 3, 4 and 5), growth factors and axonal guidance molecules [18,19]. Neurotropins up regulate the tumor cells as well as the intratumoral nerves. Hematopoietic colony stimulating factors (G-CSF and GM-CSF) play a role in tumor-nerve interaction and most importantly tumor induced pain [20]. Myelin-associated glycoprotein (MAC, Siglec-4a) present on the nerve endings and laminin-5 on the tumor cells interact and facilitate PNI of tumor cells [21].
Figure 2: Sketch diagram showing interaction between the nerve fibers and tumor cells in the pathogenesis of perineural invasion. Molecules involved in this interaction include the chemokine Fractalkine/Neurotactin (CX3CL1) expressed by neurons and its receptor CX3CR1 on tumor cells and membranebound glycoproteins (SIGLEC-4a) binding to tumor mucins (MUC-1). Neurotropins secreted by neural and tumor cells sustain growth and survival of both intratumoral nerves and cancer cells. Colony stimulating factors (G and GM-CSF) secreted by tumor cells sensitize neural cells expressing their receptors (CSFR), affecting pain perception.
Diagnosis
PNI of head and neck skin cancer is often a misdiagnosed entity. The tumor cells in the initial stage invade the small peripheral cutaneous nerves which eventually spread centrally along the larger named nerves and can reach the brainstem if untreated. Depending on the presence or absence of clinical symptoms at the time of diagnosis, it can be classified in to two categories ‘incidental PNI’ and ‘clinical PNI’. Factors predicting the PNI of skin cancer include type of tumor, size of the primary tumor, location, recurrent versus primary untreated tumor and tumor cell differentiation. The incidence of incidental PNI is more common in basal cell cancer as this represents the majority of non-melanoma skin cancer, whereas the clinical PNI is more common in patients with squamous cell cancer [3,22]. Tumors greater the 2 cm located in head and neck region particularly in the mid face and lip region due to rich cutaneous innervation are more vulnerable for PNI [6,23]. Recurrent skin cancers with prior resection and/or radiation therapy are more commonly associated with PNI than the primary untreated skin cancer and are usually of a higher histological grade (histologic grade 3 or 4) [24]. There is also increased incidence of PNI in male patients. Presence of PNI has been linked to increased incidence of regional lymph node, distant metastases and poorer prognosis [25-29].
PNI is definitively diagnosed upon histologic examination of a resected skin cancer or biopsy specimen. Most commonly, PNI is “incidental” or microscopic (mPNI); a pathological diagnosis made on specimen without the presence of preoperative symptoms suggestive of PNI. But PNI can clinically be suspected (clinical PNI, cPNI) when patients with cutaneous carcinomas manifest neural symptoms such as paresthesia, hypesthesia, pain in the distribution of a trigeminal nerve branch, or facial weakness.Early symptoms can be very subtle and often described as crawling of ants underneath the skin (formication) [30,31]. Early symptoms may go un-noticed, unless the clinician has a very high index of suspicion for PNI. If neglected, symptoms progress to pain, numbness and/or motor deficits along the distribution of the affected cranial nerve.Clinical symptoms may be erroneously ascribed to Bell palsy or trigeminal neuralgia which may further delay the diagnosis by 6 months to 2 years, but can be demonstrated radio graphically as described below [12].
Histologically, tumors cells are usually seen invading the nerve or may even extend along the length of the nerve. The tumor cells can spread retrograde toward central brain or brainstem or antegrade toward the distal nerve [32,33]. Occasionally, biopsy of the suspected cranial nerve may be necessary to confirm the diagnosis when clinically suspected preoperatively [34]. Such a biopsy is typically done at the time of resection of the cancer in attempts to confirm a clear margin of resection. Rarely, presence of concentric layers of fibrosis (peritumoral fibrosis) around the tumor cells or surrounded by the tumor cells can mimic PNI and make the diagnosis difficult [35].
Imaging
In patients with clinical symptoms suggestive of trigeminal or facial nerve involvement, imaging can be useful to confirm clinical suspicion and guide treatment planning. If neural involvement is confirmed or highly suggestive upon imaging, the extent of surgical resection to obtain a clear surgical margin, including sacrifice of portions of the facial and or trigeminal nerve, may be determined. If imaging demonstrates intracranial PNI or PNS, a lesion may be deemed to be surgically unresectable, and a primary radiation therapy treatment approach that encompasses the path of neural involvement may be considered. In addition, preoperative imaging can guide the extent radiotherapy required in the adjuvant setting. In patients with mPNI, imaging should be obtained if large nerve involvement is noted in the surgical specimen.
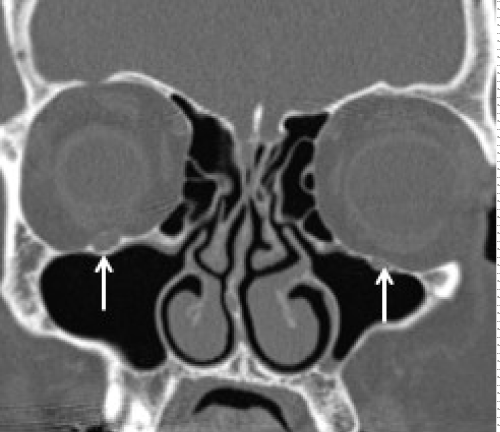
Contrast enhanced MRI is the best imaging study for the detection of PNS and also to assess the extent of disease spread along the cranial nerves.Subtle enhancement of nerve is difficult to diagnose on contrast enhanced CT until it creates a mass lesion. Hi-resolution focused gadolinium enhanced MRI or MR neurography are considered to be the most sensitive imaging for detection of PNI [31]. The MR imaging features that suggest PNI include obliteration of fat plane surrounding the cranial nerve, enhancement with or without enlargement of the nerve, mass in the cavernous sinus or Meckel cave and sometimes changes related to denervation of a group of muscles innervated by the affected cranial nerve suggest indirect signs of PNI (Figures 3-7). Although the accuracy of MR neurography is described as high as 83% in defining the extent of cranial nerve involvement [36], false negative MRI has been reported ranging from 22-47% of patients with clinical PNI in the early stages [31]. CT can detect foraminal widening and bony erosion in advanced cases [36-38] (Figure 8).
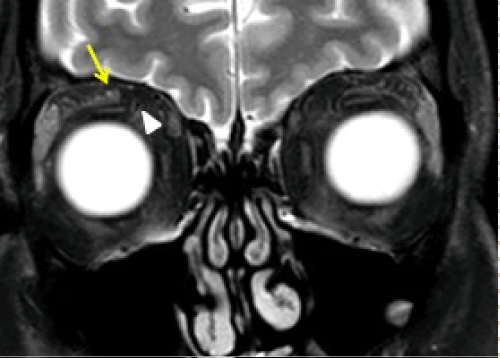
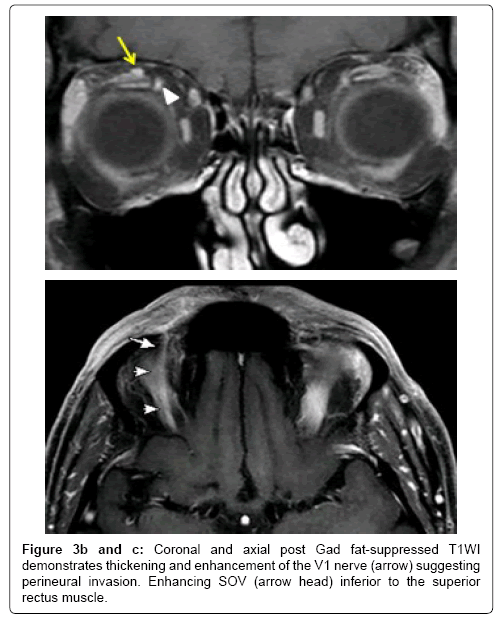
Figure 3a: 59 year old male patient with prior history of right forehead squamous cell cancer with new onset right forehead numbness and tingling sensation. A: STIR coronal image of orbit demonstrates thickening of the right V1 (ophthalmic) nerve (arrow) and also note the superior ophthalmic vein (SOV) flow void (arrow head).
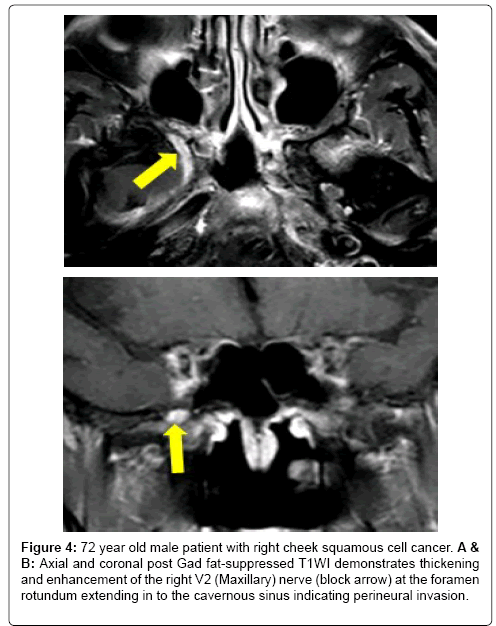
Figure 4: 72 year old male patient with right cheek squamous cell cancer. A & B: Axial and coronal post Gad fat-suppressed T1WI demonstrates thickening and enhancement of the right V2 (Maxillary) nerve (block arrow) at the foramen rotundum extending in to the cavernous sinus indicating perineural invasion.
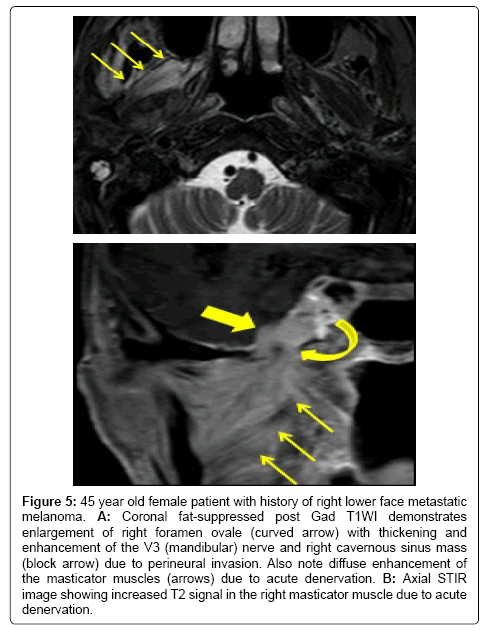
Figure 5: 45 year old female patient with history of right lower face metastatic melanoma. A: Coronal fat-suppressed post Gad T1WI demonstrates enlargement of right foramen ovale (curved arrow) with thickening and enhancement of the V3 (mandibular) nerve and right cavernous sinus mass (block arrow) due to perineural invasion. Also note diffuse enhancement of the masticator muscles (arrows) due to acute denervation. B: Axial STIR image showing increased T2 signal in the right masticator muscle due to acute denervation.
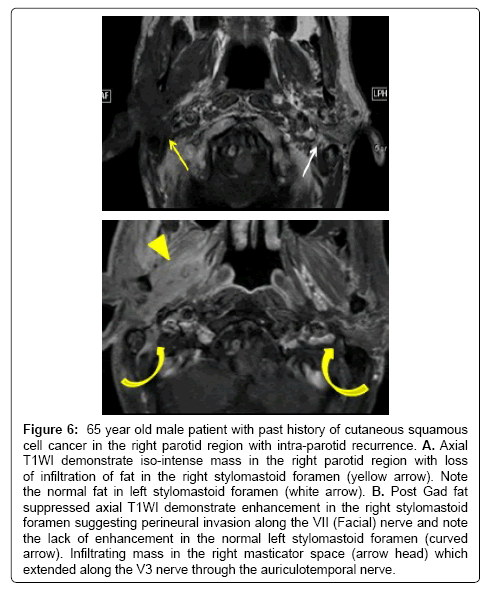
Figure 6: 65 year old male patient with past history of cutaneous squamous cell cancer in the right parotid region with intra-parotid recurrence. A. Axial T1WI demonstrate iso-intense mass in the right parotid region with loss of infiltration of fat in the right stylomastoid foramen (yellow arrow). Note the normal fat in left stylomastoid foramen (white arrow). B. Post Gad fat suppressed axial T1WI demonstrate enhancement in the right stylomastoid foramen suggesting perineural invasion along the VII (Facial) nerve and note the lack of enhancement in the normal left stylomastoid foramen (curved arrow). Infiltrating mass in the right masticator space (arrow head) which extended along the V3 nerve through the auriculotemporal nerve.
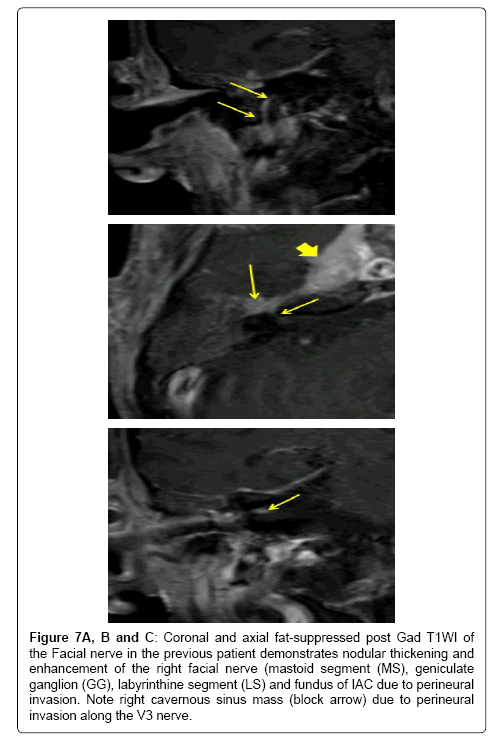
Figure 7A,B and C: Coronal and axial fat-suppressed post Gad T1WI of the Facial nerve in the previous patient demonstrates nodular thickening and enhancement of the right facial nerve (mastoid segment (MS), geniculate ganglion (GG), labyrinthine segment (LS) and fundus of IAC due to perineural invasion. Note right cavernous sinus mass (block arrow) due to perineural invasion along the V3 nerve.
It is important to thoroughly evaluate cranial nerves that are suspected for invasion on MR imaging.Non contrast T1 weighted images often reveal loss of fat plane in pterygopalatine fossa or mandibular canal. Obliteration of Meckel’s cave or infra orbital foramen also indicates presence of PNS.Asymmetric enhancement of nerve is better appreciated on post contrast MR images with fat suppression.Finally, indirect evidence of PNS can be seen as denervation changes of muscles that are supplied by the suspected cranial nerve (please find examples). In 2001, Williams et al. [39], described the zonal system (Table 1) to define the extent of the PNI along the cranial nerve to help guide the treatment options and correlate with the prognosis.
| Grade | Zone involved | Zonal anatomy |
|---|---|---|
| PN 1 | Clinical PNI imaging zone 1 | V1: Up to superior orbital fissure, V2: Up to foramen rotundum, V3: Up to foramen ovale and VII: Up to stylomastoid bell or foramen |
| PN 2 | Clinical PNI imaging zone 2 | V1, V2 and V3: From zone 1 to Gasserian ganglion. VII: Zone 1 to lateral end of IAC including geniculate ganglion and labyrinthine segment of facial nerve |
| PN 3 | Clinical PNI imaging zone 3 | All nerves proximal to ganglion into the cisterns or into the brain stem |
Table 1: Grading of Perineural Invasion (PNI) based on the zonal anatomy of the
cranial nerve. Zone 2 and 3 are classified as T4 disease.
Treatment and Prognosis
PNI is most commonly diagnosed incidentally (mPNI), and thus almost all patients have had surgery for the primary tumor resection at the time of diagnosis. Patients with negative surgical margins and focal PNI in small cutaneous nerves (less than 0.1 mm) may be adequately followed clinically without any additional resection, radiation therapy or additional imaging to help determine if more proximal nerve involvement is present. But if patients have other risk factors such as large tumor size, chronic immunosuppression, lymphovascular invasion, large nerve (greater than 0.1 mm) involvement, MRI for to search for additional nerve involvement should be considered as should adjuvant radiation therapy to the primary site and draining nodal basins Since patients with PNI are at increased risk of nodal metastases up to 15% to 20% [31,40]. For patients with questionable positive PNI margins within the resected specimen, re-excision or adjuvant radiation therapy is recommended, and adjuvant radiation therapy should definitely be recommended in the setting of positive margins, gross residual disease and/or extensive PNI. Hyperfractionation therapy is often used to reduce the risk of radiation induced complications [41]. There is no proven benefit of adjuvant chemotherapy in clinical PNI for skin cancer [42].
In patients with cPNI, treatment depends on the resectability of the primary tumor which is determined with the aid of MR imaging. If the tumor is technically resectable then treatment is usually surgery followed by post-operative radiation. In 2012, Panizza B et al. [9] evaluated 21 patients with head and neck cutaneous SCC and concluded that surgical resection with negative margins in patients with disease extending up to Gasserian or geniculate ganglion provides best chance of cure.
Compared to patients without PNI, PNI is associated with a higher rate of recurrence and 5-year disease specific death. Jambusaria et al. [43] reported a 5-year disease specific survival of 84% in patients with PNI versus 96% in those without. Local site relapse is most common, and most relapses occur within two to four years after initial resection [43]. Clinical PNI patients have an increased rate of relapse compared to those with mPNI. Garcia-Serra et al, reported local control, cause specific survival and overall survival of 87%, 65% and 50% respectively over a 5-year follow up on 59 patients with mPNI compared to 55%, 59% and 55% on 76 patients with cPNI treated with surgery and postoperative radiotherapy[40]. Imaging positive cPNI patients have highest rates of local recurrence, 43% - 75% versus 24% and lower rates of disease-specific survival, 56% - 61% versus 100% compared to image negative patients [12].
Conclusion
The radiologist and the clinician should maintain a high index of suspicion of perineural involvement when evaluating skin cancers, particularly when they are recurrent and occur in close proximation with one or more cranial nerves in the head and neck region. Patients with skin cancers that exhibit PNI are more likely to exhibit local recurrence and have decreased disease-specific survival after treatment.
References
- Ong CK, Chong VF (2010) Imaging of perineural spread in head and neck tumours. Cancer Imaging 10 Spec no A: S92-98.
- Kurtz KA, Hoffman HT, Zimmerman MB, Robinson RA (2005) Perineural and vascular invasion in oral cavity squamous carcinoma: increased incidence on re-review of slides and by using immunohistochemical enhancement. Arch Pathol Lab Med 129: 354-359.
- Leibovitch I, Huilgol SC, Selva D, Richards S, Paver R (2005) Basal cell carcinoma treated with Mohs surgery in Australia III. Perineural invasion. J Am AcadDermatol 53: 458-463.
- Donaldson MJ, Sullivan TJ, Whitehead KJ, Williamson RM (2002) Squamous cell carcinoma of the eyelids. Br J Ophthalmol 86: 1161-1165.
- Cottel WI (1982) Perineural invasion by squamous-cell carcinoma. J DermatolSurgOncol 8: 589-600.
- Lawrence N, Cottel WI (1994) Squamous cell carcinoma of skin with perineural invasion. J Am AcadDermatol 31: 30-33.
- Ratner D, Lowe L, Johnson TM, Fader DJ (2000) Perineural spread of basal cell carcinomas treated with Mohs micrographic surgery. Cancer 88: 1605-1613.
- Veness MJ, Biankin S (2000) Perineural spread leading to orbital invasion from skin cancer. AustralasRadiol 44: 296-302.
- Panizza B, Warren TA, Lambie D, Brown I (2012) The fallacy of skip lesions as an example of misinterpretations being propagated in the scientific literature. Oral Oncol 48: e33-34.
- Panizza B, Warren T (2013) Perineural invasion of head and neck skin cancer: diagnostic and therapeutic implications. Curr Oncol Rep 15: 128-133.
- Matorin PA, Wagner RF Jr (1992) Mohs micrographic surgery: technical difficulties posed by perineural invasion. Int J Dermatol 31: 83-86.
- Mendenhall WM, Amdur RJ, Hinerman RW, Werning JW, Malyapa RS, et al. (2007) Skin cancer of the head and neck with perineural invasion. Am J ClinOncol 30: 93-96.
- Ayala GE, Wheeler TM, Shine HD, Schmelz M, Frolov A, et al. (2001) In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate 49: 213-223.
- Liebig C, Ayala G, Wilks JA, Berger DH, Albo D (2009) Perineural invasion in cancer: a review of the literature. Cancer 115: 3379-3391.
- Kolokythas A, Cox DP, Dekker N, Schmidt BL (2010) Nerve growth factor and tyrosine kinase A receptor in oral squamous cell carcinoma: is there an association with perineural invasion? J Oral Maxillofac Surg 68: 1290-1295.
- Vural E, Hutcheson J, Korourian S, Kechelava S, Hanna E (2000) Correlation of neural cell adhesion molecules with perineural spread of squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg 122: 717-720.
- Solares CA, Brown I, Boyle GM, Parsons PG, Panizza B (2009) Neural cell adhesion molecule expression: no correlation with perineural invasion in cutaneous squamous cell carcinoma of the head and neck. Head Neck 31: 802-806.
- Chédotal A, Kerjan G, Moreau-Fauvarque C (2005) The brain within the tumor: new roles for axon guidance molecules in cancers. Cell Death Differ 12: 1044-1056.
- Chilton JK (2006) Molecular mechanisms of axon guidance. DevBiol 292: 13-24.
- Schweizerhof M, Stösser S, Kurejova M, Njoo C, Gangadharan V, et al. (2009) Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nat Med 15: 802-807.
- Anderson TD, Feldman M, Weber RS, Ziober AF, Ziober BL (2001) Tumor deposition of laminin-5 and the relationship with perineural invasion. Laryngoscope 111: 2140-2143.
- Ballantyne AJ (1984) Perineural invasion by SCC. J DermatolSurgOncol 10: 502-504.
- Goepfert H, Dichtel WJ, Medina JE, Lindberg RD, Luna MD (1984) Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg 148: 542-547.
- Veness MJ (2000) Perineural spread in head and neck skin cancer. Australas J Dermatol 41: 117-119.
- Jennings L, Schmults CD (2010) Management of high-risk cutaneous squamous cell carcinoma. J ClinAesthetDermatol 3: 39-48.
- Clayman GL, Lee JJ, Holsinger FC, Zhou X, Duvic M, et al. (2005) Mortality risk from squamous cell skin cancer. J ClinOncol 23: 759-765.
- Moore BA, Weber RS, Prieto V, El-Naggar A, Holsinger FC, et al. (2005) Lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Laryngoscope 115: 1561-1567.
- Cassarino DS, Derienzo DP, Barr RJ (2006) Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification. Part one. J CutanPathol 33: 191-206.
- Cassarino DS, Derienzo DP, Barr RJ (2006) Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification--part two. J CutanPathol 33: 261-279.
- Balamucki CJ, Dejesus R, Galloway TJ, Mancuso AA, Amdur RJ, et al. (2013) Impact of Radiographic Findings on For Prognosis Skin Cancer With Perineural Invasion. Am J ClinOncol .
- Galloway TJ, Morris CG, Mancuso AA, Amdur RJ, Mendenhall WM (2005) Impact of radiographic findings on prognosis for skin carcinoma with clinical perineural invasion. Cancer 103: 1254-1257.
- Nemec SF, Herneth AM, Czerny C (2007) Perineural tumor spread in malignant head and neck tumors. Top MagnReson Imaging 18: 467-471.
- Parker GD, Harnsberger HR (1991) Clinical-radiologic issues in perineural tumor spread of malignant diseases of the extracranial head and neck. Radiographics 11: 383-399.
- Esmaeli B, Ahmadi MA, Gillenwater AM, Faustina MM, Amato M (2003) The role of supraorbital nerve biopsy in cutaneous malignancies of the periocular region. OphthalPlastReconstrSurg 19: 282-286.
- Hassanein AM, Proper SA, Depcik-Smith ND, Flowers FP (2005) Peritumoral fibrosis in basal cell and squamous cell carcinoma mimicking perineural invasion: potential pitfall in Mohs micrographic surgery. DermatolSurg 31: 1101-1106.
- Gandhi, M.R., B. Panizza, D. Kennedy (2011) Detecting and defining the anatomic extent of large nerve perineural spread of malignancy: comparing "targeted" MRI with the histologic findings following surgery. Head Neck 33: 469-575.
- Nemzek WR, Hecht S, Gandour-Edwards R, Donald P, McKennan K (1998) Perineural spread of head and neck tumors: how accurate is MR imaging? AJNR Am J Neuroradiol 19: 701-706.
- Hanna E, Vural E, Prokopakis E, Carrau R, Snyderman C, et al. (2007) The sensitivity and specificity of high-resolution imaging in evaluating perineural spread of adenoid cystic carcinoma to the skull base. Arch Otolaryngol Head Neck Surg 133: 541-545.
- Williams LS, Mancuso AA, Mendenhall WM (2001) Perineural spread of cutaneous squamous and basal cell carcinoma: CT and MR detection and its impact on patient management and prognosis. Int J RadiatOncolBiolPhys 49: 1061-1069.
- Garcia-Serra A, Hinerman RW, Mendenhall WM, Amdur RJ, Morris CG, et al. (2003) Carcinoma of the skin with perineural invasion. Head Neck 25: 1027-1033.
- Hulyalkar R, Rakkhit T, Garcia-Zuazaga J (2011) The role of radiation therapy in the management of skin cancers. DermatolClin 29: 287-296, x.
- Mendenhall WM, Amdur RJ, Williams LS, Mancuso AA, Stringer SP, et al. (2002) Carcinoma of the skin of the head and neck with perineural invasion. Head Neck 24: 78-83.
- Jambusaria-Pahlajani A , Miller CJ, Quon H, Smith N, Klein RQ, et al. (2009) Surgical monotherapy versus surgery plus adjuvant radiotherapy in high-risk cutaneous squamous cell carcinoma: a systematic review of outcomes. DermatolSurg 35: 574-585.
Citation: Gaddikeri S, Bhrany A, Anzai Y (2014) Perineural Invasion of Skin Cancers in the Head and Neck: An Uncommon Phenomenon Revisited. Otolaryngology 4:169. DOI: 10.4172/2161-119X.1000169
Copyright: © 2014 Gaddikeri S et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 26992
- [From(publication date): 8-2014 - Jul 12, 2025]
- Breakdown by view type
- HTML page views: 21855
- PDF downloads: 5137