Percutaneous Ethanol Injection Treatment, Novel Solution for the Challenge of Recurrent Thyroid Pathology: A Review
Received: 22-Mar-2014 / Accepted Date: 26-Apr-2014 / Published Date: 28-Apr-2014 DOI: 10.4172/2161-0681.1000172
Abstract
Introduction: Thyroid nodules are among the most common endocrine complaints in the United States. With increasing incidence of differentiated thyroid carcinoma there has been a widespread interest in development of minimally invasive treatments such as percutaneous ethanol injection (PEI) to manage thyroid pathology. In our review of published literatures, we discuss the application of ethanol injection for treatment of benign thyroid nodules and its efficacy in treating locally recurrent papillary thyroid carcinoma.
Evolving treatment roles of PEI: PEI has been most successful at treating recurrent cystic nodules. In hyperfunctioning nodules, PEI is indicated for patients who are poor surgical and radioiodine therapy candidates. Initial trials of PEI in metastatic lymph nodes in papillary thyroid carcinoma have yielded promising therapeutic results with only minor side effects. PEI does not remove the option of future radio-frequency ablation or surgery which may become necessary in some cases.
Conclusion: In our opinion, percutaneous ethanol injection proves to be a superior treatment modality for benign thyroid nodules showing markedly higher success in cystic nodules. Its safety and efficacy in treating recurrent papillary thyroid carcinoma is significant.
Keywords: Thyroid nodules, Recurrent thyroid carcinoma, Lymph node metastasis, Re-operation, Ethanol ablation
314033Introduction
In the latter half of the twentieth century, percutaneous ethanol injection (PEI) gained popularity as a non-surgical intervention for benign parathyroid adenoma, septal ablation in hypertrophic cardiomyopathy as well as for hepatocellular carcinoma [1-3].
In the last 25 years, it has quietly inspired a surprising level of interest among clinicians treating thyroid disease. Ablation of autonomously functioning thyroid nodules with PEI was the first proposed application of the procedure in the treatment of thyroid pathology. Livraghi and colleagues published promising results of their PEI treatment trials in autonomous functioning thyroid nodules in 1990 [4]. Since those initial forays, PEI has been evaluated as a treatment option for various presentations including recurrent papillary thyroid cancer (PTC).
Evolving treatment roles of PEI
PEI has been successful in treating cystic thyroid lesions, solid non-functioning, autonomously functioning and to a certain degree, even in hyper-functioning thyroid nodules [5-8], (Table 1). The proposed mechanism of action of PEI is by inducing sclerosis of the collapsed nodule capsule [8,9]. Complete aspiration may cure a subset of cystic thyroid nodules (Table 2).
| Year | Country | Study design | No. of patients | Success rate (%) | |
|---|---|---|---|---|---|
| Andjelković et al. [6] | 2011 | Serbia | Prospective | 25 | 92 |
| Guglielmi et al. [8] | 2004 | Italy | Retrospective | 95 | 77.9 |
| Del Prete et al. [29] | 2001 | Italy | Prospective | 34 | 88.2 |
| Altinova et al. [30] | 2003 | Turkey | Retrospective | 26 | 92.3 |
Table 1: Studies evaluating percutaneous ethanol treatment in functional thyroid nodules.
| Year | Country | Study design | No. of patients | Success rate (%) | |
|---|---|---|---|---|---|
| Del Prete et al. [5] | 2002 | Italy | Prospective | 98 | 93.8 |
| Yoon et al. [9] | 2013 | Korea | Retrospective | 40 | 100 |
| Sung et al. [31] | 2011 | Korea | Retrospective | 36 | 94.4 |
| Park et al. [32] | 2011 | Korea | Retrospective | 40 | 100 |
| Lv G et al. [33] | 2014 | China | Prospective | 71 | 94.2 |
| Kim DW. [34] | 2014 | Korea | Prospective | 25 | 100 |
| In et al. [35] | 2013 | Korea | Retrospective | 64 | 81.3 |
| Sung et al. [36] | 2013 | Korea | Prospective | 50 | 100 |
| Kanotra et al. [37] | 2008 | India | Prospective | 40 | 85 |
| Kim YJ et al. [38] | 2012 | Korea | Retrospective | 217 | 90.3 |
Table 2: Studies evaluating percutaneous ethanol treatment in cystic thyroid nodules.
However, many recur and surgical removal will be considered for these mostly benign cystic nodules and solid functional nodules [10]. Thyroid surgery is safe however, there is associated risk of complications even in hands of high volume surgeons and presents a significant financial burden [8]. Therefore, PEI treatment presents an attractive minimally invasive option for these patients with benign nodules. In these patients, complete and lasting disappearance of the treated cystic nodules is a realistic goal of therapy.
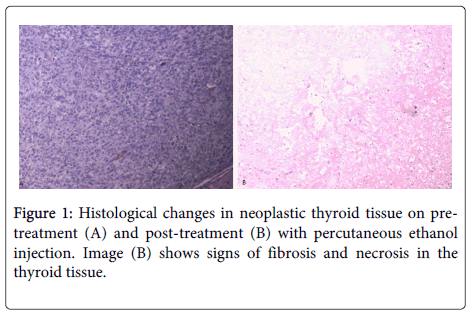
Radioactive iodine (RAI) therapy was preferred for treating hyperthyroid state. RAI is usually well tolerated but it can be associated with risks of hematologic abnormalities, reproductive disturbances, and salivary-gland disturbances with repeated doses [11]. In a recent publication, it was concluded that RAI was associated with an over 20% failure rate to treat hyperthyroid state while surgical intervention was 3.44 times more successful than RAI [12]. On the other hand, PEI may be a good treatment option for patients with small functional nodules (<5ml) who defer I-131 therapy and are poor surgical candidates [8]. The injection of ethanol acts by immediate tissue dehydration, protein denaturation, and coagulation necrosis of the vascular endothelium, platelet aggregation, vascular thrombosis and ischemic tissue necrosis (Figure 1) [8,10]. In currently published cases, ablation of the autonomous lesion was followed by return of normal thyroid function nearly 100% of these patients [6,7]. The possibility of subclinical or overt hyperthyroidism necessitates continued follow up with endocrinologist and serum thyroid function testing in the presence of clinical suspicion [6,7]. Despite evidence of some benefit, realistically, therapeutic role of PEI treatment of frankly toxic thyroid nodules is limited [8].
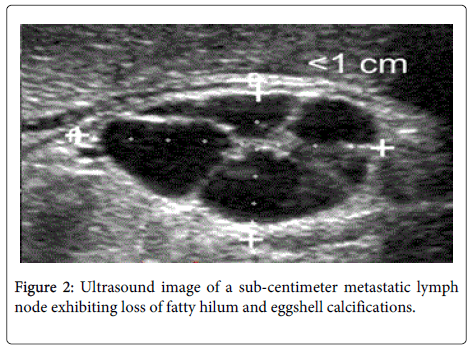
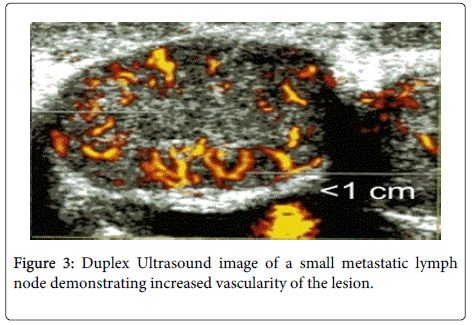
While PEI treatment for benign thyroid lesions was the area of initial sustained interest, during 2000s only investigators in Europe and Asia continued to pursue this line of application. Their colleagues in United States have instead focused on the possibility of using this treatment modality in thyroid malignancy, most commonly, PTC. PTC has one of the highest long term survival rates of any malignancy, with 90% survival at 10 years after the initial diagnosis [13,14]. However, as the other thyroid pathologies discussed in this review, it too shares the propensity to recur. Many patients who initially present with an early Stage 1 lesion must return to the operating room for treatment of their local nodal metastases multiple times after their primary operation [13,15,16]. Lobectomy or total thyroidectomy with selective cervical lymph node dissection of the affected regional nodes is the appropriate primary treatment options for PTC. Surgical interventions can be followed by radioactive Iodine-131 ablation of any remaining microscopic and macroscopic disease. In the hands of an experienced surgeon, primary total thyroidectomy has become a relatively low risk surgical procedure with a reported 2% risk of recurrent laryngeal nerve (RLN) injury [15,17,18]. Patients are monitored with ultrasound (US) routine examination for nodes suspicious for neoplastic spread (Figure 2,3). Metastatic lymph nodes are treated with selective neck lymph node dissection during primary thyroid surgery or focused dissection aimed at resecting lymph nodes missed during the initial neck dissection for the disease [15]. Prophylactic lymph node dissection has not been shown to offer a clinically significant benefit in the setting of early Stage 1 PTC to merit routine implementation [19,20]. Ironically, these patients who are most likely to suffer the cycle of local recurrence and reoperation.
Reoperation is a significantly more complex procedure compared to primary total thyroidectomy. Each revision central neck surgery presents an increasing potential of significant morbidity. Factors such as variability in natural anatomy of the RLN structure, disruption of tissue planes, surgical distortion of normal anatomic landmarks and adhesion formation caused by previous surgical interventions can contribute to the difficulty of the surgery required to resect a recurrent nodal metastasis [15,17,18,21]. Major complications most commonly associated with revision surgery in the central neck are RLN injury and hypoparathyroidism from inadvertent devascularization or removal of the parathyroid glands [13,15,22]. Additionally, due to the indolent nature of this malignancy, a significant portion of patient population presents with local recurrences in advanced age [13,15]. These patients face increased risk of injury due to the difficulty of dissection associated with redo neck surgery and additional significant health risks associated with general anesthesia. Recently, FDA approved tyrosine kinase inhibitor, sorafenib, for treatment of radioiodine-resistant metastatic differentiated thyroid cancer. However, this treatment carries a significant risk of adverse effects and is not appropriate for controlling locally persistent but limited disease [23].
The potential of PEI treatment in PTC was recognized early and a year following Livraghi work was published, a team at Mayo Clinic treated a patient with central compartment neck nodal metastases [4,24]. The patient had previously undergone three neck reoperations, the last of which was complicated by transection of the RLN. Further surgery would have come at the cost of the very real risk of bilateral RLN paralysis. The team chose to try PEI treatment of the patients’ limited recurrent disease. After the treatment, US examination showed complete disappearance of the treated metastatic lymph nodes [24]. The patient continues to be free of macroscopic disease after 20 years of follow-up. Recurrence of unsightly benign cystic nodules was a good venue to test PEI treatment’s ablation abilities; meanwhile recurrent PTC is arguably the area of greatest potential benefit for widespread adoption of this innovative treatment approach.
Unlike reoperation in the central neck, PEI treatment is a simple minimally invasive procedure and can be safely performed in an outpatient clinic setting under US guidance with local anesthetic. Therefore, ethanol ablation may have a particularly high value in situations when the risks of doing surgery are too great. Locoregional metastases of thyroid carcinoma which are not amenable to surgery, radio-iodine or irradiation can be safely injected with ethanol resulting in a local area of necrosis of pathologic tissue and vasculature [22,24,25-27]. It has proven to be especially useful in treatment of lesions with close proximity to major neurovascular structures without causing permanent nerve or vascular injury [20,22] (Table 3). Persistent vascularity of the lesion on post-treatment US and color Doppler evaluation signals to the treating physician that a repeat PEI treatment is necessary at a later time [8,22,25] (Figure 3). In this way, treatments can be carried out until satisfactory results are achieved without significant increase in risk of complications. Serum thyroglobulin level response to PEI treatment is analogous to that which occurs after surgical resection and appears to be a good predictor of successful therapy when compared with pre-treatment levels. Serum thyroglobulin levels may then be used to monitor the patient for possible recurrence [5,15].
| Year | Country | Study design | No. of patients | Success Rate (%) | LOE* | |
|---|---|---|---|---|---|---|
| Monchik et al.[14] | 2006 | US | Retrospective [case series] | 5 | 100 | 4 |
| Guenette et al.[22] | 2013 | US | Retrospective [case series] | 14 | 93 | 4 |
| Hay et al.[23] | 2012 | US | Retrospective | 88 | 100 | 3 |
| Heilo et al.[25] | 2011 | Norway | Retrospective | 66 | 79 | 3 |
| Kim et al.[26] | 2008 | Korea | Retrospective | 27 | 96 | 3 |
| Lim et al. [27] | 2007 | Korea | Prospective | 16 | 100 | 1b |
| Hay et al.[28] | 2013 | US | Prospective | 25 | 92 | 1b |
| Lewis et al.[39] | 2002 | US | Prospective | 14 | 86 | 1b |
Table 3: Studies evaluating percutaneous ethanol treatment in recurrent lymph nodes metastases in recurrent papillary thyroid carcinoma. Note: *LOE= level of evidence.
Based on existing literature and the degree of potential impact, PEI treatment may have its strongest role in treatment of recurrent PTC in metastatic lymph nodes in regions of the neck where they would be difficult to reach surgically without incurring a significant risk to the patient. At the 83rd ATA meeting in 2013, a featured presentation focused on the successful treatment of five foci of PTC in three intact thyroids [28]. Following PEI treatment, the hypervascular pattern of metastatic nodes disappeared and thyroglobulin levels dropped to undetectable levels. Each patient was followed with serial thyroglobulin levels and reevaluated with US and color Doppler for over a year without evidence of persistent or recurrent disease [28]. The group presenting this work concluded that PEI of intra-thyroid lesions is well tolerated and may offer an alternative therapy to conventional surgery for papillary thyroid microcarcinoma.
PEI treatment carries its own risk of complications. Pain at the site of injection is the most common adverse effect, typically lasts a few hours before resolution without any serious sequel [7,8,25]. Although PEI is generally a safe and effective treatment, there are sporadic reports of patients experiencing transient hoarseness after the procedure. The transient hoarseness is likely due to RLN irritation and to date, no patients experienced permanent hoarseness [7,8,25]. Rarely, PEI may cause local reaction and fibrosis if it infiltrates into the normal tissue. Overall, PEI is reported to have fewer side effects compared to thermal ablative therapies and has been proven to be effective in treating cystic thyroid nodules, non-functioning nodules, autonomous thyroid adenomas and small lesions of locally recurrent PTC.
Conclusion
To summarize, PEA is a versatile, minimally invasive option that is well tolerated with no lasting adverse effects. It is a safer and more economically attractive option compared to conventional surgical intervention. Future prospective multi-institutional studies are warranted to further compare the oncological outcomes related this approach and re-operative neck surgery.
References
- Veselka J, TomaÃ…ov P, ZemAnek D (2011) Long-term effects of varying alcohol dosing in percutaneous septal ablation for obstructive hypertrophic cardiomyopathy: a randomized study with a follow-up up to 11 years. Can J Cardiol 27: 763-767.
- Shiina S, Tateishi R, Imamura M, Teratani T, Koike Y, et al. (2012) Percutaneous ethanol injection for hepatocellular carcinoma: 20-year outcome and prognostic factors. Liver Int 32: 1434-1442.
- Veldman MW, Reading CC, Farrell MA, Mullan BP, Wermers RA, et al. (2008) Percutaneous parathyroid ethanol ablation in patients with multiple endocrine neoplasia type 1. AJR Am J Roentgenol 191: 1740-1744.
- Livraghi T, Paracchi A, Ferrari C, Bergonzi M, Garavaglia G, et al. (1990) Treatment of autonomous thyroid nodules with percutaneous ethanol injection: preliminary results. Work in progress. Radiology 175: 827-829.
- Del Prete S, Caraglia M, Russo D, Vitale G, Giuberti G, et al. (2002) Percutaneous ethanol injection efficacy in the treatment of large symptomatic thyroid cystic nodules: ten-year follow-up of a large series. Thyroid 12: 815-821.
- AndjelkoviA Z, KuzmiÄ-JankoviÄ S, Pucar D, Tavcar I, DragoviÄ T (2011) Possibilities of nontoxic autonomous thyroid nodules treatment by percutaneous ethanol injection. Vojnosanit Pregl 68 :767-73.
- Monzani F, Caraccio N, Goletti O, Lippolis PV, Casolaro A, et al. (1997) Five-year follow-up of percutaneous ethanol injection for the treatment of hyperfunctioning thyroid nodules: a study of 117 patients. ClinEndocrinol (Oxf) 46: 9-15.
- Guglielmi R, Pacella CM, Bianchini A, Bizzarri G, Rinaldi R, et al. (2004) Percutaneous ethanol injection treatment in benign thyroid lesions: role and efficacy. Thyroid 14: 125-131.
- Yoon HM, Baek JH, Lee JH, Ha EJ, Kim JK, et al. (2014) Combination therapy consisting of ethanol and radiofrequency ablation for predominantly cystic thyroid nodules. AJNR Am J Neuroradiol 35: 582-586.
- Bennedbaek FN, Hegedüs L (1999) Percutaneous ethanol injection therapy in benign solitary solid cold thyroid nodules: a randomized trial comparing one injection with three injections. Thyroid 9: 225-233.
- Padovani RP, Tuttle RM, Grewal R, Larson SM, Boucai L (2013) Complete blood counts are frequently abnormal one year after dosimetry guided radioactive iodine therapy for metastatic thyroid cancer. EndocrPract 14:1-26.
- Genovese BM, Noureldine SI, Gleeson EM, Tufano RP, Kandil E (2013) What is the best definitive treatment for Graves' disease? A systematic review of the existing literature. Ann Surg Oncol 20: 660-667.
- Tufano RP, Bishop J, Wu G (2012) Reoperative central compartment dissection for patients with recurrent/persistent papillary thyroid cancer: efficacy, safety, and the association of the BRAF mutation. Laryngoscope 122 :1634-40.
- Monchik JM, Donatini G, Iannuccilli J, Dupuy DE (2006) Radiofrequency ablation and percutaneous ethanol injection treatment for recurrent local and distant well-differentiated thyroid carcinoma. Ann Surg 244: 296-304.
- Onkendi EO, McKenzie TJ, Richards ML, Farley DR, Thompson GB, et al. (2014) Reoperative experience with papillary thyroid cancer. World J Surg 38: 645-652.
- Kim SJ, Park SY, Lee YJ, Lee EK, Kim SK, et al. (2014) Risk Factors for Recurrence After Therapeutic Lateral Neck Dissection for Primary Papillary Thyroid Cancer. Ann Surg Oncol .
- Casella C, Pata G, Nascimbeni R, Mittempergher F, Salerni B (2009) Does extralaryngeal branching have an impact on the rate of postoperative transient or permanent recurrent laryngeal nerve palsy? World J Surg 33: 261-265.
- Sheahan P, O'Connor A, Murphy MS (2012) Risk factors for recurrent laryngeal nerve neuropraxiapostthyroidectomy. Otolaryngol Head Neck Surg 146: 900-905.
- Zanocco K, Elaraj D, Sturgeon C (2013) Routine prophylactic central neck dissection for low-risk papillary thyroid cancer: a cost-effectiveness analysis. Surgery 154: 1148-1155.
- Carling T, Carty SE, Ciarleglio MM, Cooper DS, Doherty GM, et al. (2012) American Thyroid Association Surgical Affairs Committee(2012). American thyroid association design and feasibility of a prospective randomized controlled trial of prophylactic central lymph node dissection for papillary thyroid carcinoma. Thyroid 22 :237-44.
- Fontenot TE, Randolph GW, Friedlander PL, Masoodi H, Musa Yola I, et al. (2014) Gender, race, and electrophysiologic characteristics of the branched recurrent laryngeal nerve. Laryngoscope .
- Guenette JP, Monchik JM, Dupuy DE (2013) Image-guided ablation of postsurgical locoregional recurrence of biopsy-proven well-differentiated thyroid carcinoma. J VascIntervRadiol 24: 672-679.
- Thomas L, Lai SY, Dong W, Feng L, Dadu R, et al. (2014) Sorafenib in metastatic thyroid cancer: a systematic review. Oncologist 19: 251-258.
- Hay I1, Lee R, Davidge-Pitts C1, Geske J, Reading C, Charboneau W (2012) Ultrasound-guided percutaneous ethanol ablation (Upea) of selected neck nodal metastases (Nnm) in differentiated thyroid carcinoma (Dtc): A 21-year experience in 161 patients. Eur Thyroid J 1 :75a: 208.
- Heilo A, Sigstad E, Fagerlid KH, Håskjold OI, Grøholt KK, et al. (2011) Efficacy of ultrasound-guided percutaneous ethanol injection treatment in patients with a limited number of metastatic cervical lymph nodes from papillary thyroid carcinoma. J ClinEndocrinolMetab 96 :2750-5.
- Kim BM, Kim MJ, Kim EK, Park SI, Park CS, et al. (2008) Controlling recurrent papillary thyroid carcinoma in the neck by ultrasonography-guided percutaneous ethanol injection. EurRadiol 18: 835-842.
- Lim CY, Yun JS, Lee J, Nam KH, Chung WY, et al. (2007) Percutaneous ethanol injection therapy for locally recurrent papillary thyroid carcinoma. Thyroid 17: 347-350.
- Hay ID, Lee RA. (2013) Ultrasound-guided percutaneous ethanol ablation represents a promising minimally invasive alternative to observation in papillary thyroid microcarcinoma. Poster presentation at: 83rd Annual Meeting of the ATA. San Juan, Puerto Rico.
- Del Prete S, Russo D, Caraglia M, Giuberti G, Marra M, et al. (2001) Percutaneous ethanol injection of autonomous thyroid nodules with a volume larger than 40 ml: Three years of follow-up. Clinical Radiology 56;(11):895-901.
- Altinova AE, Akbay E, Yetkin I, et al. (2003) Ethanol injection as a treatment modality in autonomous thyroid Nodules: 2 Years Follow-Up. Turkish J EndocrinolMetab (3):113-118.
- Sung JY1, Kim YS, Choi H, Lee JH, Baek JH (2011) Optimum first-line treatment technique for benign cystic thyroid nodules: ethanol ablation or radiofrequency ablation? AJR Am J Roentgenol 196: W210-214.
- Park NH1, Kim DW, Park HJ, Lee EJ, Park JS, et al. (2011) Thyroid cysts treated with ethanol ablation can mimic malignancy during sonographic follow-up. J Clin Ultrasound 39: 441-446.
- Lv G1, Chen S, Li B, Chen X, Li S (2014) Efficacy Assessment of Newly Developed Open-Window Intervention Needles for the Treatment of Cystic Thyroid Nodules that can not be Aspirated. Thyroid .
- Kim DW (2014) Usefulness of Two-Stage Ethanol Ablation in the Treatment of Benign, Predominantly Cystic Thyroid Nodules. EndocrPract .
- In HS1, Kim DW, Choo HJ, Jung SJ, Kang T, et al. (2014) Ethanol ablation of benign thyroid cysts and predominantly cystic thyroid nodules: factors that predict outcome. Endocrine 46: 107-113.
- Sung JY1, Baek JH, Kim KS, Lee D, Yoo H, et al. (2013) Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology 269: 293-300.
- Kanotra SP1, Lateef M, Kirmani O (2008) Non-surgical management of benign thyroid cysts: use of ultrasound-guided ethanol ablation. Postgrad Med J 84: 639-643.
- Kim YJ1, Baek JH, Ha EJ, Lim HK, Lee JH, et al. (2012) Cystic versus predominantly cystic thyroid nodules: efficacy of ethanol ablation and analysis of related factors. EurRadiol 22: 1573-1578.
- Lewis BD1, Hay ID, Charboneau JW, McIver B, Reading CC, et al. (2002) Percutaneous ethanol injection for treatment of cervical lymph node metastases in patients with papillary thyroid carcinoma. AJR Am J Roentgenol 178: 699-704.
Citation: Bhatia P, Fontenot TE, Tsumagari K, Kandil E (2014) Percutaneous Ethanol Injection Treatment, Novel Solution for the Challenge of Recurrent Thyroid Pathology: A Review. J Clin Exp Pathol 4:172. DOI: 10.4172/2161-0681.1000172
Copyright: © 2014 Bhatia P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 17335
- [From(publication date): 6-2014 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 12616
- PDF downloads: 4719