Research Article Open Access
Performance of Mesenchymal Cell-Scaffold Constructs in Human Oral Reconstructive Surgery: A Systematic Review
Maria Paola Cristalli1, Roberta Marini2, Nicola Pranno2, Romeo Patini3, Gerardo La Monaca4 and Susanna Annibali2*1Department of Biotechnology and Medical Surgical Sciences, “Sapienza” University of Rome, Italy
2Department of Oral and Maxillo-facial Sciences, “Sapienza” University of Rome, Italy
3Dentistry Unit of Head and Neck clinical Area, Catholic University of Sacred Heart, Rome, Italy
4Department of Sense Organs, “Sapienza” University of Rome, Italy
- *Corresponding Author:
- Susanna Annibali
Department of Oral and Maxillo Facial Sciences
“Sapienza” University of Rome
6, Caserta St., 00161
Rome, Italy
Tel: +39 06 49976651;
Fax +39 06 44230811;
E-mail: susanna.annibali@uniroma1.it
Received date: April 07, 2016; Accepted date: April 29, 2016; Published date: May 06, 2016
Citation: Cristalli MP, Marini R, Pranno N, Patini R, La Monaca G, et al. (2016) Performance of Mesenchymal Cell-Scaffold Constructs in Human Oral Reconstructive Surgery: A Systematic Review. J Biotechnol Biomater 6:225.doi:10.4172/2155-952X.1000225
Copyright: © 2016 Cristalli MP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Background: Different sources of cultured cells combined with different scaffolds (allogenic, xenogeneic, alloplastic or composite materials) have been tested extensively in vitro and in preclinical animal studies, but there have been only a few clinical trials involving humans.
Aim: This study reviewed all of the English language literature published between January 1990 and December 2015 to assess the histological performance of different mesenchymal cell-scaffold constructs used for bone regeneration in human oral reconstructive procedures.
Methods: An electronic search of the MEDLINE and Cochrane Central Register of Controlled Trials databases complemented by manual searching was conducted to identify studies involving histological evaluation of mesenchymal cell-scaffold constructs in human oral surgical procedures. The methodological quality of randomized controlled clinical trials and controlled clinical trials was assessed using the Cochrane Collaboration tool for assessing the risk of bias. Heterogeneity was assessed using Review Manager software. Considering the heterogeneity, the data collected were reported by descriptive methods and a meta-analysis was applied only to the articles that reported the same outcome measures. The articles were classified and described based on the material scaffolds used.
Results: The search identified 1030 titles and 287 abstracts. Full-text analysis was performed for 32 articles, revealing 14 studies that fulfilled the inclusion criteria. Three randomized controlled clinical trials were identified as potentially eligible for inclusion in a meta-analysis. The studies were grouped according to the scaffold materials used: bone allograft (three studies), polyglycolic-polylactic scaffold (four studies), collagen sponge (two studies), and bovine bone matrix (five studies). The stem cells used in these studies had been sourced from the iliac crest, periosteum, dental pulp and intraoral sites.
Conclusions: The very small amount of available data makes it impossible to draw any firm conclusions regarding the increase in bone formation in human oral reconstructive procedures when using graft materials engineered with autogenous stem cells.
Keywords
Mesenchymal stem cell; Tissue scaffold; Bone regeneration; Tissue engineering; Ridge augmentation; Maxillary sinus lift
Introduction
Critical-size bony defects represent a major clinical challenge in oral reconstructive surgery because they jeopardize physiological bone healing to an extent that may prevent complete regeneration. In achieving bone repair of oral tissue lost due to congenital defects, degenerative disease, infections, cysts, trauma or surgical procedures, autogenous bone grafting still remains the gold standard due to its osteogenic, osteoinductive and osteoconductive properties [1,2]. However, the use of autogenous bone has significant drawbacks such as a limited intraoral supply, the requirement for an additional operation under general anaesthesia in cases of an extraoral donor site, the tendency for partial resorption, and donor-site discomfort and morbidity. For these reasons many different biomaterials have been investigated for use in bone regeneration over the years, but all of them have demonstrated poor clinical performance when used alone.
An important opportunity is offered by tissue-engineering approaches, which involve the use of adequately differentiated cells, and provide the ability to produce extracellular matrix with mineralizing capability, the presence of communications and interactions between cells and matrix, the production and release of growth factors in the regeneration area, and the presence of a scaffold, which mimics the three-dimensional (3D) structure of the bone [3].
In this field, different sources of cultured cells combined with different scaffolds (allogenic, xenogeneic, alloplastic or composite materials) are being extensively tested in vitroand in preclinical animal studies, but only a few clinical trials have been performed in humans.
The present study reviewed all English-language literature published between January 1990 and December 2015 with the aim of determining the histological performance of different mesenchymal cell-scaffold constructs used for bone regeneration in human oral reconstructive procedures.
Material and Methods
The protocol used in this systematic review was based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta- Analyses) statement [4,5].
Focus question
This study attempted to address the following question: what is the clinical and histological performance of different mesenchymal cell-scaffold constructs used for bone regeneration in oral surgical procedures in humans?
Search strategy
A comprehensive and systematic electronic search in the Cochrane Central Register of Controlled Trials (CENTRAL) and MEDLINE via OVID was carried out for articles published in the English language between January 1990 and December 2015. Only human studies were selected. The following combination of the MeSH terms was used: “stem cells”, “sinus floor augmentation”, “alveolar ridge augmentation”, “oral surgery”, “maxillary sinus”, “oral surgery procedures” and “jaw diseases”.
A supplementary manual search was performed of the following peer-reviewed journals for articles published between January 2005 and December 2015: Clinical Oral Implant Research, Journal of Oral and Maxillofacial Surgery, Clinical Implant Dentistry and Related Research, British Journal of Oral and Maxillofacial Surgery, Tissue Engineering and Biomaterials. In addition, the bibliographies of all selected articles were checked so that other potentially relevant studies were also included in the analysis.
Selection criteria
Only studies involving histological evaluations of mesenchymal cell-scaffold constructs in human oral surgical procedures were considered. The following exclusion criteria were applied:
− Case report.
− Case series with fewer than 10 surgical sites.
− Letters and narrative or retrospective reviews.
− Studies without histological results.
− Periodontal or maxillofacial procedures.
− The use of mesenchymal stromal cells (MSCs) in general surgery.
− In vitro and animal studies.
In addition, in cases of duplicate publications, the article with the most recent data was preferred.
Study selection
The screening procedure of all titles and abstracts retrieved by the electronic and manual searches was carried out by two of the authors independently. To avoid excluding potentially relevant articles, articles whose abstracts described unclear results were included in the full-text analysis.
The full texts of all potentially relevant articles were obtained, and eligible studies were identified by two reviewers. Any disagreement was checked by an independent reviewer and resolved through discussion.
Data collection
Two reviewers used specially designed data extraction forms to independently extract the following information from the included studies: year of publication, type of study, characteristics of the scaffold, source of stem cells, patient’s sample, surgical procedures and clinical, radiographic and histological data related to changes in mineralized bone. Any disagreement between them was discussed, and a third review author was consulted where necessary. The articles were classified and described based on the scaffold material utilized.
Quality assessment
A methodological quality assessment was performed in the randomized controlled clinical trials (RCTs) and in the controlled clinical trials using the Cochrane Collaboration tool for assessing the risk of bias.
Assessment of heterogeneity
Heterogeneity was assessed using Review Manager (RevMan) software [6]. The significance of any discrepancies in the estimates of the treatment effects from the different trials was assessed using Cochran’s test for heterogeneity and the I2 statistic. The chi-square test was used to evaluate the percentage of total variation across studies that were due to heterogeneity rather than chance. Heterogeneity would have been considered to be significant if the probability value was less than 0.1.
Data synthesis
A descriptive method was applied to report the data of selected articles, considering the heterogeneity in the study design, stem-cell population, surgical procedures, study period, methods for assessing the quality and quantity of regenerated bone, and the time required to perform the histological evaluation.
A meta-analysis was applied only to articles that reported the same outcome measures for the bovine bone matrix (BBM) scaffold. The mean differences were combined for continuous data using either fixedeffects models or, if the presence of heterogeneity between the studies was established, random-effects models. However, if there was a high degree of heterogeneity, the data were explored further to determine if they should be excluded from the meta-analysis [7].
Mean ± standard deviation values were calculated using the method outlined in Pudar-Hozo et al. [8]. If sufficient data were available, point estimates and 95% confidence intervals for the specific interventions were calculated.
Results
Study selection
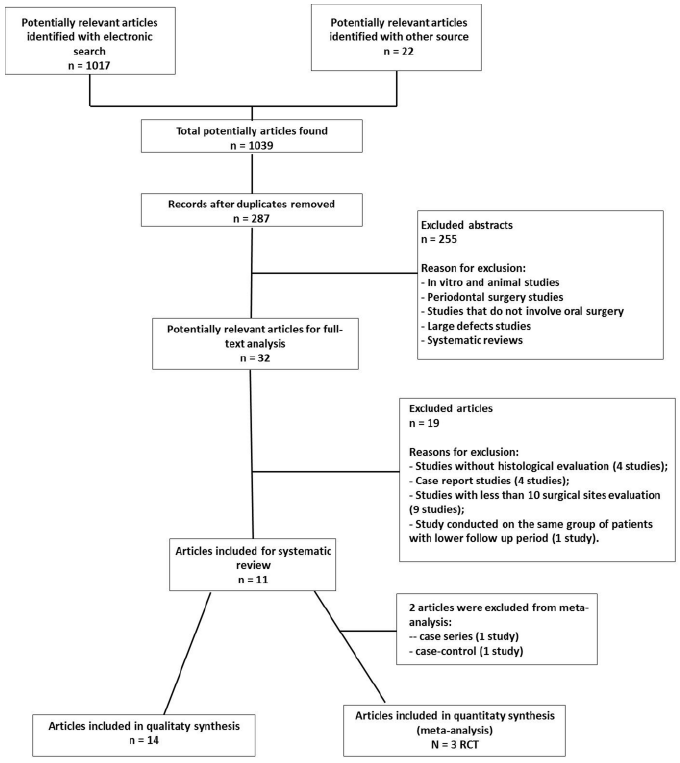
The electronic search identified 1017 studies, while an additional 13 were collected from the manual search and 9 from the references of selected articles and reviews. The full text of 32 of these 1039 articles was screened. Fourteen studies fulfilled the inclusion criteria, while 19 were excluded (Table 1) from the descriptive analysis and 2 from the meta-analysis (Figure 1). The 14 included studies comprised 3 case series, 7 case-control studies, 1 retrospective cohort study and 3 RCTs. The 3 RCTs were identified as potentially eligible for inclusion in the meta-analysis.
| Reference | Rationale for exclusion |
|---|---|
| MacAllister et al. [30] | < 10 surgical sites |
| Ueda et al. 2005 [31] | No histological evaluations |
| Ueda et al. 2008 [32] | No histological evaluations |
| D'Aquino et al. [23] | Redundant publication (Giuliani et al. [21]) |
| Yamada et al. [33] | Case report |
| Smiler et al. [34] | < 10 surgical sites |
| Kim et al. [35] | Case report |
| Soltan et al. [36] | < 10 surgical sites |
| Zizelmann et al. [27] | No histological evaluations |
| Schmelziesen et al. [37] | < 10 surgical sites |
| Beaumont et al. [38] | < 10 surgical sites |
| Hibi et al. [39] | Case report |
| Behnia et al. [40] | < 10 samples |
| Yamada et al. [41] | No histological evaluations |
| Shayesteh et al. [42] | < 10 surgical sites |
| Montesani et al. [43] | Case report |
| Strietzel et al. [44] | Case report |
| Behnia et al. [45] | < 10 surgical sites |
| Meijer et al. [46] | < 10 surgical sites |
Table 1: Reasons of excluded studies.
Study characteristics
The augmentation procedure was a sinus lift in 12 studies [9- 20]. In one study [21], bone grafting was performed in a mandibular defect after extracting the third molar. Another study [22] applied ridge augmentation and sinus lifting in the posterior maxilla and ridge augmentation in the anterior maxilla. The stem-cell populations had been harvested from the iliac crest (bone marrow stem cells) [11,13,15-17,20,22], periosteum (mesenchymal stem cells) [9,10,14,18], dental pulp (dental pulp stem cells) [21] and intraoral sites (autogenous culture-expanded bone cells) [12,19].
Bone biopsies were performed at different time periods after surgery: 3-4 months [9,13-17,19,20], 6 months [10-12,18], 8 months [22] or 3 years [21]. Histological findings were reported in terms of percentages of newly formed bone (NB) [10-13,15-17,19] or as descriptive results [9,14,18,20-22]. Radiographic evaluations were performed using computed tomography (CT) in seven studies [11-13,15,17,18,22] and by orthopantomography (OPT) in three studies [9,14,21].
The studies were divided into four groups based on the scaffold materials used: bone allograft [15,20,22], polyglycolic-polylactic (PLGA) scaffold [9,12,14,18], collagen sponge [10,21] and BBM [11,13,16,17,19]. The main characteristics of the included studies are summarized in Table 2.
| First author, year | Study design | Scaffold | Stem cell | Sample size (control/ test) | Surgical procedure | Rx Evaluation | Bone biopsy |
|---|---|---|---|---|---|---|---|
| Schimming et al. [9] | Case series | PLGA | MSCs from periosteum | 27 | Sinus lift | OPT after 3 months. | After 3 months. |
| Springer et al. [10] | Case-control | Collagen sponge/BBM | MSCs from periosteum/ ACBCs from tuberosity | 8/12 | Sinus lift | No | After 6 months in test sites and 8 months in control sites. |
| Filho Cerruti et al. [22] | Case series | Bone allografts block | hBMSCs from iliac crest + PRP | 32 | Anterior-posterior augmentation + sinus lift | CT before and 8 months after surgery. | After 8 months. |
| Fuerst et al. [11] | Case series | BBM | ACBCs from iliac crest | 22 | Sinus lift | CT after sinus lift, after implant placement and after implant uncovery. | After 6 months. |
| Mangano et al. [12] | Split-mouth case-control | PLGA | ACBCs from the posterior area of the mandible |
5/5 | Sinus lift | CT before and 6 months after surgery. | After 6 months. |
| Sauerbier et al. [13] | Case-control | BBM | hBMSCs from iliac crest | 6/12 | Sinus lift | CT cone beam | After 3 months. |
| Voss et al. [14] | Case-control | PLGA | MSCs from periosteum | 63/50 | Sinus lift | OPT before surgery, before and after implant placement, after the final prosthesis. | After 6 months. |
| Gonshor et al. [15] | Case-control | Allograft cellular bone matrix | hBMSCs | 21 | Sinus lift | CT before and 3-4 months after surgery. | After 3-4 months. |
| Rickert et al. [16] | RCT | BBM | hBMSCs from iliac crest | 12/12 | Sinus lift | No | After 3-4 months. |
| Sauerbier et al. [17] | RCT | BBM | hBMSCs from iliac crest | 11/34 | Sinus lift | CT before and after c3.5 months. | After 3.5 months |
| Trautvetter et al. [18] | Retrospective cohort | PLGA | MSCs from periosteum | 17 | Sinus lift | CT before, after 4,12,24 and 60 months after surgery. | After 6 months. |
| Hermund et al. [19] | RCT | BBM | ACBCs from tuberosity | 10/10 | Sinus lift | No | After 4 months. |
| Giuliani et al. [21] | Case-control | Collagen sponge | hDPSCs | 7/7 | Socket grafting | OPT before, 6 months, 1 and 3 years after surgery. | After 3 years. |
| Bertolai et al. [20] | Split-mouth case control | Freeze-dried bone allograft | hBMSCs from iliac crest + PRP | 20/20 | Sinus lift | No | After 3 months. |
PLGA = Polyglycolic–polylactic scaffold; MSCs = Mesenchymal stem cells; PRP = Platelet-rich plasma; OPT = Orthopantomography; BBM = Bovine bone matrix; ACBCs =Autogenous culture-expanded bone cells; hBMSCs = Human bone marrow-derived mesenchymal stem cells; CT = Computer Tomography; RCT = Randomized controlled trial; hDPSCs = Human dental pulp stem cells
Table 2: Characteristics of the included studies.
Bone allograft
Bone allografts from tissue banks were used in three studies [15,20,22]. In a case-series study, Cerruti et al. [22] treated 32 patients aged 45–83 years (median age 65 years) with 32 anterior or posterior maxillary defects to increase the amount of bone available for placing the dental implants. All procedures were performed using iliac bone allografts with autologous mononuclear cells obtained from the iliac crest or sternum bone-marrow aspirate, and platelet-rich plasma (PRP). Bone biopsies and CT were performed at 8 months after surgery, and the implants were placed in the grafted area.
Thirty of the 32 bone grafts (94.7%) were well-integrated, and all of the placed implants were functional and exhibited almost no bone loss after 2-4 years. Radiographic examinations revealed that the amount of bone was at least 6 mm in width and 10 mm in height, peaking at 14 mm in the anterior maxilla; in the posterior maxilla the width increase was not significant, and the bone height ranged between 9 mm and 15 mm. The bone biopsies reportedly showed “lines of bone formation and the presence of osteoblasts around the bone trabecula” [22].
In a case-control study, Gonshor et al. [15] compared bone formation in a two-step maxillary sinus lift procedure using either an allograft cellular bone matrix containing MSCs and osteoprogenitors (Osteocel, ACE Surgical and NuVasive) in 14 test sites, or conventional allografts (alloOss, ACE Surgical) in 7 control sites. CT scans were performed prior to and immediately following surgery and at the time of implant placement. Bone biopsy samples were harvested after 3.7 ± 0.6 months, during implant insertion. Histomorphometric evaluations revealed that the amounts of both vital and residual bone differed significantly between the two grafts. The amount of vital bone was 32.5 ± 6.8% for the allograft cellular bone graft and 18.3 ± 10.6% for the conventional allograft, and the amounts of residual graft were 4.9 ± 2.4% and 25.8 ± 13.4% in the test and control allografts, respectively.
Bertolai et al. [20] evaluated bone regeneration in 40 two-stage sinus augmentation procedures performed bilaterally in 20 patients (mean age 55.2 years). In accordance with the split-mouth design, the test side of each patient was grafted with freeze-dried bone allograft (FDBA) absorbed with MSCs, harvesting from the iliac crest and PRP, and the control side was grafted with FDBA alone. Bone biopsies were performed after 3 months at the time of implant placement. Histological analyses revealed that the control samples showed substantial persistence of the FDBA particles, separated from the trabeculae of NB bone by large amounts of fibrous connective tissue; in the test samples, the graft was adjacent to or embedded within the NB, without the interposition of fibrous connective tissue.
Polyglycolic-polylactic scaffold
Four articles had reported on the use of PLGA scaffold in the sinus lifting procedures. In a non-randomized clinical study, Shimming et al. [9] carried out 41 sinus lift procedures (17 one-stage and 24 twostage procedures) in 27 patients (45-57 years old) using PLGA fleece (Ethisorb®, Ethicon, Norderstedt, Germany), and used cultures of osteogenic cells deriving from mandibular periosteum. In all cases of the one-step procedure, radiologic (OPT) and clinical assessments were performed 3 months after surgery, which revealed excellent results in 66.6% of the patients. CT was carried out in selected cases. Bone biopsy samples were obtained at the time of implant placement, but only in 16 of the two-stage procedures, because in 8 patients no NB was detected at clinical inspections performed 3 months after augmentation. Histological analyses revealed mineralized trabecular bone, and the presence of residual biomaterial between trabeculae and osteocytes in lacunae within the bone substance.
In a split-mouth case-control study, Mangano et al. [12] compared the outcomes of 10 two-stage sinus augmentations that were performed bilaterally in 5 patients (45-65 years old) using autogenous osteoblasts seeded on PLGA scaffold (Oral Bone®, BioTissue Technologies, Freiburg, Germany) in the test sites and blocks of coral-derived porous hydroxyapatite (Biocoral, Novaxa Spa, Milan, Italy) in the control sites. CT data were acquired at baseline and at 6 months after surgery. Bone biopsies were performed at the time of implant placement after a healing period of 6 months. A radiographic comparison of the test and control sites showed vertical bone gains of 6.47 ± 1.39 mm and 9.14 ± 1.19 mm, respectively. Bone biopsies performed at both sites showed the presence of mature bone with compact and cancellous areas. Histomorphometrically the biopsy samples obtained from engineered bone revealed 37.32 ± 19.59% bone spaces and 62.67 ± 27.71% medullar spaces. Sinus grafted with hydroxyapatite comprised 54.65 ± 21.17% NB, 17.56 ± 5.03% medullar spaces and 27.78 ± 16.31% remaining material particles. Biopsies performed at both sites showed the presence of mature bone with compact and cancellous areas. From these results the authors concluded that PLGA scaffold plus autogenous osteoblasts showed a poor efficacy in promoting cellular activity and bone regeneration.
In a non-randomized clinical study, Voss et al. [14] evaluated bone regeneration in sinus lift procedures comparing the use of periosteum stem cells and PLGA scaffold (Ethisorb®, Ethicon) in 35 patients (35- 69 years old) and autogenous iliac bone in 41 patients (38-73 years old). Among the 35 patients in the study group, 15 underwent a bilateral sinus lift procedure (50 test sites) and 17 received a two-stage procedure. In the control group, 22 patients underwent augmentation of both sinuses (65 control sites). Bone biopsy samples were obtained after a healing period of 15 weeks in selected two-stage cases during the insertion of the implants. Radiologic evaluations were performed using OPT before surgery, before and after implant placement, and after the final prosthetic restoration. The clinical and radiologic follow-up lasted at least 24 months. Biopsy specimens obtained from 7 patients in the study group showed mineralized trabecular bone with remnants of biomaterial and the presence of osteocytes within the bony lacunae, while in 10 patients (16 sinuses) the grafts had acquired a connectivetissue- like consistency, and so an additional augmentation procedure with autologous bone and bone substitutes was required. No patient in the control group required a second operation, and only 1 implant failed, compared to 11 in the study group. The rate of complications (sinusitis, abscesses, loss of augmented material and loss of implants) was higher in the study group than in the control group.
Trautvetter et al. [18] evaluated the use of the same polymer scaffold in a 5-year retrospective cohort. Ten patients were treated with 17 one-step sinus lifts (bilaterally in 7 patients) using autologous tissue-engineered periosteal bone grafting (Oral Bone®, BioTissue Technologies). Radiologic examinations (OPT and CT) and clinical evaluations were carried out preoperatively and at 4, 12, 24 and 60 months post-surgery. Bone biopsy specimens were obtained in two cases after 6 months. The median bone height, measured in 27 regions of interest, increased from 6.9 mm, preoperatively, to 16.0 mm at the 4-month follow-up, and then remained significantly greater (P<0.05, median 14.2 mm) during the 5-year observation period. Histological examinations performed at 6 months confirmed the formation of full grown/mature bone, with osteocytic cells and/or osteocytes embedded in the trabecular bone lacunae and osteoblasts actively forming NB. No remnants of the biomaterial, no formation of connective tissue and no signs of necrosis or cell apoptosis were seen.
Collagen sponge
Only two studies had used collagen as a scaffold material [10,21]. Springer et al. [10] reported on a comparative study of three regenerative approaches for the two-stage sinus lift procedure. In group 1 (test group), 12 sinuses in 8 patients (51.4-65.2 years old) were augmented with periosteum-derived stem cells seeded on 4 sheets of collagen matrix (Lyostyp, Braun); in group 2A (control group), 3 sinuses in 2 patients (43 and 56 years old) were regenerated with a combination of cultured autogenous osteoblasts (taken from the maxillary tuberosity) and BBM blocks (Bio-Oss®, Geistlich Pharma, Wolhusen, Switzerland); and in group 2B (another control group), 5 sinuses in 3 patients (46-58 years old) were treated with BBM blocks alone. Bone biopsy samples were obtained during implant placement at 6 months in group 1 and at 8 months in groups 2A and 2B. None of the implants were lost and no adverse effects were seen throughout the observation period: 12–38 months in group 1 and approximately 7 years in groups 2A and 2B. In group 1 the core biopsies showed vital woven, partly lamellar bone, and small remnants of collagen matrix and an NB density of 38% (range 30.5-51%), which did not differ significantly from that in group 2A (range 32–43%).
Giuliani et al. [21] assessed the stability and quality of regenerated bone in bilateral post-extractive third molar sockets, filled with equine collagen I sponge (Gingistat, Vebas, San Giuliano Milanese, Italy) with (test site) or without (control site) third molar dental pulp stem cells, at 3 years after grafting. The sample (7 patients aged 24-40 years) was the same as that in a previously published study [23]. Clinical and radiologic evaluations were performed, and bone biopsy samples were obtained from all patients at 3 months after augmentation, using the replacement jigs used in the previous study.
The clinical assessment showed no signs of infection or morbidity in the surgical areas, and periodontal probing gains of 6.3 ± 2.1 mm and 4.5 ± 1.4 mm at the test and control sites, respectively. The completeness of regeneration and the improved vertical bone height in the test sites relative to the control sites were confirmed by OPT. Histological analyses revealed that the collagen sponge used as a scaffold was always completely reabsorbed, and that the regenerated bone at the test sites was characterized by a compacted architecture, with Haversian canals surrounded by several lamellae and osteocyte-containing lacunae. In contrast, the bone at the control sites was characterized by a cancellous (spongy) structure, with interrupted lamellae surrounding numerous large marrow-filled spaces arranged in a more-or-less regular pattern.
Bovine bone matrix scaffold
BBM was used as a scaffold in five studies: two [11,13] fulfilled the criteria for inclusion in the systematic review, and three [16,17,19] were RCTs and could be included in the meta-analysis.
In a prospective clinical study, Fuerst et al. [11] described the healing process in 22 two-stage sinus lift procedures applied to 12 patients (age 56.2 ± 9.3 years) with a mixture of bovine bone mineral granules (Bio- Oss®, Geistlich Pharma) and autogenous bone cells harvested from the anterior iliac crest (9 patients) or from the chin (3 patients). CT scans were performed after sinus grafting (CT1), after implant placement (CT2) and after uncovering the implant (CT3). Bone biopsy samples were obtained after 6 months during implant insertion. The postoperative healing period was uneventful, but 3 of the 82 placed implants were removed due to implant mobility after uncovering the implant. The graft volume was 2218.4 ± 660.9 mm3 at the time of CT1, 1694.0 ± 470.4 mm3 at CT2 and 1347.9 ± 376.3 mm3 at CT3, with significant progressive decreases of 23.62%, 20.45%, and 39.24% from CT1 to CT3. Histologically the BBM and bone were unevenly distributed: NB was woven and vital, and some of the BBM granules were surrounded by NB and some by connective tissue. A histomorphometric analysis revealed that NB and BBM were 17.9 ± 4.6% and 19.4 ± 10.1%, respectively, and that 26.8 ± 13.1% of the BBM surface was in contact with NB.
Sauerbier et al. [13] compared the NB formation in the two-stage sinus lift procedure using BBM granules (Bio-Oss®, Geistlich Pharma) and bone-marrow-aspirate-derived mesenchymal stem cells harvested from the pelvis, and processed by a FICOLL (Sigma, St Louis, MO, USA) or BMAC (Bone Marrow Procedure Pack, Harvest Technologies Corporation, Plymouth, MA, USA) method.
In total, 18 sinuses (6 in the FICOLL group and 12 in the BMAC group) were augmented in 11 patients (4 in the FICOLL group and 7 in the BMAC group). In a second-stage procedure performed after 3 months, bone biopsy samples were obtained and implants were placed. Of the 50 implants inserted (17 FICOLL and 33 BMCA), only 1 implant in the BMCA group failed before applying prosthetic loading. Histological specimens from the FICOLL and BMCA groups showed similar results: the newly formed osseous lamellae appeared as vital bone tissue containing osteocytes inside the bone lacunae, which connected the BBM particles and stabilized the graft complex. The histomorphometric analysis produced the following estimated values: 19.9% NB for BMCA and 15.5% NB for FICOLL, significantly more residual biomaterial in BMCA (31.9%) than in FICOLL (19.7%), and a significantly smaller marrow space in BMCA (47.4%) than in FICOLL (64.8%).
The three RCTs included in the meta-analysis comprised two with a parallel-group design [17,19] and one with a split-mouth design [16].
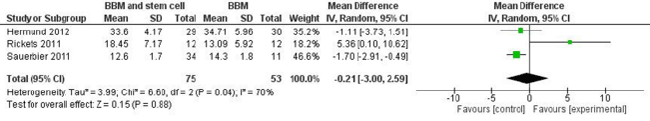
In a randomized controlled split-mouth study, Rickert et al. [16] analysed histomorphometrically the percentage of NB in 12 consecutive edentulous patients (48-69 years old) after performing bilateral maxillary sinus floor elevation. On one side the augmentation procedure was performed with BBM (Bio-Oss®, Geistlich Pharma) seeded with bone-marrow-aspirate autogenous bone cells harvested from the posterior iliac crest (test group), while on the contralateral side the augmentation procedures were performed with BBM mixed with autogenous bone that had been harvested from the retromolar area (control group), with the two sides allocated randomly. Biopsy samples were obtained after 13–16 weeks, and 66 implants were placed. One patient was excluded from the histological analysis because the biopsy sample of the control side was not obtained from an augmented site. The amount of NB formation was significantly greater in the test group (17.7 ± 7.3%) than in the control group (12.0 ± 6.6%). At 3 months after sinus augmentation, the percentage of BBM present in the samples was comparable in the test and control groups. Histological analysis showed that NB lamellae surrounded the biomaterial particles and stabilized the graft complex. No signs of inflammatory reaction were observed.
Sauerbier et al. [17] performed 45 two-stage sinus lift augmentation procedures in 26 of 40 randomized patients (38.9–67.7 years old). Thirty-four sinuses of 25 patients (test arm) were augmented with BBM (Bio-Oss®, Geistlich Pharma) in combination with pelvic bone-marrow concentrate aspirate, while 11 sinuses (control group) in 11 patients were grafted with a mixture of 70% BBM and 30% autologous bone harvested from the retromolar area. Each sinus was randomly assigned to either the test or control arm, and in 10 patients with a double sinus lift, the randomization resulted in a split-mouth model. Bone biopsy samples were obtained after a healing period of 3.41 ± 0.39 months at the time of implant insertion. The histological findings were similar in all of the specimens. No signs of inflammation were detected, vital bone tissue containing osteocytes inside the bone lacunae was observed, and the NB connected the biomaterial particles and stabilized the grafted complex. In a histomorphometric analysis the amount of NB formation did not differ significantly between the control (14.3 ± 1.8%) and test (12.6 ± 1.7%) groups. Cone-beam CT performed on 28 test and 9 control sinuses revealed that the volume of augmented bone was significantly greater in the test group (1.74 ± 0.69 mL) than in the control group (1.33 ± 0.62 mL).
Hermund et al. [19] evaluated histologically the bone formation in two-stage sinus floor augmentation procedures, comparing the use of composite graft (BBM and autogenous bone harvested using a scraper) alone (control group) or supplemented with cultivated autogenous bone cells derived from the tuberosity area (test group). Twenty maxillary sinus lift procedures were carried out in 20 patients randomly assigned to the test group (10 patients, age 60.4 ± 11.2 years) or the control group (10 patients, age 58.5 ± 8.1 years).
Implants (n=39) were placed after 4 months, and bone biopsies were performed at the same time. All implants were osseointegrated and loaded. There were no remarkable differences between the bone specimens in the two groups. In biopsy specimens from caudal portions, the mostly woven and occasionally lamellar NB was in contact with the BBM particles, which occasionally were completely incorporated within the new osseous tissue. In contrast, only a small amount of NB was found in the more apical aspects, where the biomaterial was mostly embedded within fibrovascular connective tissue.
The potential risks of bias in the studies included in the metaanalysis are summarized in Figure 2. Two trials [16,19] were judged to be at low risk of bias, whereas one [17] was judged to be at high risk of bias due to the specific study design used. This meta-analysis found insufficient evidence for determining whether there was a difference in NB formation after sinus floor augmentation performed with BBM or BBM and stem cells (Figure 3).
Discussion
The purpose of this review was to determine the clinical and histological performances of different mesenchymal cell-scaffold constructs used for oral bone regeneration in human subjects. The systematic search revealed a paucity of publications and high heterogeneity among the various studies. Indeed, while there have been numerous in vitro and animal studies of engineered stem-cell scaffolds, few researches have involved human subjects, probably due to the difficulty of obtaining consent from the relevant ethics committees. Moreover, the lack of blinding and randomization, and differences between model protocols, study designs, the included human and cellular populations, and surgical techniques made it difficult to compare the results and draw significant conclusions.
This review found that the most commonly used surgical procedure for testing the outcome of tissue-engineered scaffolds is the two-stage sinus lift [9,20]. This procedure is a good clinical model for evaluating bone regeneration, because bone formation occurs within an enclosed space, and hence with a minimal interference from external factors [17]. In addition, the two-stage sinus lift procedure is more predictable than vertical bone regeneration and allows bone biopsy specimens to be collected during implant insertion, thereby avoiding any additional discomfort for the patients [24].
While many different materials for promoting bone regeneration have been tested, none of them has satisfied all of the requirements for an ideal scaffold. Based on the current literature, an ideal scaffold should maintain an adequate 3D shape after implantation, facilitate cell adhesion and proliferation, promote cell growth, and be mechanically strong [1,3,24-26]. Furthermore, it should be biocompatible, biodegrade into no toxic by-products, easily handled and easily processed during manufacture. The scaffold should have an appropriate macrostructure (in terms of the surface geometry and a porous structure with a pore size of 100–700 μm2) and microstructure to induce cell attachment, and an adequate molecular structure to induce specific tissue responses [1,3,24-26]. Finally, it should exhibit osteoinductive/osteoconductive properties and a resorption time compatible with tissue regeneration [1]. No ideal scaffold has yet been developed, and the evidence for the use of different investigated materials remains controversial.
The three included studies [15,20,22] that used an allogenic graft as a scaffold for mesenchymal stem cells produced encouraging findings in terms of NB formation. However, it is impossible to compare their results due to differences in the types of allograft, sources of stem cells, laboratory procedures, surgical interventions and methods used to analyze NB. Indeed, the properties of allografts change significantly during decellularization, sterilization and storage processes. When the method of processing removes the viable cells (osteogenic and osteoinductive) and leaves the extracellular matrix (osteoconductive), the allograft is osteoconductive, whereas when leaving factors such as bone morphogenetic proteins and transforming growth factor-β, the demineralized bone matrix can be osteoinductive and able to recruit mesenchymal stem cells and stimulate their differentiation into osteoprogenitor cells [1].
PLGA scaffold is a widely investigated biomaterial in bone regeneration [2,9,12,14,18,27,28], although the few studies that have investigated its utility as a scaffold in tissue engineering have produced conflicting results. Insufficient clinical success in the sinus lift procedures due to poor efficacy in promoting cellular activity and bone regeneration was reported by Mangano [12] and Voss [14], whereas good clinical and radiologic results were found in the studies of Trautvetter [18] and Shimming [9]. Insufficient bone regeneration and a high resorption rate have been reported when two-stage sinus lift procedures were performed with stem cells and PLGA scaffolds [9,14,18,27,28]. Augmentation failure could be caused by the supply of oxygen and nutrients being insufficient to sustain the survival and proliferation of cells embedded within a large polymer construct, and the low pH produced by polymer resorption, which could prevent the survival of osteoblasts. Furthermore, an increase in fibrous tissue encapsulation during healing might be due to foreign-body reactions to acidic polymer degradation products induced by the hydrolysis of PLGA bulk erosion in vivo [1]. Therefore, such tissue-engineered bone should be used only in sinus lift procedures with simultaneous implant placement and at sites that provide sufficient bone.
Major advantages have been reported when using collagen sponge scaffolds, including biocompatibility, biodegradability and the ability to bind growth factors critical for osteoconduction [10,21,23]. A tissueengineered combination of a collagen matrix with autologous cells, derived from periosteum or dental pulp, tested in two of the included studies, was shown to be capable of creating NB tissue and exhibit a high mineralization rate. However, the reported data relate to small numbers of procedures and patients and short observation periods, and so they must be considered with extreme caution [10,21,23,28].
BBM is one of the most-used and well-studied grafting materials in bone reconstructive procedures. BBM exhibits excellent osteoconductive properties due to its morphological structure and mineral composition, which are similar to those of human cancellous bone, and its widespread interconnecting pore system promotes angiogenesis and the migration of osteogenic cells [29].
The present review found that more studies have investigated the use of BBM as a scaffold construct with mesenchymal cells. The availability of three RCTs made it possible to perform a meta-analysis, but this found that the available evidence is insufficient for determining whether NB formation after sinus floor augmentation differed between using BBM or BBM and stem cells. Indeed, the results were contradictory. Rickets et al. [16] reported an improved bone formation when the biomaterial was mixed with stem cells, whereas other studies found that the addition of autogenous stem cells to a graft of BBM [11,17] or to a composite graft of BBM and autogenous bone [19] did not exert statistically significant effects on the amount of NB formed.
Conclusions
The results of the present review should be interpreted with caution since few studies have histologically evaluated the performance of mesenchymal cell-scaffold constructs in human oral reconstructive procedures. The very small amount of available data makes it impossible to draw firm conclusions regarding any increase in bone formation achieved by using graft materials engineered with autogenous stem cells.
Further well-designed trials involving larger samples and longer follow-up periods are required to improve the level of evidence in order to understand whether mesenchymal cell-scaffold constructs offer significant long-term benefits to patients.
References
- Pilipchuk SP, Plonka AB, Monje A, Taut AD, Lanis A, et al. (2015) Tissue engineering for bone regeneration and osseointegration in the oral cavity. Dent Mater 31: 317-338.
- Mangano C, Sinjari B, Shibli JA, Mangano F, Hamisch S, et al. (2015)A Human Clinical, Histological, Histomorphometrical, and Radiographical Study on Biphasic HA-Beta-TCP 30/70 in Maxillary Sinus Augmentation. Clin Implant Dent Relat Res 17:610-618.
- Chung S, King MW (2011) Design concepts and strategies for tissue engineering scaffolds. Biotechnol Appl Biochem 58: 423-438.
- Langer R, Vacanti JP (1993) Tissue engineering. Science 260: 920-926.
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62: 1006-1012.
- Waugh N, Cummins E, Royle P, Kandala NB, Shyangdan D, et al. (2013) Faecal calprotectin testing for differentiating amongst inflammatory and non-inflammatory bowel diseases: systematic review and economic evaluation. Health Technol Assess 17: xv-xix, 1-211.
- Higgins JPT, Green S (2011). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration.
- Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5: 13.
- Schimming R, Schmelzeisen R (2004) Tissue-engineered bone for maxillary sinus augmentation. J Oral Maxillofac Surg 62: 724-729.
- Springer IN, Nocini PF, Schlegel KA, De Santis D, Park J, et al. (2006) Two techniques for the preparation of cell-scaffold constructs suitable for sinus augmentation: steps into clinical application. Tissue Eng12:2649-2656.
- Fuerst G, Strbac GD, Vasak C, Tangl S, Leber J, et al. (2009) Are culture-expanded autogenous bone cells a clinically reliable option for sinus grafting? Clin Oral Implants Res 20: 135-139.
- Mangano C, Piattelli A, Mangano A, Mangano F, Mangano A, et al. (2009) Combining scaffolds and osteogenic cells in regenerative bone surgery: a preliminary histological report in human maxillary sinus augmentation. Clin Implant Dent Relat Res 11 Suppl 1:e92-102.
- Sauerbier S, Stricker A, Kuschnierz J, Bühler F, Oshima T, et al. (2010) In vivo comparison of hard tissue regeneration with human mesenchymal stem cells processed with either the FICOLL method or the BMAC method. Tissue Eng Part C Methods 16: 215-223.
- Voss P, Sauerbier S, Wiedmann-Al-Ahmad M, Zizelmann C, Stricker A, et al. (2010) Bone regeneration in sinus lifts: comparing tissue-engineered bone and iliac bone. Br J Oral Maxillofac Surg 48: 121-126.
- Gonshor A, McAllister BS, Wallace SS, Prasad H (2011) Histologic and histomorphometric evaluation of an allograft stem cell-based matrix sinus augmentation procedure. Int J Oral Maxillofac Implants 26: 123-131.
- Rickert D, Sauerbier S, Nagursky H, Menne D, Vissink A, et al. (2011) Maxillary sinus floor elevation with bovine bone mineral combined with either autogenous bone or autogenous stem cells: a prospective randomized clinical trial. Clin Oral Implants Res 22: 251-258.
- Sauerbier S, Rickert D, Gutwald R, Nagursky H, Oshima T, et al. (2011) Bone marrow concentrate and bovine bone mineral for sinus floor augmentation: a controlled, randomized, single-blinded clinical and histological trial--per-protocol analysis. Tissue Eng Part A 17: 2187-2197.
- Trautvetter W, Kaps C, Schmelzeisen R, Sauerbier S, Sittinger M (2011) Tissue-engineered polymer-based periosteal bone grafts for maxillary sinus augmentation: five-year clinical results. J Oral Maxillofac Surg 69: 2753-2762.
- Hermund NU, Stavropoulos A, Donatsky O, Nielsen H, Clausen C, et al. (2012) Reimplantation of cultivated human bone cells from the posterior maxilla for sinus floor augmentation. Histological results from a randomized controlled clinical trial. Clin Oral Implants Res 23: 1031-1037.
- Bertolai R, Catelani C, Aversa A, Rossi A, Giannini D, et al. (2015) Bone graft and mesenchimal stem cells: clinical observations and histological analysis. Clin Cases Miner Bone Metab 12: 183-187.
- Giuliani A, Manescu A, Langer M, Rustichelli F, Desiderio V, et al. (2013) Three years after transplants in human mandibles, histological and in-line holotomography revealed that stem cells regenerated a compact rather than a spongy bone: biological and clinical implications. Stem Cells Transl Med 2:316-324.
- Filho Cerruti H, Kerkis I, Kerkis A, Tatsui NH, da Costa Neves A, et al. (2007) Allogenous bone grafts improved by bone marrow stem cells and platelet growth factors: clinical case reports. Artif Organs 31: 268-273.
- d'Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, et al. (2009) Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater 18: 75-83.
- Khojasteh A, Behnia H, Dashti SG, Stevens M (2012) Current trends in mesenchymal stem cell application in bone augmentation: a review of the literature. J Oral Maxillofac Surg 70: 972-982.
- Park JB (2010) Use of cell-based approaches in maxillary sinus augmentation procedures. J Craniofac Surg 21: 557-560.
- Egusa H, Sonoyama W, Nishimura M, Atsuta I, Akiyama K (2012) Stem cells in dentistry--Part II: Clinical applications. J Prosthodont Res 56: 229-248.
- Zizelmann C, Schoen R, Metzger MC, Schmelzeisen R, Schramm A, et al. (2007) Bone formation after sinus augmentation with engineered bone. Clin Oral Implants Res 18: 69-73.
- Jakobsen C, Sørensen JA, Kassem M, Thygesen TH (2013) Mesenchymal stem cells in oral reconstructive surgery: a systematic review of the literature. J Oral Rehabil 40: 693-706.
- Tapety FI, Amizuka N, Uoshima K, Nomura S, Maeda T (2004) A histological evaluation of the involvement of Bio-Oss in osteoblastic differentiation and matrix synthesis. Clin Oral Implants Res 15: 315-324.
- McAllister BS, Haghighat K, Gonshor A (2009) Histologic evaluation of a stem cell-based sinus-augmentation procedure. J Periodontol 80: 679-686.
- Ueda M, Yamada Y, Ozawa R, Okazaki Y (2005) Clinical case reports of injectable tissue-engineered bone for alveolar augmentation with simultaneous implant placement. Int J Periodontics Restorative Dent 25: 129-137.
- Ueda M, Yamada Y, Kagami H, Hibi H (2008) Injectable bone applied for ridge augmentation and dental implant placement: human progress study. Implant Dent 17: 82-90.
- Yamada Y, Hara K, Nakamura S, Ueda M, Ito K, et al. (2013) Minimally invasive approach with tissue engineering for severe alveolar bone atrophy case. Int J Oral Maxillofac Surg 42: 260-263.
- Smiler D, Soltan M, Lee JW (2007) A histomorphogenic analysis of bone grafts augmented with adult stem cells. Implant Dent 16: 42-53.
- Kim BC, Yoon JH, Choi B, Lee J (2013) Mandibular reconstruction with autologous human bone marrow stem cells and autogenous bone graft in a patient with plexiform ameloblastoma. J Craniofac Surg 24: e409-411.
- Soltan M, Smiler D, Soltan C, Prasad HS, Rohrer MD (2010) Bone grafting by means of a tunnel dissection: predictable results using stem cells and matrix. Implant Dent 19: 280-287.
- Schmelzeisen R, Schimming R, Sittinger M (2003) Making bone: implant insertion into tissue-engineered bone for maxillary sinus floor augmentation-a preliminary report. J Craniomaxillofac Surg 31: 34-39.
- Beaumont C, Schmidt RJ, Tatakis DN, Zafiropoulos GG (2008) Use of engineered bone for sinus augmentation. J Periodontol 79: 541-548.
- Hibi H, Yamada Y, Kagami H, Ueda M (2006) Distraction osteogenesis assisted by tissue engineering in an irradiated mandible: a case report. Int J Oral Maxillofac Implants 21: 141-147.
- Behnia H, Khojasteh A, Soleimani M, Tehranchi A, Atashi A (2012) Repair of alveolar cleft defect with mesenchymal stem cells and platelet derived growth factors: a preliminary report. J Craniomaxillofac Surg 40: 2-7.
- Yamada Y, Nakamura S, Ito K, Kohgo T, Hibi H (2008) Injectable tissue-engineered bone using autogenous bone marrow-derivated stromal cells for maxillary sinus augmentation: clinical application report from a 2-6-year follow-up. Tissue Eng Part A 14: 1699-1707.
- Shayesteh YS, Khojasteh A, Soleimani M, Alikhasi M, Khoshzaban A, et al. (2008) Sinus augmentation using human mesenchymal stem cells loaded into a beta-tricalcium phosphate/hydroxyapatite scaffold. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106: 203-209.
- Montesani L, Schulze-Späte U, Dibart S (2011) Sinus augmentation in two patients with severe posterior maxillary height atrophy using tissue-engineered bone derived from autologous bone cells: a case report. Int J Periodontics Restorative Dent 31: 391-399.
- Strietzel FP (2006) Tissue-engineered bone for lateral alveolar ridge augmentation: a case report. Int J Oral Maxillofac Implants 21: 131-135.
- Behnia H, Khojasteh A, Soleimani M, Tehranchi A, Khoshzaban A, et al. (2009) Secondary repair of alveolar clefts using human mesenchymal stem cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108: e1-6.
- Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA (2008) Cell based bone tissue engineering in jaw defects. Biomaterials 29: 3053-3061.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 10615
- [From(publication date):
June-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 9746
- PDF downloads : 869