Review Article Open Access
Peptides Derived from Neuronal Cell Cycle like Kinase 5 Activator p35, in Neurodegeneration; Pathology and Therapy
Philip Grant, Manju Bhaskar, Binukumar BK and Harish C Pant*National Institutes of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD 20892, USA
- Corresponding Author:
- Harish C Pant
National Institutes of Neurological Disorders and Stroke
National Institutes of Health, Bethesda, MD 20892, USA
Tel: 3014022124
E-mail: pant@ninds.nih.gov
Received date: March 17, 2017; Accepted date: June 14, 2017; Published date: June 21, 2017
Citation: Grant P, Bhaskar M, Binukumar BK, Pant HC (2017) Peptides Derived from Neuronal Cell Cycle like Kinase 5 Activator p35, in Neurodegeneration; Pathology and Therapy. J Alzheimers Dis Parkinsonism 7:338. doi:10.4172/2161-0460.1000338
Copyright: © 2017 Grant P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Neurodegenerative disorders such as Alzheimer’s or Parkinson’s are diseases of aging. Mutations, lesions, neuronal insults, the genetic and stochastic “slings and arrows” of living, accumulate and downgrade organ function. Microarray analyses of aging brain identify patterns of genetic changes that correlate with neurodegenerative phenotypes. Genes involved in synaptic transmission, mitochondrial function and protein turnover are downregulated while significant numbers of genes regulating DNA repair, stress responses and inflammation are upregulated. This impact on the brain induces, a complex, multidimensional network of abnormal interactions leading to deposits of protein aggregates such as amyloid plaques, tau and neurofilament proteins tangles. For some, this pathology leads to neuron loss, behavioral defects, cognitive decline and death. Therapeutic approaches for AD, for example, target the molecular pathways leading to plaques and tangles; these include kinases and phosphatases. Among kinases, one stands out, Cdk5/p35, essential for neuronal migration, synapse formation, function and survival. Studies have shown that aging-induced neuronal stress deregulates and hyper activates Cdk5, a ubiquitous feature of neuronal disorders such as Alzheimer’s, Amyotropic lateral sclerosis (ALS) and Parkinson’s (PD). Among its many effects, hyperactive Cdk5 is implicated in the production of abnormal phosphorylated protein aggregates and is a therapeutic target. Roscovitine and related compounds inhibit Cdk5 activity but not specifically; cell cycle Cdks and other kinases are equally affected. In our laboratory two truncated peptides CIP (126a.a) and P5 (24a.a.), derived from p35, activator, of Cdk5 have been shown to specifically inhibit hyperactive Cdk5 in vitro, in cortical neurons and in AD, ALS and PD model mice. As a result the neurodegenerative phenotype was diminished; aggregates and inflammation were reduced, abnormal behavior was improved and increased animal’s longevity. We believe these peptides are excellent therapeutic candidates for those neurodegenerative disorders expressing hyperactive Cdk5 in the brain.
Keywords
Alzheimers disease; Mutations; Microglia
Introduction
Human aging takes its toll; after age 65, a complex network of genetic and biochemical changes correlate over activates brain microglia and astrocytes [1]. Multiple signaling pathways suffer defects that evoke complex neurodegenerative phenotypes. Among these are the many neuronal pathways affected by deregulated Cdk5 (citations).
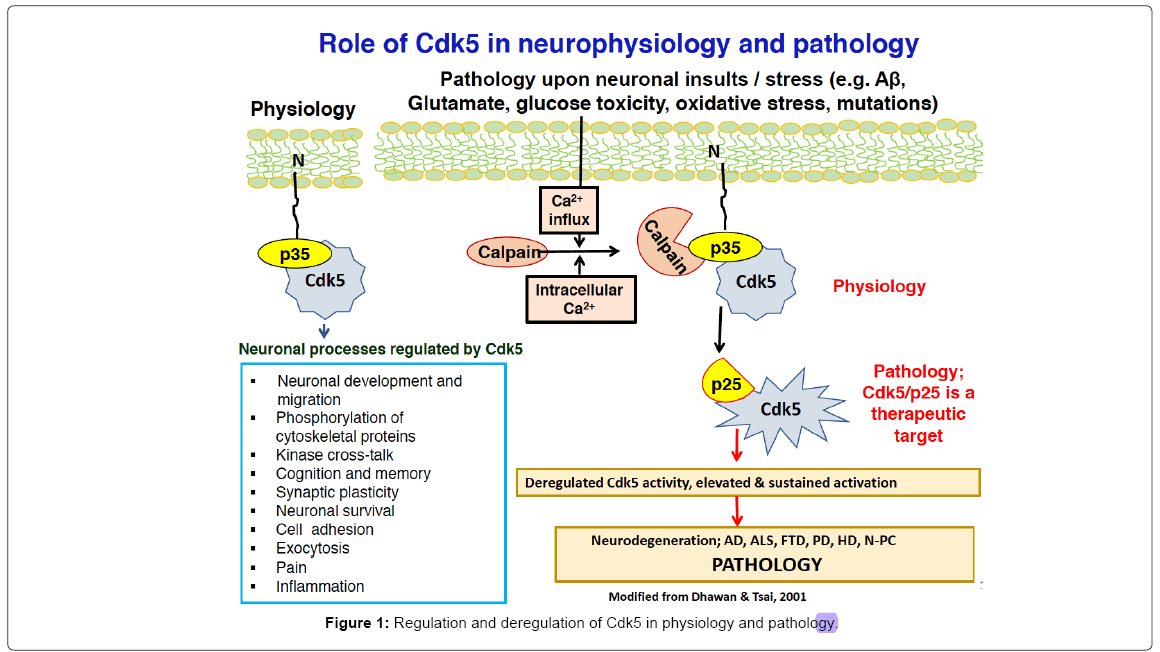
Normally, Cdk5 kinase, a Ser/Thr kinase of the cyclin dependent kinase family is essential for neuronal development, neuronal migration, cortical layering, synapse formation and behavioral functions [2]. The kinase targets a large number of neuronal protein substrates with proline directed Ser/Thr residues, which accounts, in part, for its importance in the nervous system. Its role stems from its regulation by neuronal-specific activators proteins, p35 and p39 instead of cyclins [3,4]. Cdk5, when isolated from the bovine brain is found in three states, as an large inactive complex in the cytoplasm, as an active Cdk5/p25 cytoplasmic complex and as part of a multimeric 650 kda complex bound to membranes by the p10, N-terminal myristolated domain of p35 [5] (Figure 1). In the brain, expression of p35 and p39 increases with development till adult; in fetus and adult p35 is phosphorylated by Cdk5 at Ser 8 which localizes a cytoplasmic Cdk5/p35 whereas in the fetus, phosphorylation of tyrosine 138 by tyrosine kinases, results in degradation of p35 via the proteosomeubiquitin pathway [4]. In the adult, however, Cdk5/p35 is restively more stable since neither site is phosphorylated as p35 acts to bundle microtubules and actin [6,7]. These regulatory options have profound effects on the role of Cdk5 in health and disease [8-11] (Figure 1).
It is intriguing that in several neurodegenerative disorders (AD, ALS, PD, HD) a hyperactive Cdk5 is detected in the brain at autopsy [6,7] and has been shown to be upstream of the pathways leading to plaques, tangles and inflammation in cultured neurons and model mice [8-11]. It does so when p35 is not phosphorylated and has been cleaved due to neuronal insults increasing intracellular calcium concentration inducing activation of calpain and cleavage into the myristolated p10 fragment and p25, a deregulated hyperactive Cdk5 [12]. It is assumed that aging –induced stresses affecting mitochondrial function, synaptic activity and the innate immune system of the brain may reflect upstream hyper activation of Cdk5/p25 or more likely, an interactive coupling of defects in multiple pathways. Although elevated levels of p25 have been reported in brains of AD, PD and ALS patients at autopsy, some laboratories have failed to confirm these observations; they show a downregulation of p25 instead [13,14] and more recently, a reduction of both p25 and p35 [15]. Although diverse postmortem changes may account for the differences, to date, there has been no resolution.
Hypothesis: Cdk5/p25 Activation and the Etiology of Neurodegeneration
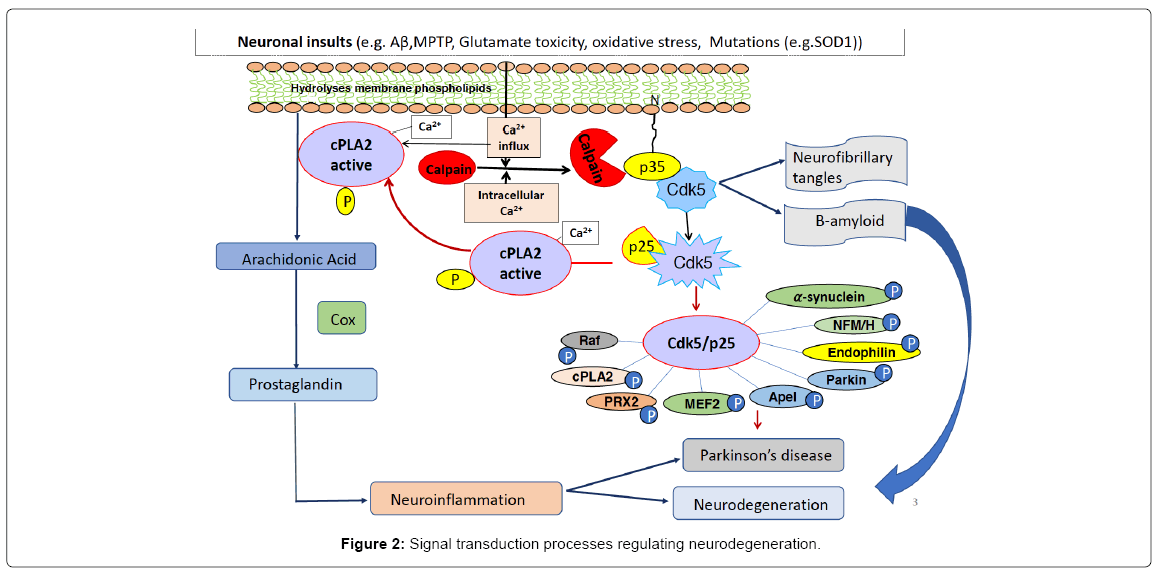
Of the many metabolic pathways compromised by aging, several have been identified as initiating neurodegenerative pathologies such as AD. The amyloid cascade hypothesis, for example, proposes that aging, via mutation or stress, results in Abeta accumulation, leading to misfolded toxic oligomers which induce inflammation, failing mitochondria, synaptic degradation, plaques and tangles [16]. A mitochondrial cascade hypothesis cites aging defects (mutations and stress) in mitochondrial metabolism as initiating events leading to defective mitochondria, oxidative stress, kinase activation, inflammation, DNA damage and the cognitive decline. Alternatively, evidence for the upstream role of hyperactive Cdk5/p25 has been shown in a number of different studies of cultured cells and model mice and has led to a hypothesis that continues to be tested (Figure 2).
The model assumes that neuronal insults and aging-induced upregulation of neuronal p25 is one of several etiological events triggering neurodegeneration and acts as age related mutations itself. The model proposes that this event leads to misfolding of proteins, oxidative defects in mitochondrial dysbioenergetics, inflammation, synaptic loss, plaques, tangles neuronal death, and behavioral decline [12]. The initiating event is a stress-induced upregulation of p25 by calpain cleavage of p35 into the P10 and p25 fragments [12]. P25 forms a more stable, deregulated cytoplasmic Cdk5/p25 hyperactive complex [12,17,18]. This toxic complex that contributes to metabolic events leading to neurodegeneration. In addition to cytoskeletal protein hyperphosphorylation, Cdk5/p25 hyperphosphorylation of other enzymes such as mitochondrial and other metabolic enzymes activities also affected and to enhance toxic effects. Perhaps the best example of p25-induced toxicity and progression of the AD phenotype is the p25 transgenic model mouse (p25Tg) [17,19,20] revealed all the AD phenotypes. We used bitransgenic (CK-p25Tg) mice (males and females) in which forebrain CK-p25 expression was tightly regulated by the tet on/off system facilitated by the CamKII (CK) promoter [17]. The tet-off system was induced by dietary alteration in doxycyclinesupplemented food in which CK-p25 expression was suppressed in the presence of doxycycline. Calmodulin-dependent protein kinase II (CaMKII/CK) is a promoter used to activate the expression of the CKp25 gene in cortical and hippocampal region of transgenic mice (p25 Tg). It was shown that CK-p25Tg mice develop significant neuronal loss in the cortex and hippocampus and display neurodegeneration and pathological tau hyper phosphorylation when p25 expression is induced. These mice are identified as p25Tg (over expressing). High p25 expression and elevated hippocampal Cdk5 activity has been observed within 1 weeks after induction.
All mice were developed and raised in the presence of doxycycline (DOX 1 mg/g in food). To induce CK-p25 expression, the mice were fed a normal diet, DOX-off. To inhibit p25 production, the mice were again fed a doxycycline diet. All experimental cohorts were treated under the same conditions and fed doxycycline diet for the same period of time Note that all animal procedures were performed in accordance with the NIH animal care committee’s regulations.
Design and Synthesis of TFP5
TFP5 is a truncated portion of p35 (activator of Cdk5), which extends 24 aa residues in length (Lys254–Ala277) conjugated with an 11-aa modifying peptide derived from the trans-activator domain of TAT protein at the C terminus (to facilitate passage through the blood-brain barrier), while FITC, fluorescein isothiocyanate (a green fluorescent tag) with linker GGG, was attached at the N terminus (to serve as a marker). A scrambled peptide (Scb) was used as a control to TFP5 (sequence shown below). Peptide 2.0 (Chantilly, VA, USA) commercially synthesized both TFP5 and Scb peptides which were used after dissolving both in saline or double distilled water.
Sequences used were as follows:
TFP5,
FITCGGGKEAFWDRCLSVINLMSSKMLQINAYARAARRAARR
Scb peptide,
FITCGGGGGGFWDRCLSGKGKMSSKGGGINAYARAARRAARR
Intraperitoneal (I.p.) injection paradigm
Five cohorts of mice were used: vehicle-injected WT, CKp25Tg+ DOX, CK-p25Tg-DOX, TFP5-injected CK-p25Tg-DOX and Scb-injected CK-p25Tg-DOX. After 12 weeks, the 3 mutant cohorts were taken off DOX to activate the expression of CK-p25. All five cohorts were age-matched and WT, CK-p25Tg+DOX, CK-p25Tg- DOX was treated with i.p. injection of vehicle, while another group of -DOX mutant mice were injected with 40 mg/kg/d TFP5. As an additional control, a cohort of –DOX mutants was injected with 40 mg/kg/d Scb peptide. All five cohorts were injected 3 days/week on week’s 13-17 for a total of 18 injections. All the mice were subjected to behavior analysis on week 18 and the mice were euthanized on week 19 when brain tissue was harvested for biochemical analysis. Based on the observation that injection of 3000 mg/kg/day of TFP5 into wild type mice in 5XFAD studies was not toxic, had no effect on body weight, behavior, appearance and longevity [21].
Conception, development and post-natal growth (at least to 12 days) are normal in the presence of doxycycline whereas removal of doxycycline induced over-expression of cortical p25, increased Cdk5 activity, neuronal loss, and progression to an AD-like phenotype with Ab plaques, hyper-phosphorylated tau tangles and inflammation [17,19]. It should be noted, in this transgenic, up regulation of p25 and hyperactivity of Cdk5 initiate inflammation via phosphorylation and activation of neuronal phospholipase 2A followed by astroglyosis and microgliosis which precede Abeta accumulation and tau phosphorylation [19]. In fact the situation has become even more confounding with the mixed results from other p25 mouse transgenic initiated at fertilization [22,23]. Robust p25 expression and Cdk5 activity at 4-5 months correlated with tau phosphorylation, axonopathy, neurodegeneration and severe motor defects but no evidence of other AD phenotypes. Moreover, later studies of the doxycyclin-regulated p25tg only added to the confusion; two roles for p25 were revealed, a low-dose, positive physiological role in memory formation, while a higher sustained p25 elevation induced neurodegeneration and ADlike phenotypes [24]. In our later examination of this transgenic, it is the latter response on which we focus.
p35-Derived Peptides as Therapeutic Candidates for Neurodegenerative Disorders
Clearly, Cdk5/p25 is a potential therapeutic target for neurodegeneration [25-27]. Although roscovitine and related compounds have been proposed and evaluated, their effects are nonspecific as they bind the common ATP site shared by other Cdks and most other kinases. Our lab has taken a different approach based on a study of truncated fragments of the p35 regulator [28]. Two peptides were identified, CIP (126 a.a) and a smaller peptide p5 (24 a.a.), derived from the p25 domain of the parent sequence, exhibited vigorous inhibition of Cdk5/p35 and Cdk5/p25 activities in test-tube experiments [29- 31]. Surprisingly, however, in cultured cortical neurons, the peptides inhibited only the Cdk5/p25 complex and spared the Cdk5/p35 kinase which retained most of its activity [31-33]. Here,E18 cortical neurons stressed by toxic Abeta display an AD phenotype, hyperactive Cdk5/ p25, hyperphosphorylated tau and neurofilaments, Abeta accumulation and apoptosis. These effects are reduced when cells are incubated in different concentrations of p5 or CIP [11,29,32]. The specificity of peptide inhibition was dramatic; whereas Cdk5/p25 activity was inhibited; Cdk5/p35 activity was unaffected as were the activities of cyclin dependent kinases [32].
Wherein does this specificity reside? We assume that the inhibitor peptides compete with the physiological regulators for the catalytic site on the kinase, as suggested by computer modeling experiments and in the test-tube, using purified enzyme complexes, inhibition by peptides is effective and comparable for both complexes. The situation in cells, with numerous interacting proteins, differs fundamentally, however. It has been demonstrated that p35, with its p10 N-terminal “domain” interacts with other proteins such as microtubules, actin, munc18 and others forming a multimeric complex [6,7,31,33,34]. We suggest that binding of macromolecules to the p10 domain favors a p35 conformational change such that it competes successfully for the Cdk5 catalytic site and sustains activity. P25, without the p10 domain, fails to compete successfully and is displaced by p5 which inhibits the kinase. An initial test of the hypothesis involved the incubation in vitro of microtubules to both Cdk5/p35 and Cdk5/p25 complexes followed by the addition of p5. Only the Cdk/p25 activity was inhibited [33]. A more extensive test of the role of the p10 domain in cultured neurons was carried out with Munc 18 a substrate of Cdk5 at the synapse [35,36]. Here, too, the activity of the Cdk5/p35 complex was spared in the presence of Munc 18 [31]. A key control was the observation that cortical neurons transfected with p67 siRNA exhibited inhibition of Cdk5/p35 in the presence of p5. Finally, the role of the p10 domain was confirmed in a pull-down experiment with GST-p10 which exhibited binding of Cdk5, p35 and p67 [31].
Therapeutic Effects of Peptides in Model Mice Showing Neurodegenerative Disorders
The real test of the hypothesis relies on studies of the effect of peptide treatment on those phenotypes in model mice resembling human disorders such as AD, ALS, PD and HD. A large, diverse selection of model mice has been engineered for each neurodegenerative disorder [37,38]. Most mouse models are prepared by introducing mutant human genes responsible for specific phenotypes. In AD, for example, transgenic bearing several known mutations of the APP pathway have been constructed which show a progression to AD resembling the phenotype in humans [38]. For the most part, most mouse models do mimic pathologies characterizing specific disorders. Behavioral criteria (memory, cognition, etc.), however, are difficult to duplicate in mice for many obvious reasons [37-39]. This raises the question as to whether such studies provide insight as to the nature and mechanisms of the human syndromes and may explain why clinical trials based on animal studies have been disappointing. Answers have been sought by comparisons of transcriptomes of brains from human patients and mimetic mouse models. Unfortunately, these reveal profound differences in the “up and down” expression of a wide range of genetic sets [40]. In general, mice are different from humans. Significantly, the authors report that mouse and human aging transcriptomes are more similar which suggests that overexpression of human gene mutations in transgenic mice distorts what is fundamentally natural aging. Nevertheless, the authors urge us to continue ways to improve the animal models.
Our approach is to carry on with tests of the hyperactive C
Cdk5/p25 hypothesis. We do so based on evidence of upregulated, deregulated Cdk5/p25 expression in AD brains [41], Cdk5/p35 activity (immunoprecipitated) associated with Lewy bodies in brains of Parkinson’s and ALS patients [42-44] and in our own work showing upregulation of p25 in brain and spinal cord of five ALS patients with matched controls (unpublished data). Results of our studies of the therapeutic effects of the two peptides in mouse models of AD, PD and ALS are consistent with the proposed hypothesis.
Proof of Concept; the CK-Tgp25 Transgenic
As previously indicated, the p25Tg transgenic mouse, when induced by doxycycline removal, exhibits a significant upregulation of p25 and Cdk5 activity. In the earlier study most data were reported after 5-12 weeks of induction and showed upregulation of Cdk5/p25 activity correlated with tau phosphorylated aggregates, a neurofibrillary pathology [17]. A later study recorded earlier time points with Cdk5 activation evident after one week induction [19]. Virtually at the same time, inflammatory signs were seen with an uptick of GFAP at one week, cytokines and chemokines of microgliosis and phosphor-tau after 4 weeks induction, followed by Abeta, most evident at 8 weeks post induction. To study the therapeutic potential of the larger CIP peptide, mutants with p25Tg AD-like phenotype were crossed with normally appearing CIP double transgenics (producing TetraTg- CIP mice controlled by a CAMK2a promoter) over expressing p25 in a background of CIP inhibitor peptide overexpression in the brain [45]. Doxycycline removal for 1 weeks initiated overexpression of p25 and Cdk5 hyper activation in forebrains of p25Tg mice which were diminished in the TetraTg CIP x p25 Tg-expressing mice as was inflammation, tau phosphorylation and amyloid deposition; AD pathology was significantly reduced as were neuronal cell loss and neurocognitive defects. This is the first successful therapeutic targeting of Cdk5/p25 hyperactivity in vivo while sparing effects on Cdk5/p35 activity.
Analysis of the therapeutic effect of the smaller p5 peptide in the same p25Tg model mouse required a different approach. Here, the p5 peptide, modified for penetration of the blood-brain barrier as TFP5 was injected intraperitoneally [21,46].
Control and experimental animals were maintained on doxycycline for 12 weeks, then doxycycline was removed and a seriesof TFP5 injections (weeks 13 to 17) were given to p25Tg animals while controls received an identical series with scrambled peptide at the same concentration. Untreated animals showed the induced increase in p25 overexpression and Cdk5 activity whereas the TFP5 treated cohorts exhibited a 40% reduction in activity. They also showed reduced tau and neurofilament (NFM/H) phosphorylation, reduced inflammation and amyloid beta expression accompanied by improved behavioral function. Significantly there was an improvement in LTD expression, a sign that synaptic activity had been restored [46]. These studies suggest that neurodegenerative disorders expressing deregulated Cdk5/ p25 may be therapeutically targeted with an appropriately designed inhibitor peptide derived from p35, the endogenous regulator in the brain.
The 5XFAD AD Model Mouse; the Effect of P5 Therapy
A different mouse model, familial 5XFAD, is a transgenic expressing human APP and PSI mutant genes (a total of five mutations). It overexpresses Abeta and amyloid plaques and exhibits significant defects in spatial memory and behavior [47]. Tau hyper phosphorylation and tangles are also evident as well as Cdk5/p25 hyperactivity. There is evidence that Abeta is toxic and induces tau hyper phosphorylation via activation of Cdk5/p25 in cortical neurons [29,33]. Moreover, intracerebroventricular injection of A beta in a mouse model hyper activates Cdk5/p25; it appears that Cdk5/p25 activation and Abeta are part of a circular feedback loop. For example, overexpression of p25 increases Cdk5-induced BACE1 transcription and the abnormal processing of APP [47,48]. Evidence from the effect of p5 peptide on the p5 overexpressing p25Tg transgenic does show the linkage between these metabolic pathways. Does it exhibit the same effect in the amyloid overexpressing model?
An injection protocol with TFP5 was established for this transgenic [21]. Control and experimental animals (6 months to 12 months of age) were injected intraperitoneally with and without TFP5 for three consecutive days (40 mg/kg) followed by a day of behavioral tests and sacrifice to dissect brains for biochemical and immunocytochemical analyses. TFP5 was shown to penetrate the blood-brain barrier; fluorescence was seen in cortex, hippocampus and cerebellum (as well as other organs) after four days. Significantly, after four days the hyper activation of Cdk5 was reduced in TFP5 treated animals to normal WT values; scrambled peptide controls had no effect. Coupled to these changes were significant improvements in behavioral tests (e.g. Y maze) as well as reductions in inflammation, hyper phosphorylation of neurofilaments and the deposition of amyloid plaques [21].
Neuronal apoptosis was also decreased by 37% in the TFP5-treated mice. Here, too, the AD phenotype in this mouse model was successfully reduced only seven days after the last treatment, without affecting the endogenous Cdk5/p35 kinase activity. Moreover, by targeting the hyperactive Cdk5/p25 kinase, the Abeta phenotype is affected, consistent with the view that kinase activity and APP processing are linked, perhaps because Cdk5 phosphorylates the thr668 site on APP, a step in Abeta processing [48-50]. Differences in phosphorylation patterns at this site between Cdk5/p35 and Cdk5/p25 were reported, the former phosphorylating both mature and immature APP while the latter only increased phosphorylation of the immature form [47]. Hence, by targeting Cdk5 we show specific effects of the peptide inhibitor on other pathological pathways underlying neurodegeneration.
Parkinson’s Disease: Effect of P5 Peptide on the MPTP Mouse Model
Parkinson’s disease is one of the consequences of aging and is increasing globally as the world population ages. This disorder, specific to substantia nigra cells, is also characterized by aggregate accumulation, synuclein-containing Lewy bodies that lead to neuronal death. Synuclein aggregates reflect dysregulation of the autophagy pathways that modulate the degradation of misfolded and abnormal proteins [51]. It is noteworthy that these aggregates also contain Cdk5 [42,43,52]. Moreover, post mortem studies of PD brains show evidence of calpain-induced Cdk5/p25 activation [53]. Mutations in a human ubiquitin-protein ligase, Parkin, also contribute to the AD phenotype [54,55]. Parkin, hyper phosphorylated by Cdk5, become dysfunctional and leads to the accumulation of damaged proteins [56]. As in AD, Cdk5 plays a key role in etiology of the PD phenotype.
Several mouse models of Parkinsons have been produced, e.g. transgenics overexpressing a dominant alpha synuclein mutant), but a more common model develops after treatment with a drug, 1-methyl- 4phenyl-1,2,3,6-tetrahydropyridine (MPTP) which specifically destroys dopaminergic neurons in the substantia nigra of the brain leading to neuronal death [57]. Neuron loss is correlated with the overexpression of Cdk5 which may be responsible for mitochondrial dysfunction and oxidative stress, coupled to deregulated protein folding and autophagy [58].
We have used this PD model to study the effect of TFP5 and TP5 peptides on the expression of the abnormal Parkinson’s phenotype [59,60]. Induction of hyperactive Cdk5/p25 was confirmed in mesencephalic cultures treated with MPTP, which was significantly reduced when cells were pretreated with TFP5, as was cell survival. Inflammation of primary mesencephalic cells was also ameliorated by peptide pretreatment, before incubation in MPTP. More to the point, potential therapeutic effects in vivo were tested in a four-dose MPTP model mouse [61]. Animals were injected intraperitoneally with TP5/ TFP5 at a high dose (80 mg/kg) for 9 days; on day 2, however, they had received the four doses of MPTP. Here, too, Cdk5/p25 activity was reduced in the substancia nigral cells, as was inflammation, dopamine levels and cell death. No protection was afforded by pretreatment with the control scrambled peptide.
It is not clear that Cdk5/p25 activation is the upstream trigger for induction of the MPTP phenotype; its mechanism of action is confounded by the fact that cross talk between Cdk5 and other kinases is a common feature in the brain. Moreover, the numerous substrate targets of Cdk5 may be affected [62] including some implicated in MPTP pathology involving mitochondrial function such as cytochrome c release, caspase-3 activation and reduction of the anti-oxidant Prx2. These aspects of mitochondrial activity were protected after TFP5 treatment.
It should be pointed out that transcriptome analyses of PD mouse brains as contrasted with human PD transcriptomes differ significantly, at least with respect to the more than 250 unregulated genes in human brains that are not matched in the mouse models [40]. Downregulated genes, however, exhibit a greater match. These discrepancies between mouse and human characterize the transcriptome data for other neurodegenerative disorders [40], which may account for the fact that primate models for PD have been introduced [40]. Future tests of the peptides in these primate models of PD might bring us closer to clinical trials in human patients.
Brain Ischemia; Cdk5 Activation and Regulation by P5 Peptides
Stroke, a leading cause of death world-wide, is responsible for short and long term cognitive impairment, i.e., declines in memory, learning and executive functions. There are reports that Cdk5 activity is upregulated in human stroke calpain upregulation and hyper activation of Cdk5/p25 have been identified in animal models of ischemia and may be responsible for downstream pathologies associated with neuronal death [63-65]. Accordingly, hyper activated Cdk5/p25 has been suggested as a target for therapeutic intervention after a stroke [66]. Several approaches such as roscovitine-like inhibitors [67] or Cdk5 silencing by Cdk5 RNAi induced neuroprotection; in the case of the latter, treatment resulted in reversal of learning defects and memory after one and four months post ischemic induction in rats [68,69]. The treatment prevented neuronal loss, inflammation, tau pathology as well as a behavioral deficit, including hippocampal long term potentiation. Upregulation of BDNF in the hippocampus may have been a contributing factor.
The pattern of Cdk5/p25 activation after an ischemic episode resembles that of other neurodegenerative disorders and invites the application of the p5 peptide therapeutic strategy so successful in AD and PD [70]. Using a hypoxia/ischemic insult in neonatal rats on postnatal day 7, brains from experimental (intraperitoneal injected p5-TAT (TP5) treated to facilitate crossing the blood-brain barrier) and sham controls (untreated) were compared as to levels of p25, p35 and Cdk5 activity at different time points post ischemia. P35 decreased after the insult whereas p25 increased robustly as did Cdk5/p25 activity. The p5- TAT treatment, however, had no effect on the levels of the regulators, but hyperactive Cdk5/p25 was diminished as seen in the reduction in levels of phospho-tau and phosphorylation of the glucocorticoid receptor [70]. After seven daily injections of p5-TAT post insult, behavioral studies also pointed to a successful improvement. Again, we see that in those neuronal disorders marked by a significant uptick of calpain activity, p25 upregulation and hyperactive Cdk5/p25 phosphorylation of substrates such as tau, treatment with an inhibitory peptide derived from the p35 kinase regulator successfully attenuates pathology, neuronal death and compromised behavior.
Regulation of Insulin Secretion in Pancreas; Cdk5 Activity and Peptide Inhibition
Cdk5 kinase activity is not restricted to the nervous system; it plays a role in diverse cell types including muscle, pancreas and even cancers, among others [71]. It is involved in transcription regulation, muscle differentiation, cell migration and adhesion and in insulin regulation of glucose uptake. Studies of its role in type 2 diabetes, have led to conflicting results. Although Cdk5 and its activators, p35 and p39 are expressed in pancreatic islets and beta cell lines, some reports claim its activation promotes insulin secretion [72,73], while others report that knockout of p35 and inhibition of Cdk5 activity promotes insulin secretion [74,75]. We have shown that duration of glucose toxicity is a key factor in regulation; short term (2 h) resulted in a modest increase in Cdk5 activity and insulin secretion whereas long term exposure results in significantly enhanced Cdk5 activity and a decline in insulin secretion [33]. Overexpression of p35 in Min6 cells plus high glucose toxicity is a stress signal that induces p25 expression and Cdk5 hyperactivation. The end result is a significant inhibition of insulin secretion. This pattern of Cdk5 deregulation resembles that seen in some neuronal disorders and suggests that inhibition of Cdk5 may promote insulin secretion under these conditions, which it does when using roscovitine or dnCdk5 transfection [33]. These non-specific results, however, fail to distinguish between inhibition of Cdk5/p35 or Cdk5/p25 activities (or both. Subsequently, we have shown that CIP, the large peptide that specifically inhibits Cdk5/p25 does rescue insulin secretion at high glucose [76,77], as does the smaller peptide TFP5 [78].
Results showing peptide inhibition of Cdk5 deregulation in nonneuronal cells invites speculation that many diverse organs and tissues in which Cdk5 plays a key role, that the peptides may also affect those disorders marked by hyperactive Cdk5 induced by toxic overexpression of p25.
Conclusion
The protein kinase Cdk5 is ubiquitous, found in most mammalian cells and tissues, where, because of its wide range of targeted substrates, is involved in key signaling pathways and kinase cross-talk. It is tightly regulated physiologically by non-cyclin activators, p35, p67 and p39 and as a Cdk5/p35 complex, is essential in the development of the nervous system, synaptogenesis, synaptic function and neuronal survival.
Under neuronal stress (aging, mutations, environmental insults) the kinase is deregulated; increased calcium flux evokes activation of the proteinase calpain, cleavage of p35 (and or p39) into a p10 myristoylated N-terminal fragment and p25, a hyperactive regulator which stably binds Cdk5, hyperactivates it and induces cellular pathology (protein aggregates) in several neuronal disorders. Similarly deregulated in other cells and tissues it provokes cell specific pathologies (e.g. insulin secretion). Accordingly, the Cdk5/p25 complex has been identified as a therapeutic target.
We have produced two small peptides, (CIP, 126 and P5, 24 aa) truncated fragments of p35, which specifically inhibit the Cdk5/p25 and Cdk5/p35 complexes in vitro but in cultured cells and in vivo in model mice, CIP and p5 specifically inhibits the abnormal Cdk5/p25 without affecting activities of Cdk5/p35 nor of related Cdks, the cell cycle kinases. Treatment with the peptides successfully reduces pathologies and behavioral defects in mouse models of AD, PD, ischemia and type 2 diabetes. Following are some of the novelties of CIP and P5 peptides; The novelty of these peptide is absence of toxicity (>3000 mgm/kg in mice) and have higher affinity with Cdk5 compared to ATP. These peptides are very stable at room temperature.
References
- Minter MR, Taylor JM, Crack PJ (2016) The contribution of neuroinflammation to amyloid toxicity in Alzheimer's disease. J Neurochem 136: 457-474.
- Hammond V, Tsai LH, Tan SS (2004) Control of cortical neuron migration and layering: Cell and non-cell autonomous effects of p35. J Neurosci 24: 576-587.
- Hisanaga S, Saito T (2003) The regulation of cyclin-dependent kinase 5 activity through the metabolism of p35 or p39 Cdk5 activator. Neurosignals 12: 221-229.
- Shah K, Lahiri DK (2014) Cdk5 activity in the brain-multiple paths of regulation. J Cell Sci 127: 2391-2400.
- Lee MH, Nikolic M, Baptista CA, Lai E, Tsai LH, et al. (1996) The brain-specific activator p35 allows Cdk5 to escape inhibition by p27Kip1 in neurons. Proc Natl Acad Sci U S A 93: 3259-3263.
- Hou Z1, Li Q, He L, Lim HY, Fu X, et al. (2007) Microtubule association of the neuronal p35 activator of Cdk5. J Biol Chem 282: 18666-18670.
- He L, Hou Z, Qi RZ (2008) Calmodulin binding and Cdk5 phosphorylation of p35 regulate its effect on microtubules. J Biol Chem 283: 13252-13260.
- Cruz JC, Tsai LH (2004) Cdk5 deregulation in the pathogenesis of Alzheimer's disease. Trends Mol Med 10: 452-458.
- Kesavapany S, Lau KF, Ackerley S, Banner SJ, Shemilt SJ, et al. (2003) Identification of a novel, membrane-associated neuronal kinase, cyclin-dependent kinase 5/p35-regulated kinase. J Neurosci 23: 4975-4983.
- Kesavapany S, Li BS, Amin N, Zheng YL, Grant P, et al. (2004) Neuronal cyclin-dependent kinase 5: role in nervous system function and its specific inhibition by the Cdk5 inhibitory peptide. Biochim Biophys Acta 1697: 143-153.
- Kesavapany S, Zheng YL, Amin N, Pant HC (2007) Peptides derived from Cdk5 activator p35, specifically inhibit deregulated activity of Cdk5. Biotechnol J 2: 978-987.
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, et al. (2000) Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405: 360-364.
- Tandon A, Yu H, Wang L, Rogaeva E, Sato C, et al. (2003) Brain levels of CDK5 activator p25 are not increased in Alzheimer's or other neurodegenerative diseases with neurofibrillary tangles. J Neurochem 86: 572-581
- Yoo BC, Lubec G (2001) p25 protein in neurodegeneration. Nature 411: 763-764.
- Engmann O, Hortobágyi T, Pidsley R, Troakes C, Bernstein HG, et al. (2011) Schizophrenia is associated with dysregulation of a Cdk5 activator that regulates synaptic protein expression and cognition. Brain 134: 2408-2421.
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science 297: 353-356.
- Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH (2003) Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron 40: 471-483.
- Patrick GN, Zhou P, Kwon YT, Howley PM, Tsai LH. (1998) p35, the neuronal-specific activator of cyclin-dependent kinase 5 (Cdk5) is degraded by the ubiquitin-proteasome pathway. J Biol Chem 273: 24057-24064.
- Sundaram JR, Chan ES, Poore CP, Pareek TK, Cheong WF, et al. (2012) Cdk5/p25-induced cytosolic PLA2-mediated lysophosphatidylcholine production regulates neuroinflammation and triggers neurodegeneration. J Neurosci 32: 1020-1034.
- Shukla V, Seo J, Binukumar BK, Amin ND, Reddy P, et al. (2017) TFP5, a peptide inhibitor of aberrant and hyperactive Cdk5/p25, attenuates pathological phenotypes and restores synaptic function in CK-p25Tg mice. J Alzheimers Dis 56: 335-349.
- Shukla V, Zheng YL, Mishra SK, Amin ND, Steiner J, et al. (2013) A truncated peptide from p35, a Cdk5 activator, prevents Alzheimer's disease phenotypes in model mice. FASEB J 27: 174-186.
- Ahlijanian MK, Barrezueta NX, Williams RD, Jakowski A, Kowsz KP, et al. (2000) Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of Cdk5. Proc Natl Acad Sci U S A 97: 2910-2915.
- Bian F, Nath R, Sobocinski G, Booher RN, Lipinski WJ, et al. (2002) Axonopathy, tau abnormalities, and dyskinesia, but no neurofibrillary tangles in p25-transgenic mice. J Comp Neurol 446: 257-266.
- Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai LH (2005) Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron 48: 825-838.
- Tsai LH, Lee MS, Cruz J (2004) Cdk5, a therapeutic target for Alzheimer's disease? Biochim Biophys Acta 1697: 137-142.
- Glicksman MA, Cuny GD, Liu M, Dobson B, Auerbach K, et al. (2007) New approaches to the discovery of Cdk5 inhibitors. Curr Alzheimer Res 4: 547-549.
- Kanungo J, Zheng YL, Amin ND, Pant HC (2009) Targeting Cdk5 activity in neuronal degeneration and regeneration. Cell Mol Neurobiol 29: 1073-1080.
- Amin ND, Albers W, Pant HC (2002) Cyclin-dependent kinase 5 (Cdk5) activation requires interaction with three domains of p35. J Neurosci Res 67: 354-362.
- Zheng YL, Li BS, Amin ND, Albers W, Pant HC (2002) A peptide derived from cyclin-dependent kinase activator (p35) specifically inhibits Cdk5 activity and phosphorylation of tau protein in transfected cells. Eur J Biochem 269: 4427-4434.
- Kesavapany S, Amin N, Zheng YL, Nijhara R, Jaffe H, et al. (2004) p35/cyclin-dependent kinase 5 phosphorylation of ras guanine nucleotide releasing factor 2 (RasGRF2) mediates Rac-dependent Extracellular Signal-regulated kinase 1/2 activity, altering RasGRF2 and microtubule-associated protein 1b distribution in neurons. J Neurosci 24: 4421-4431.
- Amin ND, Zheng Y, Bk B, Shukla V, Skuntz S, et al. (2016) The interaction of Munc 18 (p67) with the p10 domain of p35 protects in vivo Cdk5/p35 activity from inhibition by TFP5, a peptide derived from p35. Mol Biol Cell 27: 3221-3232.
- Zheng YL, Kesavapany S, Gravell M, Hamilton RS, Schubert M, et al. (2005) A Cdk5 inhibitory peptide reduces tau hyperphosphorylation and apoptosis in neurons. EMBO J 24: 209-220.
- Zheng YL, Amin ND, Hu YF, Rudrabhatla P, Shukla V, et al. (2010) A 24-residue peptide (p5), derived from p35, the Cdk5 neuronal activator, specifically inhibits Cdk5-p25 hyperactivity and tau hyperphosphorylation. J Biol Chem 285: 34202-34212.
- Bhaskar K, Shareef MM, Sharma VM, Shetty AP, Ramamohan Y, et al. (2004) Co-purification and localization of Munc18-1 (p67) and Cdk5 with neuronal cytoskeletal proteins. Neurochem Int 44: 35-44.
- Shetty KT, Kaech S, Link WT, Jaffe H, Flores CM, et al. (1995) Molecular characterization of a neuronal-specific protein that stimulates the activity of Cdk5. J Neurochem 64: 1988-1995.
- Shuang R, Zhang L, Fletcher A, Groblewski GE, Pevsner J, et al. (1998) Regulation of Munc-18/syntaxin 1A interaction by cyclin-dependent kinase 5 in nerve endings. J Biol Chem 273: 4957-4966.
- Wong PC, Cai H, Borchelt DR, Price DL (2002) Genetically engineered mouse models of neurodegenerative diseases. Nat Neurosci 5: 633-639.
- Janus C, Welzl H (2010) Mouse models of neurodegenerative diseases: Criteria and general methodology. Methods Mol Biol 602: 323-345.
- Reaume AG, Howland DS, Trusko SP, Savage MJ, Lang DM, et al. (1996) Enhanced amyloidogenic processing of the beta-amyloid precursor protein in gene-targeted mice bearing the Swedish familial Alzheimer's disease mutations and a "humanized" Abeta sequence. J Biol Chem 271: 23380-23388.
- Ashe KH (2005) Mechanisms of memory loss in Abeta and tau mouse models. Biochem Soc Trans 33: 591-594.
- Li MD, Burns TC, Morgan AA, Khatri P (2014) Integrated multi-cohort transcriptional meta-analysis of neurodegenerative diseases. Acta Neuropathol Commun 2: 93.
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, et al. (1999) Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402: 615-622.
- Brion JP, Couck AM (1995) Cortical and brainstem-type Lewy bodies are immunoreactive for the cyclin-dependent kinase 5. Am J Pathol 147: 1465-1476.
- Nakamura S, Kawamoto Y, Nakano S, Akiguchi I, Kimura J (1997) p35nck5a and cyclin-dependent kinase 5 co-localize in Lewy bodies of brains with Parkinson's disease. Acta Neuropathol 94: 153-157.
- Nakamura S, Kawamoto Y, Nakano S, Ikemoto A, Akiguchi I, et al. (1997) Cyclin-dependent kinase 5 in Lewy body-like inclusions in anterior horn cells of a patient with sporadic amyotrophic lateral sclerosis. Neurology 48: 267-270.
- Sundaram JR, Poore CP, Sulaimee NH, Pareek T, Asad AB, et al. (2013) Specific inhibition of p25/Cdk5 activity by the Cdk5 inhibitory peptide reduces neurodegeneration in vivo. J Neurosci 33: 334-343.
- Oakley H, Cole SL, Logan S, Maus E, Shao P, et al. (2006) Intraneuronal beta-amyloid aggregates, neurodegeneration and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci 26: 10129-10140.
- Lopes JP, Oliveira CR, Agostinho P (2007) Role of cyclindependent kinase 5 in the neurodegenerative process triggered by amyloid-beta and prion peptides: Implications for Alzheimer’s disease and prion-related encephalopathies. Cell Mol Neurobiol 27: 943-957.
- Wen Y, Yu WH, Maloney B, Bailey J, Ma J, et al. (2008) Transcriptional regulation of beta-secretase by p25/Cdk5 leads to enhanced amyloidogenic processing. Neuron 57: 680-690.
- Liu F, Su Y, Li B, Zhou Y, Ryder J, et al. (2003) Regulation of amyloid precursor protein (APP) phosphorylation and processing by p35/Cdk5 and p25/Cdk5. FEBS Lett 547: 193-196.
- Chang Y, Ostling P, Akerfelt M, Trouillet D, Rallu M, et al. (2006) Role of heat-shock factor 2 in cerebral cortex formation and as a regulator of p35 expression. Genes Dev 20: 836-847.
- Su LY, Li H, Lv L, Feng YM, Li GD, et al. (2015) Melatonin attenuates MPTP-induced neurotoxicity via preventing Cdk5-mediated autophagy and SNCA/a-synuclein aggregation. Autophagy 11: 1745-1759.
- Takahashi M, Iseki E, Kosaka K (2000) Cdk5 and munc-18/p67 co-localization in early stage neurofibrillary tangles-bearing neurons in Alzheimer type dementia brains. J Neurol Sci 172: 63-69.
- Alvira D, Ferrer I, Gutierrez-Cuesta J, Garcia-Castro B, Pallàs M, et al. (2008) Activation of the calpain/Cdk5/p25 pathway in the girus cinguli in Parkinson's disease. Parkisonism Relatd Disord 14: 309-313.
- Dawson TM, Ko HS, Dawson VL (2010) Genetic animal models of Parkinson's disease. Neuron 66: 646-661.
- Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, et al. (2014) Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J 33: 2798-2813.
- Avraham E, Rott R, Liani E, Szargel R, Engelender S (2007) Phosphorylation of Parkin by the cyclin-dependent kinase 5 at the linker region modulates its ubiquitin-ligase activity and aggregation. J Biol Chem 282: 12842-12850.
- Smith D1 (2003) Cdk5 in neuroskeletal dynamics. Neurosignals 12: 239-251.
- Wang W, Xu M, Wang G, Galili G (2017) Autophagy: An important biological process that protects plants from stressful environments. Front Plant Sci 7: 2030.
- Binukumar BK, Shukla V, Amin ND, Grant P, Bhaskar M, et al. (2015) Peptide TFP5/TP5 derived from Cdk5 activator P35 provides neuroprotection in the MPTP model of Parkinson's disease. Mol Biol Cell 26: 4478-4491.
- Binukumar BK, Pelech SL, Sutter C, Shukla V, Amin ND, et al. (2016) Profiling of p5, a 24 amino acid inhibitory peptide derived from the CDK5 activator, p35 CDKR1 against 70 protein kinases. J Alzheimers Dis 54: 525-533.
- Jackson-Lewis V, Przedborski S (2007) Protocol for the MPTP mouse model of Parkinson's disease. Nat Protoc 2: 141-151.
- Lim AC, Qu D, Qi RZ (2003) Protein-protein interactions in Cdk5 regulation and function. Neurosignals 12: 230-238.
- Rashidian J, Rousseaux MW, Venderova K, Qu D, Callaghan SM, et al. (2009) Essential role of cytoplasmic Cdk5 and Prx2 in multiple ischemic injury models, in vivo. J Neurosci 29: 12497-12505.
- Hayashi T, Warita H, Abe K, Itoyama Y (1999) Expression of cyclin-dependent kinase 5 and its activator p35 in rat brain after middle cerebral artery occlusion. Neurosci Lett 265: 37-40.
- Love S (2003) Neuronal expression of cell cycle-related proteins after brain ischaemia in man. Neurosci Lett 353: 29-32.
- Slevin M, Krupinski J (2009) Cyclin-dependent kinase-5 targeting for ischaemic stroke. Curr Opin Pharmacol 9: 119-124.
- Menn B, Bach S, Blevins TL, Campbell M, Meijer L, et al. (2010) Delayed treatment with systemic (S)-roscovitine provides neuroprotection and inhibits in vivo Cdk5 activity increase in animal stroke models. PLoS ONE 5: e12117.
- Gutiérrez-Vargas JA, Múnera A, Cardona-Gómez GP (2015) Cdk5 knockdown prevents hippocampal degeneration and cognitive dysfunction produced by cerebral ischemia. J Cereb Blood Flow Metab 35: 1937-1949.
- Gutiérrez-Vargas JA, Múnera A, Cardona-Gómez GP (2016) Targeting CDK5 post-stroke provides long-term neuroprotection and rescues synaptic plasticity. J Cereb Blood Flow Metab pii: 0271678X16662476.
- Tan X, Chen Y, Li J, Li X, Miao Z, el. (2015) The inhibition of Cdk5 activity after hypoxia/ischemia injury reduces infarct size and promotes functional recovery in neonatal rats. Neurosci 290: 552-560.
- Contreras AV, Monge-Cazares T, Alfaro-Ruiz L, Hernandez-Morales S, Miranda-Ortiz H, et al. (2011) Resequencing, haplotype construction and identification of novel variants of CYP2D6 in Mexican Mestizos. Pharmacogenomics 12: 745-756.
- Lilja L, Yang SN, Webb DL, Juntti-Berggren L, Berggren PO, et al. (2001) Cyclin-dependent kinase 5 promotes insulin exocytosis. J Biol Chem 276: 34199-34205.
- Lilja L, Johansson JU, Gromada J, Mandic SA, Fried G, et al. (2004) Cyclin-dependent kinase 5 associated with p39 promotes Munc18-1 phosphorylation and Ca(2+)-dependent exocytosis. J Biol Chem 279: 29534-29541.
- Wei FY, Nagashima K, Ohshima T, Saheki Y, Lu YF, et al. (2005) Cdk5-dependent regulation of glucose-stimulated insulin secretion. Nat Med 11: 1104-1108.
- Ubeda M, Kemp DM, Habener JF (2004) Glucose-induced expression of the cyclin dependent protein kinase 5 activator p35 involved in Alzheimer's disease regulates insulin gene transcription in pancreatic beta-cells. Endocrinology 145: 3023-3031.
- Zheng YL, Li C, Hu YF, Cao L, Wang H, et al. (2013) Cdk5 inhibitory peptide (CIP) inhibits Cdk5/p25 activity induced by high glucose in pancreatic beta cells and recovers insulin secretion from p25 damage. PLoS ONE 8: e63332.
- Binukumar BK, Zheng YL, Shukla V, Amin ND, Grant P, et al. (2014) TFP5, a peptide derived from p35, a Cdk5 neuronal activator, rescues cortical neurons from glucose toxicity. J Alzheimers Dis 39: 899-909.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 4343
- [From(publication date):
August-2017 - Jul 04, 2025] - Breakdown by view type
- HTML page views : 3412
- PDF downloads : 931