Research Article Open Access
Perfume Spray Analysis of Sn Doped ZnO-CNT NPs and its Morphological Characterizations
Saravanakkumar D1,4, Sivaranjani S1,2, Ayeshamariam A3*, Ravikumar B4, Pandiarajan S4 and Jayachandran M51Research and Development Center, Bharathiar University, Coimbatore, 641046, India
2Department of Physics, SBM College of Engineering Technology, Dindigul, 624001, India
3Department of Physics, Khadir Mohideen College, Adirampattinam, 614701, India
4Department of Physics, Devanga Arts College, Aruppukkottai, 626101, India
5Department of Physics, Sethu Institute of Technology, Pullor, Kariapatti, 626106, India
- *Corresponding Author:
- Ayeshamariam A
Department of Physics
Khadir Mohideen College
Adirampattinam, 614701, India
Tel: 9486738806
Fax: +91-04373-240810
E-mail: aismma786@gmail.com
Received Date: May 04, 2017; Accepted Date: June 02, 2017; Published Date: June 12, 2017
Citation: Saravanakkumar D, Sivaranjani S, Ayeshamariam A, Ravikumar B, Pandiarajan S, et al. (2017) Perfume Spray Analysis of Sn Doped ZnOCNT NPs and its Morphological Characterizations. J Powder Metall Min 6: 168. doi:10.4172/2168-9806.1000168
Copyright: © 2017 Saravanakkumar D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Powder Metallurgy & Mining
Abstract
The nano composite Sn doped ZnO-CNT having peculiar property in various physical science fields has been synthesized using simplified cost-effective spray pyrolysis method which forms the nano powder unusually. The work has been bringing to the fundamental study such as XRD and SEM with EDAX using sophisticated instrument to confirm expected elements present with strong bond by evaluating and serious care on diffraction peaks which becomes sharper, surface morphology and energy dispersive amplitude. All crystal phases are anatase formation and no characteristic peaks of Cu metal or oxide at 2θ values which matches with JCPDS 36-1451 corresponding to ZnO and JCPDS 26-1079 corresponding to MWCNT.TEM and SAED investigated the entitled compound that particles size, shape and presence of oxygen under ZnO and CNT remand, 2% of Sn was doped with this source solution.
Keywords
ZnO-CNT; FE-SEM; EDAX; SAED; TEM; UV-Vis; Gas sensor; Antibacterial
Introduction
At current trends, combination of metal oxide and novel materials such as ZnO-CNT, ZnO-Graphene important headlines in the research areas particularly in the field of nano science and technology usually have enhanced properties. Because, to meet the society in the world with commercial approach, the materials, particularly novel materials having the unique fundamental properties such as durability, high energy density, conductivity, light weight, cost-effective, highefficiency, chemical and thermal stability and environment friendly [1-3]. In and around the world the present research activities on nano structured ZnO-CNT and its alloy application for the gas sensitivity and antibacterialism is enhanced. Gas sensor and antibacterial study mainly focus adsorbed species or free radicals or ROS (reactive oxygen species) O− (ads), super oxide anion (O2Ô??), O2− (ads) O−2, hydrogen peroxide (H2O2) and hydroxyl radical (OH-) and size and shape of the particles means reducing grain size and increase surface to volume ratio efficiency and porous nano structure. The modified decorated CNT tube with metal oxide nano particles gives high sensitivity, high S/V ratio and fast response. Generally Chemisorbed oxygen diminishes the electrical conductivity by taking an electron from the conduction band of the material. It releases electrons back to the semiconductor and leads to increase on the conductivity of the sensor. Further, as for as ZnO nano particles concern by its application , its electrical conductivity and optical property and delivering of super oxide ions can be tuned by adding with single walled or multi walled One-dimensional aligned CNTs or two-dimensional randomly oriented CNT or threedimensional randomly aggregated bulk CNT [4-6]. In that working principle, the rate of change in resistance on the surface per unit zone due to interaction between the active sites and tested gas species. On detection of toxic gases, active sites for redox processes and delivering free charges as carriers that increases the electrical conductivity of the ZnO/CNT nano composites. To improve the gas sensing capacity the material should be in various dimensions such that 2 dimensional nano plane or sheet and nano belt such as graphene, 1 dimensional nano fibrers, nano tube and nao wire o dimensional nano particles. In recent years, there are much interest in researching and producing new antimicrobial agents from various sources to struggle with microbial resistivity. So a greater attention has been enforced to antimicrobial activity particularly against to Clostridium , genus of Gram-positive bacteria, includes numerous important human pathogens which obligate anaerobes can able to producing endospores. Normally, when the recommended human drug is absorbed pinpointed towards the affected zone where confined to a tiny area of the inner organ and is recovered after therapy using a suitable external applied magnetic field where over antibiotic treatment using antibiotics drugs will be bad factor for the emergence of antibiotic-resistant bacteria. Anti-biotic resistance increases for over dose but for sufficient dose antibiotic resistance will be decreases [7,8]. In the present work, ZnO-CNT nano composite at different annealing temperatures were prepared by cost-effective SCP (Spray-Copper plate) technique and succeeding calcination and their gas sensing performance as well as anti-microbial studies performance were investigated. The nano structure and surface morphological properties based on entitled nano powder were analysed by XRD, FESEM, TEM, EDAX, SAED, UV and antibacterial well diffused method respectively. It was clear that the ZnO-CNT nano composite exhibited good methanol sensing properties also having good antibacterial agent for both gram positive and gram negative bacteria [9,10]. Furthermore the formation mechanism of methanol adsorption and desorption and then anti-bacterial inhibition by releasing super oxide ions releasing mechanism was also discussed.
Experimental Section
Zinc acetate Dehydrate Zn(O2CCH3)2(H2O)2, Glycine NH2Ô?ÉCH2Ô?ÉCOOH and Tin Chloride (SnCl2.2H2O) were analytical grade and used for the synthesis of Sn: ZnO-CNT nano composite without further purification elsewhere. Like a coal gas, consisting mainly of components such as CH4, CO, CO2, H2, and N2, glycine consisting of components such as CH2, CH, OH, C=O, C-C, COH, C-N, NH2. In experimental typical procedure, 11.12 gram Zn (O2CCH3)2(H2O)2 and or 1.2 gram of Tin Chloride and 1.5 gm of glycine were dissolved in 50 ml of mill Q water stirred for 2 hrs by using magnetic stirrer with hot plate temperature at 30°C. Then stirred precursor solution mixed together and again stirred vigorously for 4 hours at 120°C [11]. Then the mixture was cooled up to ambient temperature. Afterwards the mixture solution poured into the 50 ml well cleaned perfume spray bottle for spraying. Copper plate with dimension 10 × 8 × 0.5 cm at 200°C mounted on the hot plate oven for 12 hour. Then the readymade solution was spraying by hand pressing on the hot plate periodically by 10 seconds intervals. The solid component settled on the surface as powder format. Afterwards spraying, the hot copper plate with sample cooled at room temperature. The solid powder sample exploited from plate using stainless knife and collected at crucibles. Next the crucibles with sample subjected to calcinations at 150°C using muffle furnace for 1 hours next two three steps 250°C, 350°C and 450°C (Table 1). In this article we have analysed the results of morphological studies of TEM with SAED pattern and FESEM for the two samples of asprepared and 450°C and its structural studies were reported in Table 1. The crystalline phases of the materials were investigated using X-ray powder diffraction (XRD) PAN analytical X-ray diffractometer using CuKα radiation (λ=1.5406 nm). The morphological of the materials was observed using a scanning electron microscope (SEM, JEOL JSM 6500-F). A transmission electron microscope (TEM JEOL, JEM – 2010-F) was used to characterize the Sn doped with ZnO-CNT powdered particles.
| Sample details | Crystallite size (nm) | Strain lines/m2 | Dislocation density (1/D2) × 1014 lines/m2 |
|---|---|---|---|
| As prepared | 53.92 | -0.00348 | 3.439 |
| 450°C | 60.13 | -0.00392 | 2.765 |
Table 1: Structural studies of Sn doped ZnO-CNT at as-prepared sample and its annealing temperature at 450°C -Card No 361451.
Results and Discussion
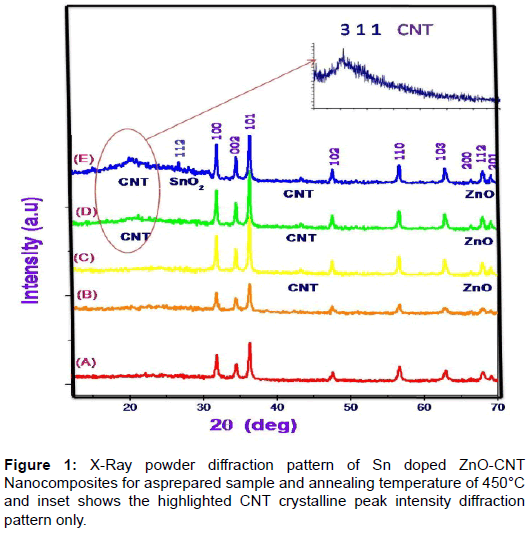
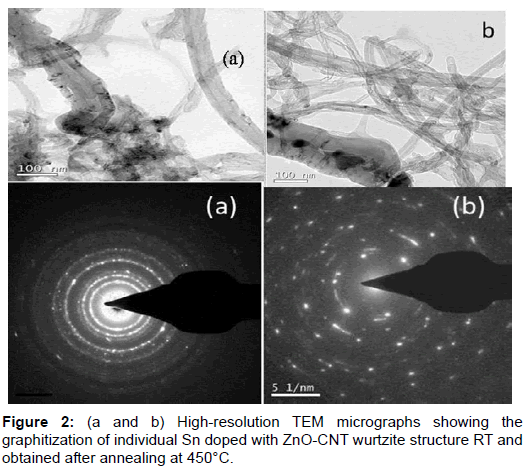
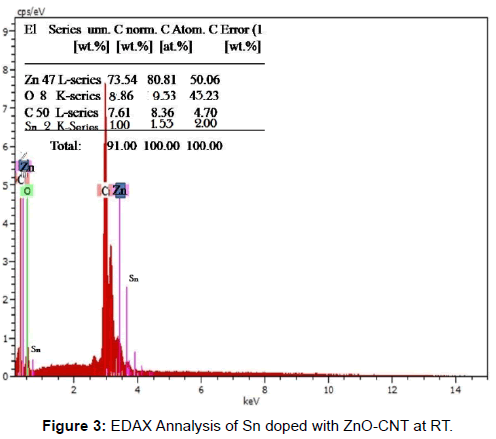
X- ray diffraction patterns of as prepared Sn doped with ZnO-CNT nanocomposite powder by SCP (Spray-Copper plate) technique are shown in Figure 1. From this crystallographic structural of the product, it can be see that all the diffraction peaks can be subjected to the pure hexagonal wurtzite structure of ZnO correlated to (JCPDS Card NO: 36-1451) where the diffraction peak related to the (100), (002), (101), (102), (110), (103), (200), (112) and (201) [12]. The peak at 2θ=26.79° and 43.21°can be attributed to MWCNT (002) is sharp and less intense with hexagonal carbon structure. But at annealing temperature 350° C the appreciable peak have obtained means the improvement in the peak of MWCNT (002), (200) (JCPDS No.26-1079, a=b=0.2456 nm, c=1.0044 nm). Therefore, the degree is puffed up as the temperature increased. This increases in the intensity nearly to 5% due to the zinc vacancies by the combination of MWCNT [13]. Furthermore oxygen vacancies also make contribution to enhancing the peaks intensity yield the improvement in the periodicity of the crystalline nature of MWCNT. Moreover the negatively charged MWCNT hexagonal carbon incorporates with oxygen vacancies. The XRD results confirmed that the ZnO on the MWCNTs has been in the nano crystalline anatase phase. Because MWCNTs show a diffraction peak at 25.79 and 44.21 which are between the diffraction peaks of ZnO and MWCNTs; these are assigned to the interaction between (100) and (102) facets of MWCNTs and ZnO respectively. Additionally, there is no diffraction peaks at 27° and 31° observed indicating that the ZnO deposited on the MWCNTs was free from rutile and brookite contaminations [14]. At low annealing temperature there was no impurity phase is observed and there is no remarkable shift in diffracted peak which denotes that no transitional products are detected when spinning of electron calculated using Scherrer equation, the average crystallite size is observed to be around 21 nm. At various annealing temperature 350°C it is observed that the location of the peaks shift towards lower angle. The facts such as large number of unit cell combined together and aggregated arrangement of large number of unit cells are the reason for variation in the crystalline size. According to Zener spinning effect, A dispersion of particles may slows the motion of a grain boundary towards the small crystallite size formation by decreasing the rate of retarding force and restrict the more number of unit cell groupies means limiting the crystallite size [15]. Figure 2a represents the transmission electron microscope (TEM) images of ZnO/CNT samples at room temperature which looks like an animal’s bone. From these images we confirm that the nano tube is composed of ZnO nano paricles and some the nano particles combined to other so many ZnO nano particles. The approximate size of the ZnO nano particle is ~ 22 nm which agree with the expected particle size the agglomerated ZnO cluster strongly binds with the carbon nano tube. Tubes are clearly observed on the figure, representing that the tube is composed of a well crystalline nature of ZnO oxide nanoparticles i.e the sample composed of multi-walled carbon Nanotubes [16]. The inset picture rear TEM image is SAED pattern explain the nano rod and nano tube are obviously poly crystalline nature in nanostructure. It can be concluded from the Figure 2a reveals that nano tube with ununiformed diameter and rough surface and inset SAED picture reveals that the oxide compound presence and combined poly crystallinity Sn doped ZnO-CNT as prepared samples. Figure 2b shows that an improvement in crystallinity nature after the annealing process at 450° C and agglomeration due to Zener spinning effect has minimized appreciably. It can be conclude that annealing process plays vital role in formation of entitled compound. Field emission scanning electron microscopes shows the surface morphology nature of three samples in Figure 4a and 4b. Before annealing, the sample at room temperature Sn doped ZnOCNT nano culture is very weak with best formation of CNT. In the Figure 2b, after annealing at 450°C, Sn doped ZnO-CNT nano cluster combined as expect as result was there with least agglomeration. An expected appreciable improvement was observed as in the TEM analysis due to annealing effect. Further it shows that all the materials have uniform distribution nano particles of size 25 nm from TEM and nearly 50-60 nm from XRD. Sn doped ZnO-CNT composite as-prepared sample have 22 nm dia and for annealing at 450°C, CNT composite of 60 μm length and some agglomeration of primary nano particles that distributed particle size ranging from 50-60 nm due to the oxide stabilization and existence of Sn presence with Zn-C bonding. These are confirmed by XRD due to peaks around 26.79° and 43.21°correspond to plane 0 0 2 and 2 0 0. According to EDX analysis Figure 3, 80.81 wt% 10.4 wt% and 8.36% was obtained for Zn, O, MWCNTs and Sn, respectively. The average composition of ZnO/MWCNTs contains a ZnO:C:Sn atomic ratio of 91:09:02. This experimental observation is very close to expected CNT composition of 09 wt% [17] (Figures 1-4).
Conclusion
The Sn doped ZnO-CNT nano material has been prepared by simple perfume spray pyrolysis method on copper substrate. The effect of the structural, morphological, and sensor properties have been studied extensively. The XRD, SEM and TEM images evidenced the formation of crystalline Sn doped ZnO-CNT with nano grained structure. The SEM examination of the present compound reveals that, there are numerous particles appear on the surface of the nano tubes which contributes in homogeneity over the surface of carbon nanotube due to ZnO: Sn formation.
References
- Ayeshamariam A, Saravanakkumar D, Kashif M, Sivaranjani S, Ravikumar B (2016) Analysis on the effect of ZnO on Carbon nanotube by spray pyrolysis method. Mechanics of Advanced Materials and Modern Processes 2: 1-8.
- Milella E, Cosentino F, Licciulli A, Massaro C (2001) Preparation and Characterization of Titania/Hydroxyapatite Composite Coatings, Obtained by Sol–Gel Process. Biomaterials 22: 1425-1431.
- De G, Licciulli A, Massaro C, Tapfer L, Catalano M, et al. (1996) Silver nanocrystals in silica by sol-gel processing. J non-cry Sol 194: 225-234.
- Elangovan E, Ramamurthi K (2004) Optoelectronic properties of spray deposited SnO2:F thin films for window materials in solar cells. J Optoele Adv Mat 6: 197-203.
- Ayeshamariam A, Bououdina M, Sanjeeviraja C (2013) Optical, electrical and sensing properties of In2O3nanoparticles, Mat Sci Semi Proc 16: 686-695.
- Ayeshamariam A, Kashif M, Bououdina M, Hashim U, Jayachandran M, et al. (2014) Morphological, structural, and gas-sensing characterization of tin-doped indium oxide nanoparticles. Cer Int 40: 1321-1328.
- Huang CS, Yeh CY, Chang Y, Hsieh Y, Ku C, et al. (2009) Field emission properties of CNT–ZnO composite materials, Diamond & Related Materials 18: 452-456.
- Wang JY, Shi R, Lin J, Zhu YF (2010) Significant photocatalytic enhancement in methylene blue degradation of TiO2 photocatalysts via graphene-like carbon in situ hybridization, Appl Catal B: Environ 100: 179-183.
- Mahmood A, Ahmed N, Raza Q, Khan TM, Mehmood M, et al. (2010) Effect of thermal annealing on the structural and optical properties of ZnO thin films deposited by the reactive e-beam evaporation technique. Physica Scripta 82: 065801.
- Tan S, Chen B, Sun X, Fan W, Kwok H, et al. (2005) Blue shift of optical band gap in ZnO thin films grown by metal-organic chemical-vapor deposition. Journal of Applied Physics 98: 013505.
- Xu C, Sun X, Zhang X, Ke L, Chua S (2004) Photoluminescent properties of copper-doped zinc oxide nanowires. Nanotechnology 15: 856
- Chen HX, Ding JJ, Guo WG, Chen GX, Ma SY (2013) Blue-green emission mechanism and spectral shift of Al-doped ZnO films related to defect levels. RSC Advances 3: 12327-12333.
- Guo B, Qiu Z, Wong K (2003) Intensity dependence and transient dynamics of donor–acceptor pair recombination in ZnO thin films grown on (001) silicon, Applied Physics Letters 82: 2290-2292.
- Lee JH, Ko KH, Park BO (2003) Electrical and optical properties of ZnO transparent conducting films by the sol–gel method. Journal of Crystal Growth 247: 119-125.
- Hong R, Huang J, He H, Fan Z, Shao J (2005) Influence of different post-treatments on the structure and optical properties of zinc oxide thin films. Applied Surface Science 242: 346-352.
- Zak AK, Majid WHA, Abrishami ME, Yousefi R (2011) X-ray analysis of ZnO nanoparticles by Williamson-Hall and size-strain plot methods. Solid State Sciences 13: 251-256.
- Prathap P, Revathi N, Reddy AS, Subbaiah YPV, Reddy KTP (2011) Synthesis of conducting Zn1-xMgxO: Al layers by spray pyrolysis for photovoltaic application. Thin Solid Films 519: 7592-7595.
Relevant Topics
- Additive Manufacturing
- Coal Mining
- Colloid Chemistry
- Composite Materials Fabrication
- Compressive Strength
- Extractive Metallurgy
- Fracture Toughness
- Geological Materials
- Hydrometallurgy
- Industrial Engineering
- Materials Chemistry
- Materials Processing and Manufacturing
- Metal Casting Technology
- Metallic Materials
- Metallurgical Engineering
- Metallurgy
- Mineral Processing
- Nanomaterial
- Resource Extraction
- Rock Mechanics
- Surface Mining
Recommended Journals
Article Tools
Article Usage
- Total views: 4033
- [From(publication date):
July-2017 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 3162
- PDF downloads : 871