PD-L1/PD-1 Check Point in Anaplastic Large Cell Lymphoma, ALK+: A Case Report with Immunohistochemical and Molecular Study
Received: 22-Mar-2018 / Accepted Date: 28-Mar-2018 / Published Date: 05-Apr-2018 DOI: 10.4172/2476-2253.1000114
Abstract
Anaplastic large cell lymphoma, ALK+ (ALK+ ALCL) is a T-cell lymphoma consisting of large and pleomorphic lymphoid cells, often with horseshoe-shaped nuclei, with a chromosomal translocation involving the ALK gene and expression of ALK protein and CD30. ALK+ ALCL must be distinguished from primary cutaneous ALCL and from other subtype of T-cell or B-cell lymphoma with anaplastic features and/or CD30 expression. PD-L1/PD-1 check point physiologically plays a key role in induction and maintenance of immune tolerance to self-antigens and limits normal immune response against microorganisms. PD-L1 has been demonstrated in the whole spectrum of normal hematopoietic and non-hematopoietic cells, as well as in a large variety of epithelial cells. Moreover, it is also very commonly expressed by a large number of malignant cell types of epithelial and hematopoietic cell origin, and its activation is one of the major mechanisms exerted by neoplastic cells to evade elimination by the host immune system. Here we report a case of an ALK+ ALCL, with confirmed FISH rearrangement of ALK gene, PD-L1-positive and discuss the molecular mechanism involved in its immunohistochemical profile.
Keywords: ALK+ ALCL; PD-L1; PD-1; Lymphomas; Lymphoproliferative Diseases; FISH
Abbreviations
PD-1: Programmed Death-1; PD-L1: Programmed Death-Ligand 1; ALK+ ALCL: Anaplastic Lymphoma Kinase-Positive Anaplastic Large Cell Lymphoma; NPM: Nucleophosmin; cHL: classical Hodgkin’s Lymphoma; FFPE: Fixed Formalin Paraffin Embedded; FISH: Fluorescence In-Situ Hybridization.
Introduction
According the WHO classification of lymphoid malignancy, updated in 2017, Anaplastic Large Cell Lymphoma, ALK+ (ALK+ ALCL) is a T cell lymphoma consisting of large and pleomorphic lymphoid cells, often with horseshoe-shaped nuclei, with a chromosomal translocation involving the anaplastic lymphoma kinase (ALK ) gene and expression of ALK protein and CD30. ALK+ ALCL must be distinguished from primary cutaneous ALCL and from other subtype of T-cell or B-cell lymphoma with anaplastic features and/or CD30 expression [1].
In fact, different sub-entities of ALCL, though sharing the presence of CD30+ large atypical lymphocytes, substantially differ for clinical presentation (i.e. systemic, primary cutaneous, associated with breast implants) and behaviour [2]. Among systemic ALCLs, those harbouring translocations of ALK gene have shown a more favourable clinical course as compared to those lacking ALK gene lesions (ALKALCL) [3]. Breast implant-associated ALCL (BI-ALCL) has an ALK - negative phenotype and, similarly to primary cutaneous ALCL (cALCL) [4], cases confined to the peri-implant breast sarcoma fluid without invasion of the fibrous capsule have shown an excellent prognosis [5].
ALK+ ALCL accounts for 10-20% of childhood lymphomas and 3% of adult non-Hodgkin lymphomas. It frequently involves both lymph node and extra-nodal sites such as skin, bone, soft tissue, lung and liver. Several morphological patterns are described; however, all cases contain a variable proportion of cell with eccentric, horseshoe-shaped or kidney-shaped nuclei.
The tumour cells are CD30-positive on the cell membrane and in the Golgi region and express one or more T-cell antigens; however, some cases may have an apparent so-called null-cell phenotype.
All normal postnatal human tissues are negative for ALK protein expression and its ectopic immunohistochemical expression results in the affected CD4+ T lymphocytes of ALK+ ALCL from chromosomal translocations involving the ALK gene on chromosome 2 and several different partners, most frequently the nucleophosmin (NPM ) gene, on chromosome 5. Moreover, the NPM/ALK chimeric protein is not only constitutively expressed but is also chronically activated through autophosphorylation. NPM/ALK mediates its oncogenicity by activating a number of signal transduction proteins, including STAT3. The continuous activation of these signal transmitters leads to the persistent expression of genes and the protein products of which are involved in key cell functions, such as the promotion of cell proliferation and protection from apoptosis [6].
Moreover, most cases that have the t (2;5) (p23;q35) translocation, show ALK staining is both cytoplasmic and nuclear, and aberrant cytoplasmic expression of NPM. Furthermore, in cases with variant translocations, i.e. fusion of ALK with partners other than NPM, the ALK staining is usually cytoplasmic and rarely membranous and NPM nuclear as expected.
Programmed death-ligand 1 (PD-L1) plays a key role in induction and maintenance of immune tolerance to self-antigens and limits normal immune response against microorganisms, to protect the involved tissues from excessive damage incurred during such a response and to prevent its potential autoimmune complications. PDL1 has been identified in the whole spectrum of normal hematopoietic and non-hematopoietic cells, including macrophages, dendritic cells, activated T and B-lymphocytes, endothelial, muscle, and glial cells, as well as a large variety of epithelial cells [6].
Normal cells use the PD-1/PD-L1 interaction as a mechanism of protection against immune recognition by inhibiting the action of Tcells. More specifically, inactivation of cytotoxic T-cells induced by PD-L1 down-regulates the immune response such that the inactive Tcell is exhausted, ceases to divide, and might eventually die by programmed cell death, or apoptosis.
The PD-1/PD-L1 checkpoint pathways is one of the major mechanisms of immune escaping exerted by several cancer types in which up-regulation of PD-L1 is observed. Therefore, from a therapeutic point of view, the administration of anti-PD-1 or anti-PDL1 antibodies has been associated with durable responses of several metastatic solid tumors, as for example, lung carcinoma and melanoma.
In hematological malignancies this novel therapeutic strategy is far less documented, however many Authors investigated large cohort of Hodgkin and B-cell lymphomas for expression of PD-L1 [7].
Among lympho-proliferative disease, PD-1/PD-L1 inhibitors have been successful, so far, only in the treatment of classical Hodgkin lymphoma (cHL), which typically exhibits an over-expression of PDL1 due to alterations in chromosome 9p24.1. Such positive outcomes led to the US Food and Drug Administration approval of Nivolumab use in relapsed cHL in 2016 as the first hematological indication. Although the results in other lymphoid malignancies have not been so striking, blockade of the PD-1/PD-L1 axis has led to meaningful responses in other lymphoma types such as diffuse large B-cell lymphoma, follicular lymphoma or several T-cell lymphomas [8].
Due to the success of checkpoint blockade therapy in the treatment of different solid tumor, and given that the assessment of PD-L1 expression through immunohistochemical staining is considered as the finest biomarker test [9], many Authors reviewed current knowledge of PD-L1 immunohistochemical expression, in both non-Hodgkin lymphoma cells and their surrounding immune cells. This body of literature establishes the cell surface expression of PD-L1 as a critical determinant for the identification of non-Hodgkin lymphoma patients eligible for immune checkpoint blockade therapies [10].
Case Presentation
A 53-year-old female patient went to the emergency room reporting pain and swelling in the left supraclavicular region. A CT scan of the neck and chest showed a 6 cm diameter solid lesion with inhomogeneous contrast enhancement and multiple enlarged lymph nodes. The mass impinged and dislocated the left internal jugular vein, and showed encasement of the thyrocervical trunk with compression and thrombosis of the subclavian vein. A CT Scan of the abdomen and pelvis showed involvement of the paraaortic and interaortocaval lymph nodes. The major lesion of the neck showed positive at Pet-CT scan examination (SUV max 52).The patient was therefore admitted to our hospital. At physical examination, the lesion appeared painful, swollen and erythematous. Surgical lymphadenectomy of left-side axillary region was then performed.
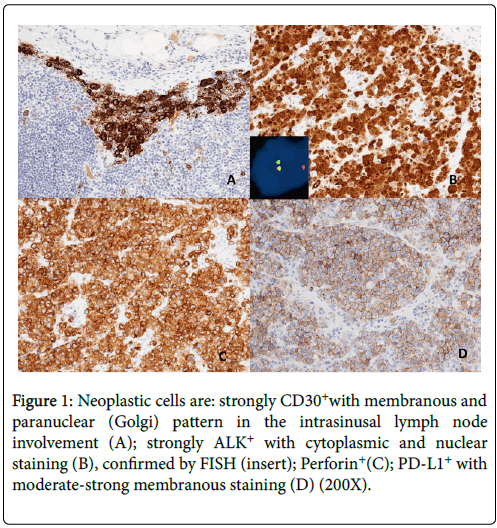
Histopathologically, lymph nodes were characterized by a diffuse and Intrasinusal infiltrate of highly pleomorphic cells with horseshoeshaped and simil-Reed-Sternberg nuclei, with prominent nucleoli. The tumor cells were immunohistochemically positive for CD30, ALK/p80 (clone SP8 Thermoscientific) with cytoplasmic and nuclear staining, Perforin, Granzyme B, CD4 and demonstrated negative immunostaining for EMA, LMP1,other T-cell, pan-B-cell and epithelial markers. Almost all tumor cells (± 90%) showed expression for PD-L1 (22C3 DAKO, Agilent Technologies) with specific, moderate-strong and membranous staining pattern (Figure 1) [7,11]. PD-L1 expression was also evaluated in non-neoplastic cells; adjacent nodal lymphocytes and histiocytes were negative [12].
Figure 1: Neoplastic cells are: strongly CD30+with membranous and paranuclear (Golgi) pattern in the intrasinusal lymph node involvement (A); strongly ALK+ with cytoplasmic and nuclear staining (B), confirmed by FISH (insert); Perforin+(C); PD-L1+ with moderate-strong membranous staining (D) (200X).
Immunohistochemistry was performed with the automated device Dako Omnis (Agilent Technologies) to ensure repeatability and reproducibility. Appropriate positive and negative controls were routinely included for intra-laboratory optimization of the tests. Even though, in routine practice, even according to WHO Classification, Fluorescence In-Situ Hybridization (FISH) is not mandatory if ALK staining is positive, to confirm immunohistochemical result, we performed FISH using an ALK Dual Color Break-Apart Probe (ZytoVision). A signal pattern consisting of one orange/green fusion signal, one orange signal, and a separate green signal indicated one normal 2p23.1-p23.2locus and one 2p23.1-p23.2 locus affected by a translocation (insert in B of Figure 1).
Staging was completed by bone marrow biopsy that showed no evidence of lymphoma involvement.
Discussion
Given the clinical implications of the assessment of PD-L1 expression through immunohistochemistry for selecting patients for immunotherapy, between the wide spectrums of lymphoproliferative diseases, Marzec et al. reported a consistent overexpression of PD-L1 in ALK+ ALCL cell lines [6]. This report was confirmed in biopsies from ALK+ ALCL tumors, with frequencies of PD-L1+ cases varying from 34 to 100% of the analyzed cases [13-15].
What is particularly interesting, Marzec et al. showed that activation of the transcription factor STAT3 by the NPM-ALK fusion protein could be responsible for the increased expression of PD-L1 at the cell surface of ALK+ tumor cells. Moreover, the same group showed that NPM-ALK induces the activation of the IL-10 and TGF-β cytokines. Since IL-10 can also activate JAK/STAT signaling via STAT3, and thus induce the up-regulation of PD-L1 [16], one may reasonably speculate that NPM-ALK directly up-regulates PD-L1 via STAT3 or IL-10 [6].
There was significant heterogeneity among the available tests for PD-L1. Specifically, no definitive cut-off for PD-L1 positivity was identifiable, with more than one threshold being reported for most antibodies [17]. However, the standardization of all pre-analytical factors (e. g tissue fixation) and analytical processing, through the use of an automated platform, improve the reproducibility and reliability of immunohistochemistry.
Conclusion
In conclusion, however further studies would be necessary for the validation of reporting criteria, pathologists and haematologists should continue to collaborate for the optimization of the PD-L1 immunohistochemical assays for the patients affected by different subtypes of lymphomas and considered for immunotherapy.
Furthermore, they should work together to make molecular biotechnologies widely available for pathology laboratories, to provide an even more precise classification of lymphoproliferative diseases.
Acknowledgement
The authors are deeply grateful to the laboratory staff for excellent technical assistance.
Conflict of Interest
All the authors declare no competing interest.
References
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, et al. (2017) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues: World Health Organization Classification of Tumours. [4thedn], Lyon, France: IARC Publications.
- Miranda RN, Aladily TN, Prince HM, Kanagal-Shamanna R, de Jong D, et al. (2014)Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol 32: 114-120.
- Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, et al. (1994) Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science 263: 1281-1284.
- Savage KJ, Harris NL, Vose JM, Ulrich F, Jaffe ES, et al. (2008) ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood 111: 5496-5504.
- Clemens MW, Medeiros LJ, Butler CE, Hunt KK, Fanale MA, et al. (2016) Complete Surgical Excision Is Essential for the Management of Patients With Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J Clin Oncol 34: 160-168.
- Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, et al. (2008) Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad Sci U S A 105: 20852-20857.
- Menter T, Bodmer-Haecki A, Dirnhofer S, Tzankov A (2016) Evaluation of the diagnostic and prognostic value of PDL1 expression in Hodgkin and B-cell lymphomas. Hum Pathol 54: 17-24.
- Jelinek T, Mihalyova J, Kascak M, Duras J, Hajek R, et al. (2017) PD-1/PD-L1 inhibitors in haematological malignancies: update 2017. Immunology 152: 357-371.
- Ilie M, Hofman V, Dietel M, Soria JC, Hofman P, et al. (2016) Assessment of the PD-L1 status by immunohistochemistry: challenges and perspectives for therapeutic strategies in lung cancer patients. Virchows Arch 468: 511-525.
- Gravelle P, Burroni B, Pericart S, Rossi C, Bezombes C, et al. (2017) Mechanisms of PD-1/PD-L1 expression and prognostic relevance in non-Hodgkin lymphoma: a summary of immunohistochemical studies. Oncotarget 8: 44960-44975.
- Vranic S, Ghosh N, Kimbrough J, Bilalovic N, Bender R, et al. (2016) PD-L1 Status in Refractory Lymphomas. PLoS One 11: e0166266.
- Kim WY, Jung HY, Nam SJ, Kim TM, Heo DS, et al. (2016) Expression of programmed cell death ligand 1 (PD-L1) in advanced stage EBV-associated extranodal NK/T cell lymphoma is associated with better prognosis. Virchows Arch 469: 581-590.
- Andorsky DJ, Yamada RE, Said J, Pinkus GS, Betting DJ, et al. (2011) Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res 17: 4232-4244.
- Wilcox RA, Feldman AL, Wada DA, Yang ZZ, Comfere NI, et al. (2009) B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood 114: 2149-2158.
- Brown JA, Dorfman DM, Ma FR, Sullifan EL, Munoz O, et al. (2003) Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol 170: 1257-1266.
- Gupta M, Han JJ, Stenson M, Maurer M, Wellik L, et al. (2012) Elevated serum IL-10 levels in diffuse large B-cell lymphoma: a mechanism of aberrant JAK2 activation. Blood 119: 2844-2853.
- Udall M, Rizzo M, Kenny J, Doherty J, Dahm S, et al. (2018) PD-L1 diagnostic tests: a systematic literature review of scoring algorithms and test-validation metrics. Diagn Pathol 13: 12.
Citation: Bianchi A, Vallese S, Annibali O (2018) PD-L1/PD-1 Check Point in Anaplastic Large Cell Lymphoma, ALK+: A Case Report with Immunohistochemical and Molecular Study. J Cancer Diagn 3: 114. DOI: 10.4172/2476-2253.1000114
Copyright: © 2018 Bianchi A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6348
- [From(publication date): 0-2018 - Oct 31, 2025]
- Breakdown by view type
- HTML page views: 5441
- PDF downloads: 907