Peculiar Histopathological Alterations of Enterocytes in a Patient Co-Infected with Severe Acute Respiratory Syndrome Coronavirus 2 and Mycobacterium Tuberculosis: A Case Study
Abstract
The ongoing novel coronavirus disease 2019 (COVID-19) is principally defined by its respiratory symptoms. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can affect the gastrointestinal tract (GIT) and although the pathogenesis of COVID-19 is understood, the exact pathological alterations following infection require further investigation. Here, we report our histopathological findings from a right hemicolectomy specimen from a patient coinfected with COVID-19 and Mycobacterium tuberculosis. Our observations showed that the novel SARS-CoV-2 can affect the GIT, causing epithelial injury and pathological alterations attributed to its ability to infect absorptive enterocytes by interacting with the Angiotensin Converting Enzyme-2 (ACE2) receptor. These pathological findings are regarded as viral cytopathic changes and should be considered when evaluating gastrointestinal specimens from COVID-19 infected patients.
Keywords: SARS-CoV-2; Angiotensin converting enzyme-2; Gastro intestinal tract
Introduction
The ongoing coronavirus disease 2019 (COVID-19) pandemic caused by the novel severe acute respiratory syndrome coronavirus 2 (SARSCoV- 2) has rapidly spread worldwide. It has infected more than 100 million individuals and led to more than 2 million deaths. SARS-CoV-2 is associated with the development of a systemic disease that typically affects the respiratory tract. The most common symptoms are fever, dry cough, fatigue, headache, and myalgia. SARS-CoV-2 can also affect the gastrointestinal tract (GIT), but the incidence of COVID-19 in the GIT as the main presentation or as a concurrent infection with the respiratory tract is low. The range of GIT symptoms include diarrhea, abdominal pain, nausea, vomiting, and anorexia [1]. While the virus is highly contagious and spreads predominantly by respiratory droplets and aerosols, SARSCoV- 2 has been isolated from stool samples, suggesting that the virus could be transmitted via the fecal oral route [2]. There is a good understanding of the pathogenesis of GIT infections caused by SARS-CoV-2 due to continuous investigations. However, the histopathological alterations of the gastrointestinal mucosa have not been well elucidated. We observed incidental peculiar pathological alterations of the terminal ileal mucosa and the mesenteric lymph nodes, which were part of a right hemicolectomy specimen obtained as a result of a bowel perforation event secondary to a GIT infection with Mycobacterium tuberculosis in a COVID-19 positive patient. Our aim was to share these microscopic findings with practicing pathologists and improve the awareness of what they can expect to see in gastrointestinal biopsies and resections of COVID-19 infected patients.
Case Report
Materials and methods
A right hemicolectomy specimen was received fixed in 10% neutral buffered formalin, appropriately sectioned, and examined. Representative sections were then processed; they were microtome cut at a thickness of 4 μm and hematoxylin eosin stained. These sections were placed on charged slides and prediluted solutions of Cluster of Differentiation 10 (CD10; 56C6, Dako), Epithelial Cell Adhesion Molecule (Ep-CAM; Ber-EP4, Ventana Medical Systems Inc., Tucson, AZ, USA), Epithelial Membrane Antigen (EMA; GP1.4, Leica), E-cadherin (clone 36, Ventana), and Ki-67 (clone MM1, Leica, diluted 1:50) were evaluated by immunohistochemistry. Sections on regular slides were stained with periodic acid Schiff histochemical stain and Ziehl-Neelsen stain.
Pathological findings
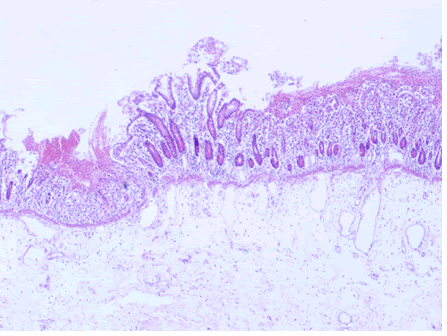
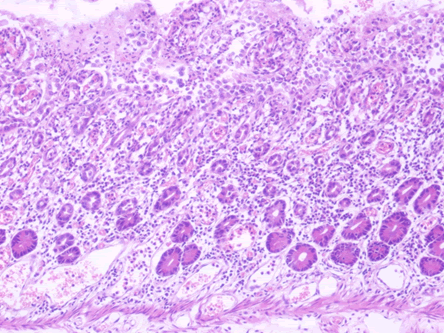
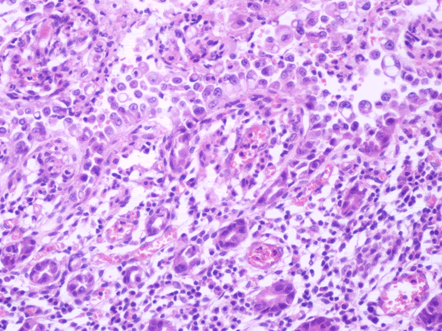
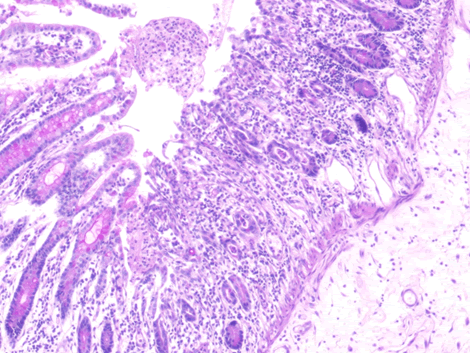
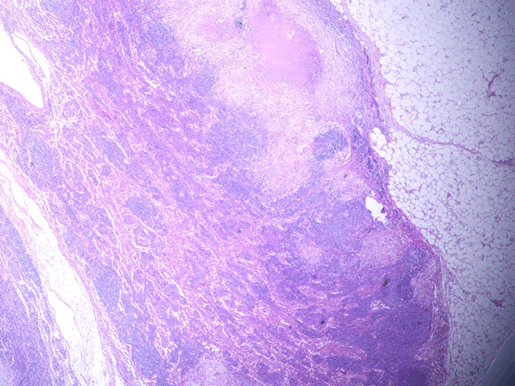
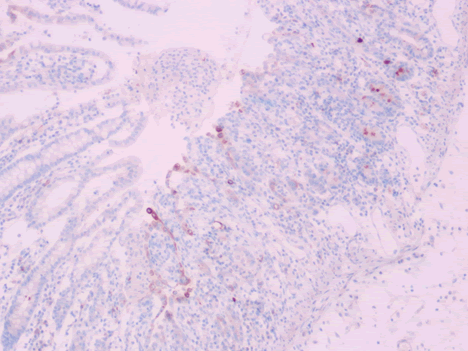
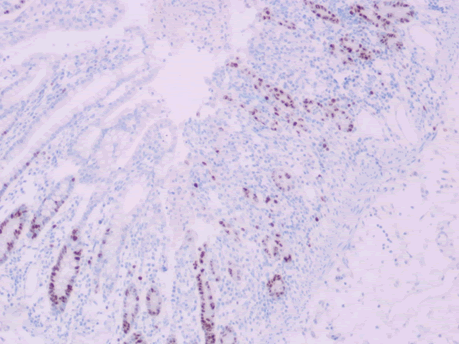
Microscopic examination of the terminal ileum, cecum, ascending colon, and appendix revealed multiple caseating epithelioid granulomas scattered throughout the wall. In addition, the overlying mucosal tissue was ulcerated, with granulation tissue formation. Numerous acid fast bacilli within the epithelioid granulomas were observed following Ziehl-Neelsen staining. The remainder of the terminal ileum mucosa showed patchy peculiar architectural alterations intertwined with normal intervening mucosa, and the villi of the affected mucosa were short, wide, and blunt (Figures 1 and 2).There was a striking "hobnail" appearance with focal detachment/denudation of enterocytes, erosions, and fibrinopurulent exudate (Figure 3). In addition, the loss of microvilli and goblet cells was also observed (Figure 4). Moreover, the underlying lamina propria was expanded by marked edema and congestion with occasional thrombosis (Figure 5), and crypt regeneration and elongation were observed. The high power view showed striking cytological alterations of the absorptive enterocytes, including a cuboidal to rounded appearance with striking nuclear enlargement, coarse chromatin pattern, prominent nucleoli, and thickening of the nuclear membrane (Figure 6). A subset of absorptive cells showed binucleation. Predominantly, subnuclear intracytoplasmic vacuoles with a Signet ring-like appearance were apparent (Figure 7). Periodic acid Schiff staining showed positive intracytoplasmic material in scattered enterocytes, which might represent viral glycoproteins (Figure 8). Furthermore, sections from the mesenteric lymph nodes showed caseating granulomatous inflammation. In addition, the nodal trabecular and medullary sinuses were expanded with histocytes that showed prominent erythrophagocytosis (Figure 9).
Immunohistochemical evaluation
Our observations of the immunehistochemical stains are summarized in (Table 1).
| Antibody Applied | Normal Intestinal Mucosa | SARS-CoV-2 Infected Intestinal Mucosa |
|---|---|---|
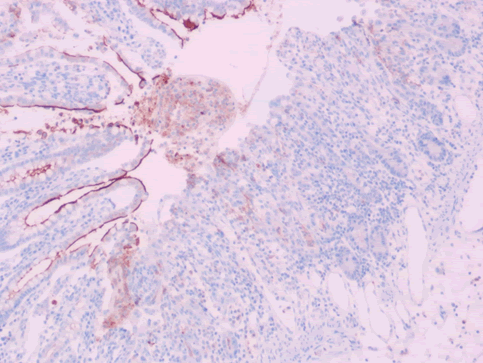
| CD10 | linear brush-border staining pattern of enterocytes | Diminished to complete loss (Figure 10) |
| Ep-CAM | Basolateral staining of enterocytes | Diminished to complete loss (Figure 11) |
| E- cadherin | Diffuse membranous staining of enterocytes | Diminished to complete loss (Figure 12) |
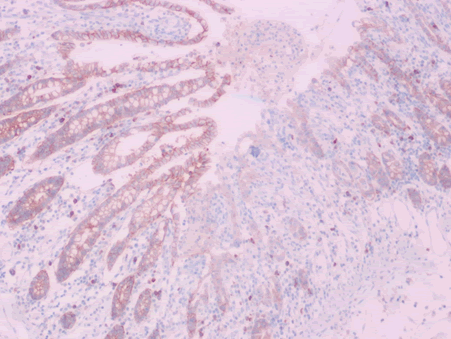
| EMA | Limited staining of the luminal surface of the crypts | Aberrant cytoplasmic expression in a subset of infected cells (Figure 13) |
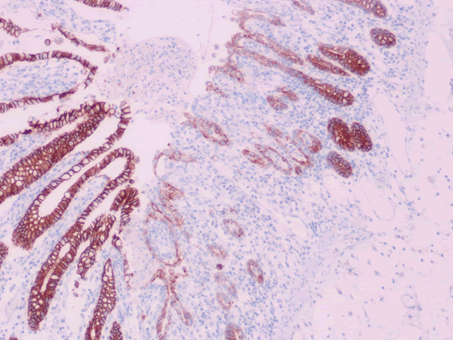
| Ki-67 labeling index | Limited staining in the base of crypts | Extended upward staining to the surface of villi (Figure 14) |
Table 1: Immunohistochemical evaluation of the patient.
Discussion
The novel SARS-CoV-2 causes COVID-19, which has rapidly spread worldwide, threatening global health. SARS-CoV-2 is a single stranded, enveloped RNA virus that belongs to the betacoronavirus 2b lineage [1,3]. Its diameter is approximately 65 nm-125 nm. Structurally, it has four main proteins, including the Spike (S), small Envelope (E), Membrane (M) glycoproteins, and the Nucleocapsid (N) protein. The S glycoprotein is a transmembrane protein that protrudes from the viral surface and facilitates binding and entry of the virus into host cells by interacting with the Angiotensin Converting Enzyme-2 (ACE2) receptor [4]. According to the Human Protein Atlas database (proteinatlas.org), ACE2 is widely expressed in various human organs and tissues, including the oral and nasal mucosa, nasopharynx, lung, small intestine, colon, liver, spleen, kidney, and brain. ACE2 expression is approximately 100-fold higher in the GIT than in the respiratory system. This makes the GIT highly susceptible to SARS-CoV-2 infections. ACE2 is expressed on the luminal surface of the absorptive enterocytes of the small intestine and colon, with lower expression in crypt epithelial cells [5]. A previous study showed that both the RNA and protein expression of ACE2 is higher in the small intestine than in the colon [1]. Importantly, ACE2 plays an important role in the regulation of dietary amino acid homeostasis, innate immunity, microbial ecology, and susceptibility to colitis [5].
SARS-CoV-2 has a propensity for the GIT, similar to other members of the coronavirus family, although it affects the GIT less than it affects the respiratory system. Notably, up to 30% of patients with pulmonary infection complain of GIT symptoms, mostly in association with respiratory symptoms; however, only 4% of patients complain of GIT symptoms alone [6]. In one study, the most common GIT symptoms were nausea and vomiting (41.6%), diarrhea (17.2%), abdominal pain, and anorexia [1]. Several studies have documented the presence of SARSCoV- 2 RNA in stool or anal/rectal swabs in patients with COVID-19 and suggested that the virus can replicate and exist in the GIT [7].
The pathogenesis of SARS-CoV-2 associated GIT infections mainly relies on the entry of the virus into the cytoplasm of absorptive enterocytes through its interaction with ACE2. Successful virus entry depends on the ACE2 receptor and endogenous serine proteases, such as furin and cellular Transmembrane Protease Serine 2 (TMPRSS2) which cleaves the S protein into two segments (S1 and S2) [4,7]. Both furin and TMPRSS2 are widely distributed in the small bowel mucosa. This cleavage is critical for the attachment of the virus to both the ACE2 receptor and the cellular membrane. This attachment is followed by endocytosis of the viral genomic material (RNA). Subsequently, viral mRNA is translated into new structural proteins with subsequent insulation in the endoplasmic reticulum-Golgi intermediate compartment, from which they form small vesicles that eventually undergo exocytosis [4,7]. This explains the presence of intracytoplasmic vesicles/vacuoles that are noted in the affected enterocytes from our samples, some of which contain periodic acid- Schiff positive material that represents the newly formed SARS-CoV-2 structural glycoproteins. Furthermore, the partial or complete blockage of the ACE2 receptor by a large viral load impairs the host cell nourishment supply and capabilities necessary to ensure a balanced immune response. The transport of amino acids, particularly tryptophan, is also impaired. This results in aberrant mechanistic target of rapamycin activation and impaired expression of antimicrobial peptides from Paneth cells, which eventually leads to alterations in the gut microbial environment [5,7]. The infected cells probably undergo subcellular alterations that might lead to certain pathological changes, as seen in our case. One of these alterations was the loss of brush-border of the enterocytes, which is known to occur in the setting of active enteritis and is the leading cause of diarrhea. This alteration is best evaluated by performing CD10 immunohistochemical staining. CD10 is a membrane-associated neutral peptidase that is normally observed as a linear brush-border staining pattern of the small intestinal mucosa. Variable loss of brush-border immunostaining for CD10 is usually observed in the setting of active enteritis [8]. Another alteration was the reduced expression of E-cadherins which are a major constituent of adherens junctions and play an essential role in intestinal homeostasis by providing mechanical integrity and maintaining cell polarization. Additionally, E-cadherins are necessary for the proper maturation and differentiation of secretory cell lineages, including Paneth and goblet cells. Downregulation of E-cadherin function due to decreased expression occurs secondary to the effect of inflammatory cytokines that lead to the activation of signaling pathway mediators, such as GTPases. This in turn, destabilizes adherens junctions and disrupts cell contacts, thus, facilitating pathogen invasion. Loss of E-cadherin function has been reported in inflammatory conditions of the intestine, such as Crohn’s disease and ulcerative colitis [9]. This explains the obvious detachment and "hobnailing" appearance of the enterocytes as well as the diminished immunostaining for E-cadherin seen in our patient. Similarly, possible alterations in the expression of EpCAM, which is a transmembrane glycoprotein, may occur secondary to SARS-CoV-2 infection. EpCAM plays an important role in intercellular adhesion, cell signaling, proliferation, differentiation, and cell polarity. Furthermore, EpCAM is enriched in the basolateral membrane of intestinal absorptive cells [10]. Diminished or loss of immunostaining for this adhesion molecule was also noted. This suggests that significant subcellular alterations are induced by SARS-CoV-2 infection. In addition, M. tuberculosis coinfection certainly played a significant synergistic role in causing the overwhelming SARSCoV- 2 infection in this case.
Review of literature
Few studies describing the pathological findings of SARS-CoV-2 in the intestine have been reported to date. Liu et al. described an autopsy finding from a patient with COVID-19 who developed alternating segmental dilation and stenosis of the small intestine [11]. Xiao et al. Performed an endoscopy of the GIT in a patient with COVID-19. They observed damage to the esophageal mucosa, numerous plasma cells, and lymphocytes in the lamina propria of the stomach, duodenum, and rectum. Furthermore, viral nucleocapsid proteins were detected in the cytoplasm of the infected cells [12]. A reported case of acute hemorrhagic colitis showed a slight expansion of the lamina propria due to edema with normal cellularity, and intact crypts were found [13]. A study of 67 autopsies performed on patients who died due to COVID-19 infection revealed no gross or microscopic abnormalities of 16 small intestines and 17 colons examined. However, hemophagocytic histiocytes (with engulfment of red blood cells) were noted in the spleen (9/22 cases), bone marrow (4/6 cases), thoracic lymph nodes (9/11 cases), liver Kupffer cells and epicardial inflammation (1 case). According to this observation, the study suggested that SARSCoV- 2 may induce a macrophage activation syndrome-like state, which might occur secondary to the cytokine storm [14].
Conclusion
COVID-19 is a threatening disease that causes systemic illness which primarily affects the respiratory system but may affect the GIT. While the pathogenesis of this disease becomes more evident, it appears that the pathological alterations of the small intestinal epithelium and the mesenteric lymph nodes are associated with the direct injury induced by the virus on the host cells, as well as the effect of the aberrant inflammatory response produced by the host’s immune system. We suggest that SARSCoV- 2 might cause cellular and subcellular alterations that induce certain microscopic changes in the affected tissue. The pathological alterations of the intestine associated with SARS-CoV-2 infection are important to be known by practicing anatomic pathologists; thus, they deserve close attention and further investigation.
References
- Ma C, Cong Y, Zhang H (2020) COVID-19 and the digestive system. Am J Gastroenterol 115:1003-1006.
- Hunt RH, East JE, Lanas A, Malfertheiner P, Satsangi J, et al. (2021) COVID-19 and gastrointestinal disease. Gastro Dig Dis 39:119-139.
- Lu R, Zhao X, Li J, Niu P, Yang B, et al. (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 395:565-574.
- Astuti I, Ysrafil (2020) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab Syndr 14:407-412.
- Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, et al. (2012) ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487:477-481.
- Wong SH, Lui RN, Sung JJ (2020) Covid-19 and the digestive system. J Gastroenterol Hepatol 35:744-748.
- Mönkemüller K, Fry L, Rickes S (2020) COVID-19, coronavirus, SARS-CoV-2 and the small bowel. Rev Esp Enferm Dig 112:383-388.
- Lloyd JM, Owens SR (2011) CD10 immunohistochemistry stains enteric mucosa, but negative staining is unreliable in the setting of active enteritis. Mod Pathol 24:1627-1632.
- Schneider MR, Dahlhoff M, Horst D, Hirschi B, Trülzsch K, et al. (2010) A key role for E-cadherin in intestinal homeostasis and Paneth cell maturation. PLOS ONE 5:e14325.
- Huang L, Yang Y, Yang F, Liu S, Zhu Z, et al. (2018) Functions of EpCAM in physiological processes and diseases (Review). Int J Mol Med 42:1771-1785.
- Liu Q, Wang RS, Qu GQ, Wang YY, Liu P, et al. (2020) Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi 36:21-23.
- Xiao F, Tang M, Zheng X, Liu Y, Li X, et al. (2020) Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 158:1831-1833.
- Carvalho A, Alqusairi R, Adams A, Paul M, Kothari N, et al. (2020) SARS-CoV-2 gastrointestinal infection causing hemorrhagic colitis: Implications for detection and transmission of COVID-19 disease. Am J Gastroenterol 115:942-946.
- Bryce C, Grimes Z, Pujadas E, Ahuja S, Beasley M, et al. (2021) Pathophysiology of SARS-CoV-2: Pathophysiology of SARS-CoV-2: Targeting of endothelial cells renders a complex disease with thrombotic micro angiopathy and aberrant immune response. Mod Pathol 2021:1-7.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1961
- [From(publication date): 0-2021 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 1406
- PDF downloads: 555