Peri-implantitis X biomaterials: is there a co-relation?
Received: 08-Feb-2024 / Manuscript No. johh-24-127297 / Editor assigned: 10-Feb-2024 / PreQC No. johh-24-127297 (PQ) / Reviewed: 24-Feb-2024 / QC No. johh-24-127297 / Revised: 29-Feb-2024 / Manuscript No. johh-24-127297 (R) / Accepted Date: 05-Mar-2024 / Published Date: 05-Mar-2024 DOI: 10.4172/2332-0702.1000413
Abstract
Is there a co-relation between the use of biomaterials and the development, or not, of peri-implantitis? To answer such relevant clinical question, Pubmed data base was consulted (keywords “peri-implantitis” and “biomaterials”) resulting in 340 studies (2013-2023). The review was registered in PROSPERO, #CRD42023412334. Filters applied: Clinical Trials (CT), Randomized CT. Inclusion/exclusion criteria were applied, resulting in 12 studies. The studies were assessed for quality and bias (Newcastle-Ottawa Scale of Evaluation of Quality). 576 patients were submitted to randomize CT/CT, 723 implants evaluated. Prior to surgeries, patients were submitted to periodontal/radiographic examination (average post-operatory interval of 20.5 months). Probing pocket depths were recorded prior/during the follow-up period. Biomaterials used in the selected studies included chlorexidine coating of the internal chamber of the implant (8.33%), showing significant smaller number of bacterial units. However, no long-term analysis of the patients was performed. Bone substitutes materials were used in 58.33% of the studies, in combination (or not) with concentrated growth factors (CGF). The results were contradictory: 50% found no differences in the outcomes. The other 50% of the studies with bone substitutes with CGF showed significant improvements in both clinical and radiographic assessments. Titanium granules were present in a minor number of studies from the final sample (16.6%), showing no difference in bone marker levels. Biphasic calcium phosphate ceramic granules were used in to fill the bone loss after peri-implantitis. Other studies (16.6%) preferred to compare the use of chitosan brushes to the use of traditional currettes, showing reduced signs of inflammation after the baseline treatment and 3 months after maintenance. After the analysis of the results, we concluded that the area of biomaterials is broad and the study of peri-implantitis encompasses different lines of research. Thus, we were not able to establish a co-relation between the use of biomaterials and peri-implantitis.
Keywords
Implants; Peri-implantitis; Biomaterials
Introduction
Biomaterials are a term that has considerably changed over the past 50 years [1]. Its definition depends on the purpose and use of the material. For instance, a biomaterial can be used for helping the repair process of bone or the surrounding tissues after a surgery. Or even, it can also be a material with certain properties which will avoid or diminish either bacterial or fungi growth on its surface, being used alone or in group with other materials.
Therefore, according to Keane & Badylak [1], the appropriate contribution of each biomaterial depends upon the application in question, but not limited to this, since it also depends on the strategy of tissue replacements and variables linked to the patients, such as age and comorbidities, among others. Thus, biomaterials encompass other specific areas, such as tissue engineering and the development of new materials. Thus, it is not uncommon to find research groups involving professionals from different areas, such as Engineers, Biologists, Medical Doctors, Doctors in Dentistry, among others, since important findings regarding biomaterials need the contribution of knowledge from different experts [1]. In Dentistry, several subareas of knowledge focus their attention on the development of new biomaterials, which would help bone regeneration, for instance. Since the use of dental implants has become common in the daily practice, researches involving biomaterials and different strategies on the surface of implants have also become frequent in Dentistry in the past decades.
Peri-implantitis is a clinical outcome which has multi-factorial etiology and distinct clinical characteristics of inflammation [2]. Among its etiology, some factors such as hygiene maintenance care and clinical conditions inherent to the patients are commonly mentioned [2].
In the literature, peri‐implantitis is believed to be caused by bacterial pathogens in susceptible individuals, leading to the loss of supporting bone and eventually, the implant. With such loss of the implant, the patient is impaired with the loss of the prosthetic treatment and subsequently, financial burden [3]. It is widely known that the knowledge of such etiological factors and featured clinical characteristics of the patient may be crucial in the outcome of the implant therapy [2]. However, peri-implantitis still represent a clinical challenge in daily practice.
Therefore, with the current researches using biomaterials being performed in several areas, including Dentistry, the purpose of this study was to perform a review of peri-implantitis and the usage of biomaterials, having in mind the following research question: is there a co-relation between the use of biomaterials and the development (or not) of peri-implantitis?
Materials and methods
This review was registered in PROSPERO (International Prospective Register for Systematic Reviews), #CRD42023412334.
Pubmed data base was chosen to perform this review of the literature. It is considered one of the most complete databases in Health Science, including publications from all over the world in peer-review journals, with high impact factors. The keywords “peri-implantitis” and “biomaterials” were used to perform the search during the month of March, 2023, starting on March 28th and ended on April 30th. Everyday of this period, two reviewers performed the search. The authors of this present review of the literature established the period of three weeks for the first search, since Science has become a vivid field, with many studies being published every week. Therefore, the purpose of performing the first search, everyday, during 3 weeks was to minimize the possibility of a novel study to be excluded, which could happen if the search was performed on a single day only.
It is important to mention that the selection of studies was performed by the reviewers independently and blindly. The search was performed by two reviewers; both PhD in oral pathology, during the exact same period and the results of this search was tabled in Microsoft Excel.
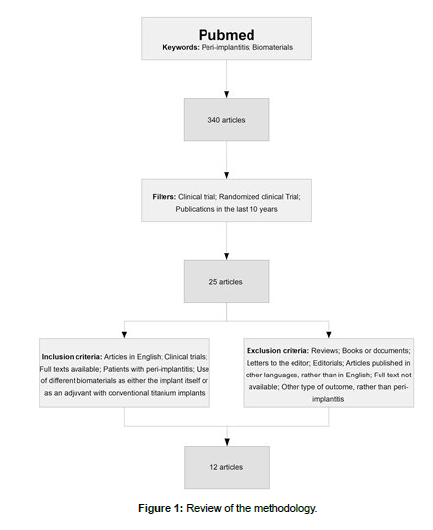
In the first review, 340 articles resulted from the search with the two keywords being applied for such search. Then, the following filters were applied in Pubmed: Clinical Trial, Randomized Clinical Trial and publications in the last 10 years. As follows, the second search resulted in 25 articles. To these articles, the inclusion and exclusion criteria were applied, resulting in a total of 12 articles, to which the extraction of data was performed.
The review of the methodology is found in (Figure 1). PRISMA Guidelines were followed for the exclusion and inclusion of studies.
Eligibility criteria
According to the PRISMA guidelines, the inclusion and exclusion criteria in a systematic review of the literature need to be specified clearly. Therefore, the following inclusion criteria were applied to the studies.
Inclusion criteria
• Articles in English
• Clinical trials
• Full texts available
• Patients with peri-implantitis
• Use of different biomaterials as either the implant itself or as an adjuvant with conventional titanium implant.
Exclusion criteria
• Reviews
• Books or documents
• Letters to the editor
• Editorials
• Articles published in other languages, rather than in English
• Full text not available
• Other type of outcome, rather than peri-implantitis
Information sources and search strategies
As previously mentioned, Pubmed was the chosen database for this systematic review. Using the keywords “peri-implantitis” and “biomaterials”, two independent reviewers performed the search in the data base, for three weeks (March 28h. to April 30th.).
At first, the title and abstracts of all the studies in the first search were read. Then, the inclusion and exclusion criteria were applied to such studies.
The evaluation of the studies and the bias risk was according to the Newcastle-Ottawa Scale of Evaluation of Quality (NOS), with scores given by such scale, following (a) representativeness of the exposed group, (b) selection of the non-exposed group, (c) evaluation of the exposition and (d) outcome of interest that was not present in the beginning of the study; Also such scale has scores for (e) the category outcome, such as the evaluation of the outcome and regarding the follow-up (enough and proper). The selected studies were evaluated according to such scores, to determine the quality of the study and the bias risk.
After the selection of the articles, according to the up-mentioned inclusion and exclusion criteria, a total of 12 articles were submitted to the extraction of data and statistical analysis.
Results
The scope of biomaterials and peri-implantitis is very broad, since many different materials are used, as well as different techniques. The studies included in this study can be found in (Table 1) [4, 9, 10, 11, 12, 13, 14, 5, 8, 6, 15].
| Authors | Title | Journal | Pubmed ID | Type |
|---|---|---|---|---|
| Carinci et al. 2019 | A New Strategy Against Peri-Implantitis: Antibacterial Internal Coating. | Int J Mol Sci. 2019 Aug 9;20(16):3897 | PMID: 31405061 | Clinical Trial |
| Derks et al. 2022 | Reconstructive surgical therapy of peri-implantitis: A multicenter randomized controlled clinical trial. | Clin Oral Implants Res. 2022 Sep;33(9):921-944 | PMID: 35804491 | Randomized controlled trial |
| Koldsland & Aass (2020) | Supportive treatment following peri-implantitis surgery: An RCT using titanium curettes or chitosan brushes. | J Clin Periodontol. 2020 Oct;47(10):1259-1267 | PMID: 32767565 | Randomized controlled trial |
| Isler et al., (2022) | Efficacy of concentrated growth factor versus collagen membrane in reconstructive surgical therapy of peri-implantitis: 3-year results of a randomized clinical trial | Clin Oral Investig 2022 Aug 26(8): 5247-5260 | PMID:9381616 | Randomized controlled trial |
| Isler et al., (2018) | Regenerative surgical treatment of peri-implantitis using either a collagen membrane or concentrated growth factor: A 12-month randomized clinical trial. | Clin Implant Dent Relat Res. 2018 Oct;20(5):703-712 | PMID:30118569 | Randomized controlled trial |
| Renvert et al. (2018) | Surgical treatment of peri-implantitis lesions with or without the use of a bone substitute-a randomized clinical trial. | J Clin Periodontol. 2018 Oct;45(10):1266-1274 | PMID: 30003583 | Randomized controlled trial |
| Monje et al. (2023) | Significance of barrier membrane on the reconstructive therapy of peri-implantitis: A randomized controlled trial | J Periodontol. 2023;94:323–335. | PMID: 36399349 | Randomized controlled trial |
| Wohlfahrt et a l. (2014) | Sulcus fluid bone marker levels and the outcome of surgical treatment of peri-implantitis | J Clin Periodontol 2014; 41: 424–431 | PMID 24417563 | Randomized controlled trial |
| Jepsen et al., (2016) | Reconstruction of Peri-implant Osseous Defects: A Multicenter Randomized Trial | J Dental Res 2016, Vol. 95(1) 58–66 | PMID 26450511 | Randomized controlled trial |
| Hussain et al. (2022) | Treatment of residual pockets using an oscillating chitosan device versus regular curettes alone—A randomized, feasibility parallel-arm clinical trial | J Periodontol. 2022;93:780–789 | PMID 34710240 | Randomized controlled trial |
| Wohlfahrt et al. (2019) | Treatment of peri-implant mucositis with a chitosan brush-A pilot randomized clinical trial | Int J Dent Hyg. 2019 May;17(2):170-176 | PMID 30582880 | Randomized controlled trial |
| Klimecs et al. (2018) | Bone Loss around Dental Implants 5 Years after Implantation of Biphasic Calcium Phosphate (HAp/βTCP) Granules | J Healthc Eng. 2018 Dec 5;2018:4804902 | PMID:30631412 | Clinical Trial |
Table 1: Articles included in the study, after the inclusion/exclusion criteria
From the selected articles, a total of 576 patients were submitted to randomized controlled studies/ clinical trials, which studied different materials or techniques regarding the treatment of peri-implantitis. A total of 723 implants were evaluated in the 12 studies, with different techniques and methodologies.
Of all these patients, the majority of the studies were very careful in the pre-selection of patients, using criteria such as overall health issues, such as absence of chronic diseases which could impair the results, such as diabetes, for example, and other commorbities. In addition, in the majority of the studies, patients were submitted to periodontal/radiographic examination prior to surgeries and the most common post-operatory period was of 12 months, being the average post-operatory interval of 20,5 months. Probing pocket depths were recorded at different sites prior and during the follow-up period, to establish in addition to the patient’s radiography, whether the test treatment was successful or not.
Biomaterials used in the selected studies included chlorexidine coating of the internal chamber of the implant (8.33%), as an attempt to prevent bacterial loading at the peri-implant level. The idea behind the use of coatings in dental implants is to reduce or eliminate bacterial biofilm, mainly of species related to long-term implant failure. When chlorexidine coating of the internal chamber of the implant was used, a significant smaller number of bacterial units were found, when compared to the control group [4]. However, no long-term analysis of the patients was performed.
Bone substitutes materials were used in 58.33% of the studies, in combination (or not) with concentrated growth factors. The results from those studies were contradictory, since 50% found no differences in the outcomes when bones substitutes in combination with concentration growth factor were used. On the other hand, the other 50% of the studies that used bones substitutes with concentration growth factor showed significant improvements in both clinical and radiographic assessments. In studies with different strategies to study peri-implantitis and the use of biomaterials, titanium granules were also present in a minor number of studies from the sample (16.6%). Biphasic calcium phosphate ceramic granules were used in a single study to fill the bone loss after peri-implantits was established in patients with dental implants. From those studies, additional insertion of porous titanium granules showed no difference in bone marker levels between the test and control group, either at baseline or at 12 months follow-up period [5].
Other studies (16.6%) preferred to compare the use of chitosan brushes, as to the use of traditional currettes, as to the treatment of residual periodontal pockets, when peri-implantitis was already established. In those studies, the use of chitosan brushes as to subgingival biofilm removal and soft tissue curretage, for the treatment of already established periodontitis may be positive [6, 7, 8].
Regarding the Newcastle-Ottawa Scale of Evaluation of Quality, all of the 12 studies received a total of 9 stars, according to the items evaluated. Only one study, part of the early group of the 25 studies, when the inclusion and exclusion criteria were being applied, received a low score, due to the high bias. However, such article was eliminated since it did not provide full text. Therefore, all the 12 final included articles were rated by both reviewers with maximum NOS stars.
Discussion
To date, peri-implantitis is a very challenging clinical outcome of restorative therapy using implants. The diagnosis of peri-implantitis is based on several criteria [3], but generally includes probing exceeding 6 mm, bleeding to probing and radiographic evaluation, with bone loss equal or above 3 mm. The prevalence of peri-implantitis varies in the literature, according to the studied population and age.
According to Pazmino-Garaicoa et al., 2023 [16], most studies regarding peri-implantitis mentioned resolution of under 50% of the cases, which means that the resolution of peri-implantitis is still unpredictable and infrequently achieved.
In addition, current strategies to reconstruct lost tissues due to peri-implantitis are also largely unpredictable [3]. In such scenario, the use of biomaterials may act in the control of soft tissue inflammation, as well as in bone development. Chan et al., 2023 [3] also mention that methods such as fixation to better stabilize the biomaterials could be beneficial.
The scope of biomaterials and peri-implantitis is very broad, since many different materials are used, as well as different techniques. However, the samples of this review were rated with 9 stars, according to the NOS. This means that all the evaluated studies were solid, with eligible cases, with appropriate samples and with adequate control groups.
When different techniques are considered with the concept of biomaterials, implants may be coated [4], with the purpose of reducing bacterial growth around the implants. Bone substitute materials, in addition to membranes may also be used [11, 13], to establish whether the long-term evaluation of such patients present any differences when peri-implantitis is considered.
Also, biomaterials such as chitosan brushes or devices were also analyzed in some articles regarding their effectiveness for treating mild peri-implantitis [7, 8]. It was noticed that the use of chitosan brushes resulted in reduced signs of inflammation, after the baseline treatment and 3 months after maintenance. This means that this type of treatment with such device may be safe and efficient when considering debridement of dental implants [8].
The statistical analysis of all the included studies was not easy to perform. Therefore, this study, which started as a systematic review of the literature, ended up as a scoping review, with the impossibility of further statistical analysis due to the broad data of the studies regarding biomaterials and peri-implantitis.
In addition, other difficulties were found during this review: there seems to hardly any standardization when regarding biomaterials used in fighting peri-implantitis. This includes not only the materials used in the studies, but also the post-operative period of evaluation of patients.
Such fact has also been present in a recent systematic review of the literature [16], in which primary therapeutic outcomes, including treatment success, rate of disease resolution and/or recurrence, as well as a variety of secondary outcomes for peri-implantitis were evaluated. In addition, the authors of this study stated that the use of standardized parameters to evaluate disease resolution should be considered in future clinical studies.
After the analysis of the 12 studies from the sample, we were able to conclude that since the area of biomaterials is broad and the study of peri-implantitis encompasses different lines of research (use of bone substitutes, coating implants with antibacterial properties substances, using chitosan brushes when peri-implantitis is already established), we were not able to establish a co-relation between the use of biomaterials and the occurrence or not of peri-implantitis.
This can be performed in a future clinical study, but some advisement is necessary: 1) to establish whether patients have no periimplantitis or have already presented the disease, now under control; 2) to define what biomaterial will be used; 3) to perform a randomized control clinical trial; 4) to ensure that patients do not present other conditions that may contribute to the appearance of peri-implantitis, mainly regarding to the present microbiota before implant installation; 5) sharp clinical and image follow-up of such patients.
Conclusion
After the review of the literature, we were not able to establish a co-relation between the use of biomaterials and the development (or not) of peri-implantitis, due to the broad lines of research, absence of standardization of materials and period of evaluation of patients in clinical studies.
References
- Keane TJ, Badylak SF (2014) Biomaterials for tissue engineering applications. Semin Pediatr Surg 23: 112-8.
- Apaza-Bedoya K, Correa BB, Schwarz F, Bianchini MA, Benfatti CAM, et al. (2023) Prevalence, risk indicators, and clinical characteristics of peri-implant mucositis and peri-implantitis for an internal conical connection implant system: A multicenter cross-sectional study. J Periodontol 23-355.
- Chan H-L, Betancourt AR, Liu CC, Chiang YC, Schmidlin PR (2023) A conceptual review on reconstructive peri-implantitis therapy: Challenges and opportunities. Clin Exp Dent Res (5): 735-745.
- Carinci F, Dorina Lauritano D, Pazzi D, Candotto V, Oliveira PS, et al. (2010) A New Strategy against Peri-Implantitis: Antibacterial Internal Coating. Int J Mol Sci 20: 3897.
- Jepsen K, Jepsen S, Laine ML, Anssari Moin D, Pilloni A, et al. (2016) Reconstruction of Peri-implant Osseous Defects: A Multicenter Randomized Trial. J Dent Res 95: 58-66.
- Wohlfahrt JC, Aass AM, Koldsland OC (2019) Treatment of peri‐implant mucositis with a chitosan brush-A pilot randomized clinical trial. Int J Dent Hyg 17: 170-176.
- Koldsland OC, Aass AM (2020) Supportive treatment following peri-implantitis surgery: An RCT using titanium curettes or chitosan brushes. J Clin Periodontol 47: 1259-1267.
- Hussain B, Karaca EO, Kuru BE, Gursoy H, Haugen HJ, et al. (2022) Treatment of residual pockets using an oscillating chitosan device versus regular curettes alone-A randomized, feasibility parallel-arm clinical trial. J Periodontol 93: 780-789.
- Derks J, Ortiz-Vigón A, Guerrero A, Donati M, Bressan E, et al. (2022) Reconstructive surgical therapy of peri-implantitis: A multicenter randomized controlled clinical trial. Clin Oral Implants Res 33: 921-944.
- Isler SC, Soysal F, Ceyhanl T, Bakırarar B, Unsal B (2022) Efficacy of concentrated growth factor versus collagen membrane in reconstructive surgical therapy of peri-implantitis: 3-year results of a randomized clinical trial. Clin Oral Investig 26: 5247-5260.
- Isler SC, Soysal F, Ceyhanlı T, Bakırarar B, Unsal B (2018) Regenerative surgical treatment of peri-implantitis using either a collagen membrane or concentrated growth factor: A 12-month randomized clinical trial. Clin Implant Dent Relat Res 20: 703-712.
- Renvert S, Roos-Jansåker AM, Persson GR (2018) surgical treatment of peri-implantitis lesions with or without the use of a bone substitute-a randomized clinical trial. J Clin Periodontol 45: 1266-1274.
- Monje A, Pons R, Vilarrasa J, Nart J, Wang H-L (2023) Significance of barrier membrane on the reconstructive therapy of peri-implantitis: A randomized controlled trial. J Periodontol 94: 323-335.
- Wohlfahrt JC, Aass AM, Granfeldt F, Lyngstadaas SP, Reseland JE (2014) Sulcus fluid bone marker levels and the outcome of surgical treatment of peri-implantitis. Clin Periodontol 41: 424-431.
- Klimecs V, Grishulonoks A, Salma I, Neimanel L, Locs J, et al. (2018) Bone Loss around Dental Implants 5 Years after Implantation of Biphasic Calcium Phosphate (HAp/βTCP). J Healthc Eng 1-7.
- Pazmino-Garaicoa C, Couso-Queiruga E, Monje A, Avila-Ortiz G, Castilho RM, et al. (2023) Disease resolution following treatment of peri-implant diseases: A systematic review. Int J Periodontics Restorative Dent.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Paradella TC, Sousa FACG (2024) Peri-implantitis X biomaterials: is there a co-relation? J Oral Hyg Health 12: 413. DOI: 10.4172/2332-0702.1000413
Copyright: © 2024 Paradella TC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2319
- [From(publication date): 0-2024 - Nov 20, 2025]
- Breakdown by view type
- HTML page views: 1969
- PDF downloads: 350