Research Article Open Access
Patient Satisfaction and Complications of Over-the-Counter Bleaching Products
Cecilia Heinisch1, Karin Larsson1, Johanna Mattsson1, Stig Karlsson2, Torgny Alstad2 and Ellen Bruzell3*1Sahlgrenska Academy, University of Gothenburg, Sweden
2Institute of Odontology, University of Gothenburg, Sweden
3Nordic Institute of Dental Materials (NIOM), Oslo, Norway
- *Corresponding Author:
- Dr. Ellen Bruzell
Senior Scientist, NIOM
Sognsveien 70A, NO-0855
Oslo, Norway
Tel: +4767512200
Fax: +4767591530
E-mail: ebr@niom.no
Received Date: December 20, 2014; Accepted Date: March 30, 2015; Published Date: April 30, 2015
Citation: Heinisch C, Larsson K, Mattsson J, Karlsson S, Alstad T, et al. (2015) Patient Satisfaction and Complications of Over-the-Counter Bleaching Products. J Oral Hyg Health 3:176. doi: 10.4172/2332-0702.1000176
Copyright: © 2015 Heinisch C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Oral Hygiene & Health
Abstract
Objective: To compare two home-based over-the-counter (OTC) bleaching tray products with similar hydrogen peroxide (HP) concentrations with respect to tooth lightening efficacy and potential to induce negative side effects in tobacco- and non-tobacco users as well as degree of satisfaction with product handling. The HP concentrations were near the regulation limit for bleaching products of 6% set by the European Union in 2011.
Materials and Methods: The clinical trial was randomized, single-blind and included 41 patients (93% completed) who were allocated into three bleaching groups: non-tobacco users treated with either 16% (corresponding to 5.8% HP) or 22% (corresponding to 7.9% HP) carbamide peroxide (CP) and tobacco users treated with 22% CP. Total bleaching time was 10.5 hours within 14 days. Tooth shade was visually assessed using a commercial shade guide. Side effects and satisfaction with the bleaching outcome and product handling were reported through a perception scale and a questionnaire.
Results: Tooth lightening was similar (p>0.05) and significantly increased (p<0.05) for all groups. Patient age (> 31 years) was negatively correlated (p<0.05) and tobacco use was positively correlated (p<0.05) with bleaching efficacy. A percentage of 97% (n=38) of the patients experienced some form and degree of side effect, most frequently tooth hypersensitivity and gingival irritation. Patient satisfaction with the bleaching result was positively correlated with the user-friendliness of the product (p<0.05).
Conclusion: A large number of side effects were reported after bleaching treatment with OTC products after a short treatment time. Prior to bleaching, patients should be informed of the major individual differences in both bleaching efficacy and degree and number of side effects.
Keywords
Tooth bleaching; over-the-counter (OTC); hydrogen peroxide; carbamide peroxide; efficacy; side effects; tobacco; Visual Analogue Scale
Introduction
Hydrogen peroxide (HP), the active agent in bleaching products can, depending on the reaction conditions, form (per-)hydroxyl anions, superoxide anions and other radicals. Both HP and free radicals can diffuse various distances into tooth substance depending on their lifetimes and reaction environment. Although the action mechanism is not fully understood, the radical-involved oxidative process can cause breaking of molecule bonds in stains in dentin and enamel. The resulting products appear less colored [1]. Bleaching products that are administered by the patient at home, either initially supervised by a dentist or many types that are purchased over-the-counter (OTC), contain carbamide peroxide (CP) to increase product stability. In an oral, watery environment, CP is decomposed to 36% HP and 65% urea. HP further decomposes into water and oxygen, while the urea breaks down to ammonia and carbon dioxide. Ammonia may contribute to a beneficial basic solution which can increase the radical formation reaction rate [1].
Of the number of tooth bleaching methods available, research is scarce on the behavior of OTC products. These products consist of a large variation of methods, products, active bleaching ingredients, concentrations and additives and they can have different pH. The OTC bleaching products that utilize pre-fabricated trays typically contain between 16% and 35% CP [2]. The tray is fit to the teeth by softening in hot water and placing it on the teeth before the material thermosets. The height of the tray rim is not adjustable, which increases the risk for overfilling and for contact with the gingiva. In comparison, dentist supervised at-home bleaching is administered using CP concentrations of 10 - 16% CP [2] using custom-made trays, once or twice a day or during the night for about 2 weeks. Dentist-supervised at-home based bleaching products containing 10% CP have been extensively evaluated. However, research is scarce regarding efficacy of and side effects caused by CP concentrations of 20% or higher although products containing up to 35% CP are available on the international market. According to the European Union (EU)-directive on cosmetic products [3] adopted in Scandinavia on Nov 1st 2012 only bleaching products of <6% HP can be used on patients, and not at all on patients under the age of 18 years. An appropriate clinical examination has to be carried out, i.e. the products can be sold to and used by dental professionals only.
It is generally accepted that efficacy of the bleaching treatment increases with concentration of the active bleaching ingredient, the probability for contact between bleaching agent and discolorations, and contact time [4]. Further, efficacy is dependent on several factors such as age of patient, discoloration characteristics and duration of bleaching sessions [5]. According to an efficacy and safety guidelines for tooth bleaching, the product chosen must be capable of providing a shade at least two increments lighter (Vita shade guide) [4].
Dental bleaching has been reported to cause various local side effects, including tooth hypersensitivity, gingival soreness/irritation, tooth pain and sore throat [6]. The two former occurs most frequently [1,7-11]. Such effects can be both patient and/or treatment related and may be dependent on bleaching agent concentration, application time [7,10], inadequate gingival isolation [12], and pre-existing conditions such as tooth sensitivity, erosion/abrasion or gingivitis [4,11] as well as cervical caries and multiple restorations [4].
The study was conducted as a senior dental student project prior to the time of enforcement of the EU directive [3]. The aim was to compare two OTC bleaching tray products of different CP concentrations, with regards to efficacy and side effects in tobacco- and non- tobacco users, products’ user friendliness and patient satisfaction with such OTC products. One product had a bleaching agent concentration encompassed by the EU directive [3] (16% CP/5.8% HP) and the other had a slightly higher concentration (22% CP/7.9% HP).
Materials and Methods
Subjects
A randomized, single-blind clinical trial, initially including 41 patients, 17 males and 24 females, age range 18-65 years, was performed at the Department of Prosthodontics and Dental Materials Science, Institute of Odontology, University of Gothenburg, Sweden between February 7th and April 13th 2011. The patients were recruited by advertisement in the nearby public environment. Patient records were not available.
Inclusion and exclusion criteria
Eligible patients were at least 18 years old, without crowns, dental veneers, implants, orthodontic appliance or large fillings (in teeth to be bleached). Pregnant and lactating patients were excluded. Patients with small, white composite fillings were informed that these would not be bleached, after which they decided themselves whether or not to participate. Hypersensitivity prior to treatment was recorded.
Informed consent
Informed consent, both oral and written, was obtained from all participants. Patients were informed that they could withdraw from the study at any time. The study was performed according to the Declaration of Helsinki [13].
OTC bleaching products
SimpleSmile Startkit Sensitive (Group beconfiDent Europe AB, Helsingborg, Sweden) containing 16% CP corresponding to 5.8% HP (called SS) and WhiteNow (DentaWorks, Helsingborg, Sweden) containing 22% CP corresponding to 7.9% HP (called DW) were ordered from the manufacturers’ web shops.
Study groups and randomization
Thirty of the patients, non-tobacco users, were randomly divided into two equally large groups, one to be treated with SS of 16% CP (group SS16%) and one to be treated with DW of 22% CP (group DW22%). Eleven participants were tobacco users, either smokers or Swedish “snus” users, and assigned to use product DW (group DW-T22%). Each non-smoking participant was assigned one of the two bleaching products according to a predetermined randomization scheme. A person who was not involved in the study was responsible for the randomization. One of the two products, contained in opaque bags, was drawn. The product coding list was not broken until all data was collected. Each participant was assigned a number code at the initial visit. The patients informed of their age by choosing an age interval in a questionnaire (see Side effect assessment) (Appendix).
Study design
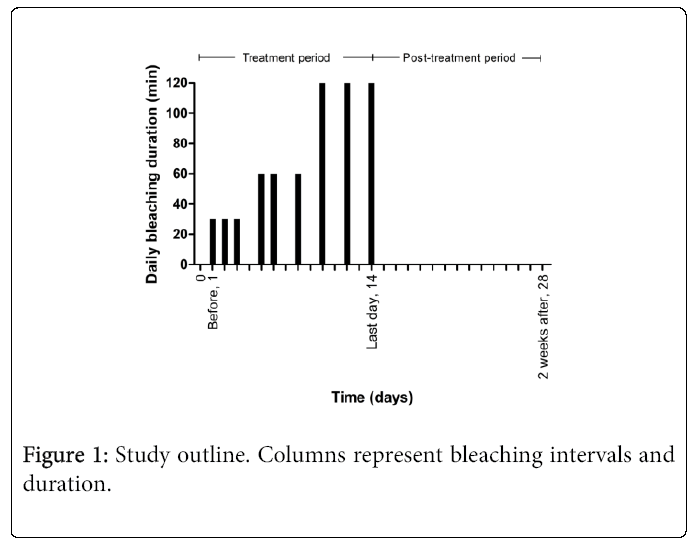
At the first of three examination visits (called “before treatment”, “last day of treatment” and “2 weeks after treatment”) each patient was screened for inclusion/exclusion criteria (Figure 1). The patients were given the bleaching products along with the user instructions. These included how to fit the pre-fabricated tray to their upper jaw. Patients were asked to refrain from drinking coffee, red wine or other intensely colored edibles during the study period. The manufacturers’ shortest recommended treatment time (range 10-30 hours) of 10.5 hours was chosen. Bleaching was instructed to be performed on nine separate days within a 14 day period (Figure 1), starting on the day of the first visit. Patients were informed to strictly follow the instructions from the manufacturer and the treatment schedule.
Side effect assessment
At the “before treatment” visit, patients were instructed to report, in a questionnaire, their experience, intensity and type of any negative side effects during bleaching days 1-3 and days 10-13 throughout the bleaching treatment. The questionnaire was handed in on the “last day of treatment” visit. Presence of any side effect at the “last day of treatment” and between this time point and “2 weeks after treatment” were communicated verbally and recorded by the project administrator (Figure 1).
The questionnaire included four questions designed in accordance with Visual Analogue Scale (VAS), namely degree of tooth hypersensitivity, gingival irritation, ease of use of the products and satisfaction with the bleaching result. Patients were asked to indicate a score with a mark on a 100 mm long scale (score range: 0-10). One end (0) represented the answers “no pain”/ “very easy to use the product”/ “not happy with the bleaching result”. The other end (10) represented “worst pain imaginable”/ “very difficult to use the product”/ “very content with the bleaching result” [14].
Tooth shade assessment
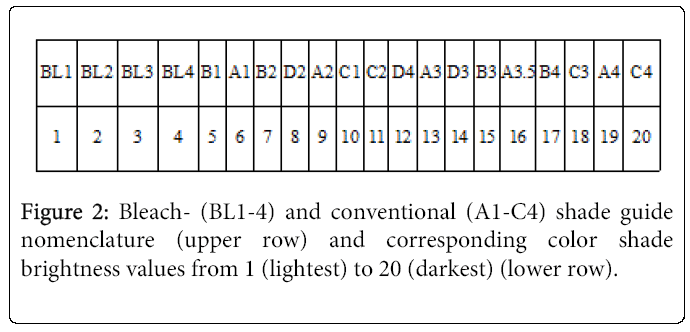
Tooth shade was determined at all three examination visits (Figure 1). Shade change was registered on the first four maxillary teeth in the first quadrant. Three senior dental students determined the tooth shade by visual inspection using the VITA Classical A1-C4 shade guide (Vita Zahnfabrik, H Rauter GmbH & Co. KG., Bad Säckingen, Germany) and Ivoclar Vivadent Bleach Shade Guide (Ivoclar Vivadent AG, Schaan, Liechtenstein) which was complemented with the four extra tabs; Ivoclar Vivadent bleach shade guide. The examiners were calibrated on color measurement prior to the study. Two examiners, individually, scored the shade of each test tooth by selecting the closest matching shade tab on the guide (Figure 2). The tabs of the shade guide were arranged from BL1 to C4, corresponding to color shade brightness from 1 to 20, in which a lower number indicated a lighter tooth shade. Care was taken to perform shade determinations under as similar ambient lighting conditions as possible. The patients wore no lipstick to avoid disturbing the tooth shade perception of the examiners.
Statistical analysis
Independent sample t-test and one-way ANOVA were used for comparison of groups. Paired sample t-test was used for comparison of bleaching effect within individuals. The General Linear Model was used to analyze the influence of several variables on bleaching efficacy and side effects. SPSS for Windows, version 19.0, was used for the statistical analyses. P<0.05 was regarded as statistically significant.
Results
Inclusion and withdrawal
Of the 38 (93%) patients who completed the study, five patients (two in DW22%, three in DW-T22%) terminated the bleaching procedure before the end of treatment due to side effects (two in DW22%, two in DW-T22%). One patient withdrew due to a reason unrelated to the treatment (DW-T22%). All 38 patients were examined at the “2 weeks after treatment” visit.
Efficacy
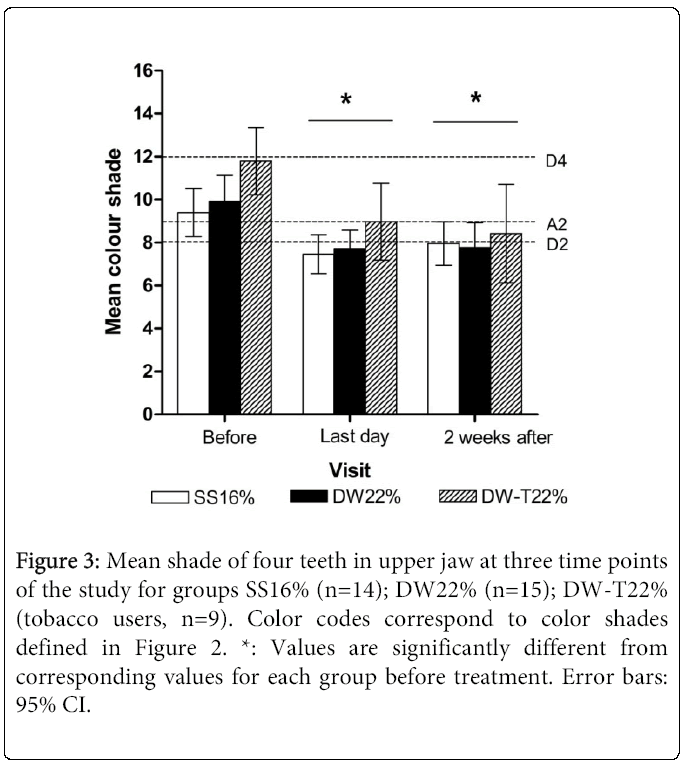
The mean shades of the four investigated teeth and the mean shade of any individual tooth in each group were significantly lighter between “before treatment” and “last day of treatment” visits for all three groups (p<0.05), but were unchanged between the two last visits (p>0.05) (Figure 3). At the “before treatment” visit, the shades of teeth 13 and 14 were significantly darker in those allocated to the tobacco group (DW-T22%) than those allocated to the non-tobacco group of the same bleaching concentration (DW22%) (p<0.05). No significant difference in mean bleaching efficacy between the products was observed (p>0.05) (Figure 3). The older patients (age range 31-65, n=4) experienced less change in lightening than the younger patients (p<0.05). The shade change between the two first visits was significantly increased for tobacco users (p<0.05).
Figure 3: Mean shade of four teeth in upper jaw at three time points of the study for groups SS16% (n=14); DW22% (n=15); DW-T22% (tobacco users, n=9). Color codes correspond to color shades defined in Figure 2. *: Values are significantly different from corresponding values for each group before treatment. Error bars: 95% CI.
Side effects
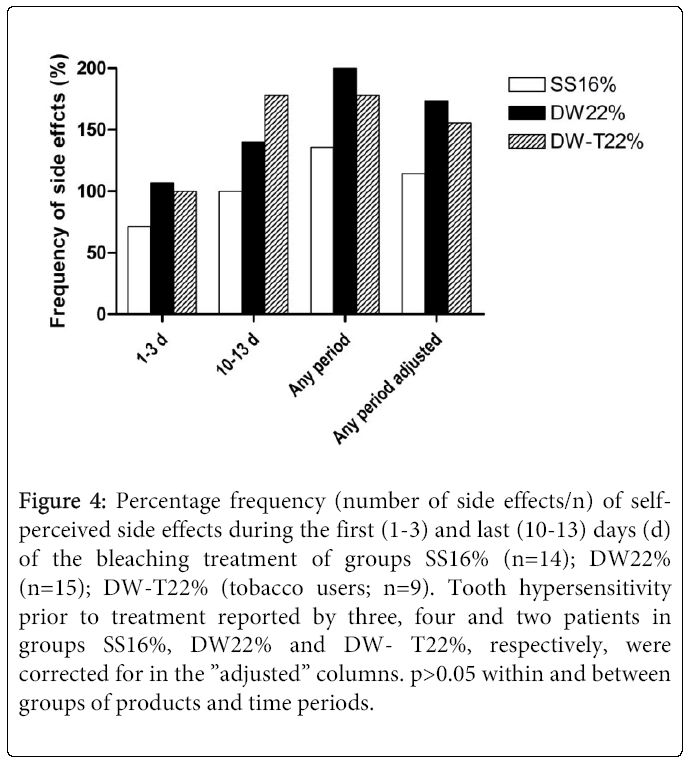
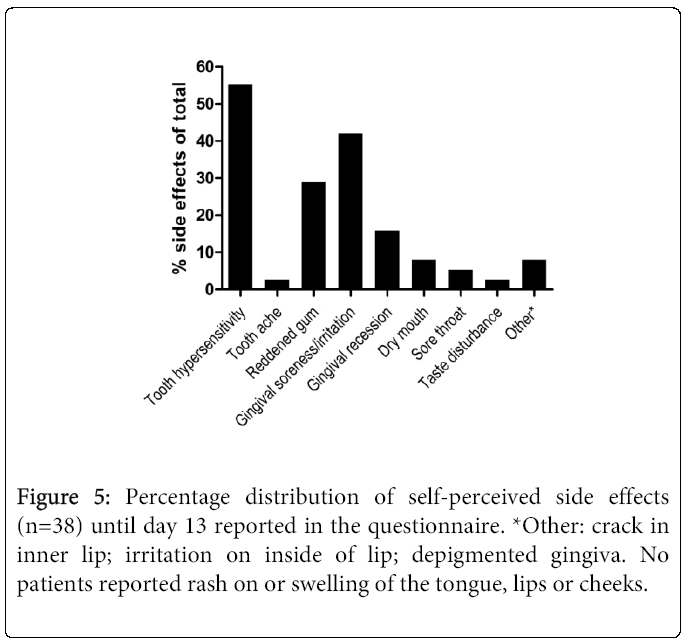
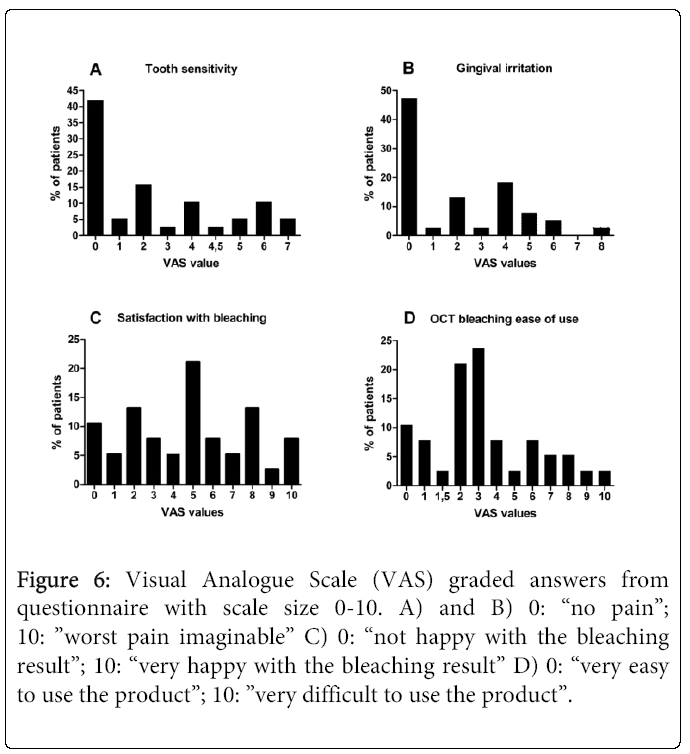
Thirty-seven (SS16%, n=14; DW22%, n=15; DW-T22%, n=9) of 38 (97%) patients reported some form of negative side effect during the study period (Figure 4). Most frequently experienced/reported were tooth hypersensitivity and gingival soreness/irritation (Figure 5). Of those who experienced tooth hypersensitivity, nine had reported this problem at the first visit. These patients were equally distributed between groups (p>0.05). Side effects were present in 12 (32%; n=38) patients at the “last day of treatment” visit as reported in the questionnaire, but had ceased by the “2 weeks after” visit (communicated verbally) (Figure 1). A percentage of 10.5 (n=38) of the patients terminated the treatment prematurely (i.e. prior to the “last day of treatment” visit) due to onset of side effects. None of these patients reported problems at the two last visits. A similar number of self- perceived side effects were reported after treatment with the two products (p>0.05) (Figure 4). Significantly fewer tobacco users compared to non-tobacco users experienced tooth hypersensitivity between treatment days 1-3 (p<0.05). The mean VAS values (±SD) of tooth hypersensitivity and gingival irritation were 2.25 ± 2.43 (range: 0-7) and 2.03 ± 2.31 (range: 0-8), respectively (Figures 6a and 6b). Those who reported hypersensitivity prior to treatment had no increased risk for developing hypersensitivity during treatment (p>0.05).
Figure 4: Percentage frequency (number of side effects/n) of selfperceived side effects during the first (1-3) and last (10-13) days (d) of the bleaching treatment of groups SS16% (n=14); DW22% (n=15); DW-T22% (tobacco users; n=9). Tooth hypersensitivity prior to treatment reported by three, four and two patients in groups SS16%, DW22% and DW- T22%, respectively, were corrected for in the ”adjusted” columns. p>0.05 within and between groups of products and time periods.
Figure 6: Visual Analogue Scale (VAS) graded answers from questionnaire with scale size 0-10. A) and B) 0: “no pain”; 10: ”worst pain imaginable” C) 0: “not happy with the bleaching result”; 10: “very happy with the bleaching result” D) 0: “very easy to use the product”; 10: ”very difficult to use the product”.
Patient assessment of product
The mean VAS score (±SD) of satisfaction with bleaching result and ease of product use were 4.74 ± 3.01 (range: 0-10) and 3.46 ± 2.61 (range: 0-10), respectively (Figures 6c and 6d). No correlation was found between bleaching efficacy and the patients’ satisfaction with the bleaching result (p>0.05) (Figures 3 and 6c). However, patient satisfaction was positively correlated with user-friendliness of the product (p<0.05) (Figures 6c and 6d).
Discussion
Efficacy
The use of VITA Classical shade guide is frequent in bleaching efficacy studies and in practice as it detects clinically relevant shade changes with claimed sufficient accuracy [15,16] and shade changes that can be perceived by the patient [9]. A disadvantage of the visual method compared to the more objective method of e.g. spectrophotometry is that visual inspection is a subjective process with related bias, and could be influenced by the examiner’s expectations. However, there are certain disadvantages associated with the use of spectrophotometer: measurements are only accessible on anterior teeth, not on rotated or malaligned teeth; color data can only be read in one direction; fogging of the optical device may give false dark readings; not every wavelength is read; translucency is not uniform across the tooth; and curved tooth surface may negatively impact upon the uniform reflectance of light to the spectrophotometer [17].
Even the relatively short treatment duration applied in this study (Figure 1) compared with the more typical duration of 2 weeks when 10% CP night treatment is used [18] caused significant tooth shade lightening (Figure 3). The similar result in bleaching efficacy between the groups was likely due to the small concentration difference (5.7% vs. 7.9% HP) and the small study size. Various bleaching product manufacturers state in their product information that concentration may vary by 20%. If the concentrations of the products used in this study varied by the same percentage, the HP concentrations would have been overlapping. The authors are not aware of the use of any other oral CP-containing product that could have enhanced the bleaching process in any of the subjects. Failure to demonstrate a difference in degree of tooth lightening after bleaching with similar concentrations has been reported in several studies [2]. The increase in tooth shade lightening in the current study was comparable to that reported after bleaching with 10% CP 3-5 hours once or twice a day for 5-6 days [4]. Matis et al. [19] concluded, generally, that lower concentrations applied over longer periods of time achieve the same final results with fewer adverse effects. Since no change in lightening was observed two weeks after terminated bleaching treatment, and the individual variations were large (Figure 3), a longer follow-up with a larger patient group would be desirable.
Side effects
Fifteen to 78% of patients using 10% CP reported increased tooth sensitivity [1,8]. In a comparison of three different products containing 10% CP tooth sensitivity was reported by 64% (n=24) and 4% of the patients terminated the treatment due to discomfort [9]. Another study [20] reported tooth hypersensitivity so severe that 14% of the participants discontinued the bleaching procedure (10% CP), a number close to that found in the current study. The experience of increased tooth hypersensitivity varies among studies, and prevalence/incidence in the range 0-100% has been reported [8] although a majority of studies report prevalence of 15-80% [1]. Tooth hypersensitivity is, in most cases, transient within a few days after the end of the bleaching treatment [8-10,20], yet longer periods up to 39 days [10] and later [11] have been reported.
The large number of local side effects reported (Figures 4,5 and 6b) compared to the above- mentioned studies may be explained by the following: first, OTC-products with pre- fabricated trays were used. Particularly, the fact that 11 and 17 patients reported reddening of or irritated gingiva, respectively, until “the last day of treatment” visit (p>0.05; data not shown) (Figure 5) indicated that the trays were not well fitted. Second, patients may have been particularly aware of side effects due to the university clinic setting. Third, the bleaching product could have contained adjuvants such as thickeners, stabilizers and flavorings and/or have had low pH (2-3) [21], factors that may have contributed to local side effects. Pre-treatment conditions that could have influenced the onset of side effects, such as gingivitis, abrasion and aberration [11], were not examined nor recorded. A placebo-control group was not included. We hypothesize that it is likely that use of OTC products without any clinical examination and follow-up will contribute to an even higher number of and more pronounced side effects.
The absence of significant differences in side effects between the products was inconsistent with studies showing that a higher concentration is more likely to result in increased tooth hypersensitivity [12]. However, real differences in small effect parameters between small groups cannot be expected and less so when perhaps overlapping concentrations of treatment products are used. Further, the bleaching duration may have been too short to detect a potential difference between the products.
A percentage of 68 (n=37) of the patients’ side effects were transient in 2-3 days. However, side effects may have been underreported due to the design of the questionnaire (Appendix). The short treatment duration (10.5 hours) may have contributed to the cessation of side effects before the end of the treatment period. Within each group there was a tendency of reporting a higher, although non-significant number of side effects in the last than first bleaching period (Figure 4). This observation was likely related to the number of bleaching applications that were completed during the two bleaching periods, 1-3 and 7-9 applications during the first and last part, respectively. No unexpected or objectively severe adverse effects were reported.
The bleaching tray may overlap soft tissue and cause burns, irritation and bleaching of the gingiva due to bleaching gel contact [1]. A higher number of patients (63%) reported gingival soreness/irritation in this study in which pre-fabricated trays were used compared to a clinical trial, in which 10% CP in custom-made trays were used, affecting up to 34% of the patients [8,9]. Some of the patients in the current study may have failed to fit the tray properly and, therefore, increased the risk for gingival irritation. In general, the use of OTC products will increase the risk for poorly fitted trays.
Large individual differences were shown in the patients’ experience of side effects. The percentage of patients (10.5%) who terminated the study prematurely due to discomfort was slightly lower than that found in similar studies: 14% [20] and 20% [10] discontinued treatment with 10% CP (dentist-supervised at-home treatment) whereas 14% interrupted at-home treatment with various CP concentrations [11].
VAS measurement
VAS is a measurement concept that grades a characteristic or attitude over a range across a continuum of values that cannot easily be directly measured, such as the degree of intensity of an experience. The method is commonly used in pain assessment in conjunction with bleaching. The wording of the scale’s end-points describing the most intense (“painful”; “severe pain”; “unbearable pain”, etc.) and least intense (“no pain”, “absolutely no pain”, “acceptable pain”, etc.) experience will influence the grading of the answers [22]. Thus, it is difficult to compare VAS scores between studies. In this study 66% (n=38) of the patients reported tooth hypersensitivity between scores 1 and 7 on VAS sometime during the study (Figure 6a). The mean VAS score was comparable to those found both after OTC bleaching with 5.3% HP bleaching strips (2.62 ± 1.46) 7 days after treatment and treatment with 10% CP in a custom-made tray (3.38 ± 1.66) [23]. Further, gingival irritation scores were overlapping with those in the current study: 0.85 ± 1.82 and 0.38 ± 0.87 after 5.3% HP and 10% CP treatments, respectively. The wording of the “worst”-end of the scale in the Auschill study was “severe discomfort” as opposed to “worst pain imaginable” in the present study. It would have been expected that such a “strong” wording in the current study would have led to a lower average VAS score. Other studies have reported tooth sensitivity as mean VAS score of about 4, such as 24 hours after treatment with 28% HP in combination with light (worst: “unbearable sensitivity”) [24] and after bleaching with 35- 38% HP combined with light (worst: “severe discomfort”) [25]. The corresponding mean VAS score for gingival sensitivity in the latter study was 1.11 ± 0.9, i.e. overlapping with that of the current study. It is noteworthy that patients in the current study terminated the treatment without reaching the maximum on the VAS [10]. However, taking the wording “the worst pain imaginable” into consideration, their experience was likely to be rather painful. Although no significant difference in sensitivity between groups was found, all patients who terminated the treatment prematurely were in the DW22% and DW-T22%- groups.
Tobacco use
Tobacco users were both cigarette smokers and Swedish “snus” users, and the potential influence of each tobacco product on bleaching efficacy is not known. The relative number of each tobacco product user was not reported. The patients were not asked whether they continued to use tobacco during the bleaching treatment. The finding that tobacco users reported significantly less hypersensitivity than did non-tobacco users (days 1-3) was contradictory to the non-significant correlation between tobacco use and hypersensitivity in an at-home bleaching study group (n=143) [11]. The above observations emphasize that a significant finding in small study groups needs to be confirmed. The influence of Swedish “snus” use on oral and general health is currently a topic of debate as long-time follow-up studies are lacking. More studies show an increased risk than showing no risk for oral cancer caused by “snus” use [26]. With reference to increased risk for carcinogenicity of combined use of tobacco/excess alcohol consumption and bleaching [1] tobacco users should be specifically advised against bleaching as they may be tempted by apparently good cosmetic results (Figure 3) and, as indicated in this study, less hypersensitivity during the first bleaching days.
Patient satisfaction
Contrary to previous findings [27], the current investigation did not reveal a positive correlation between bleaching efficacy and patient satisfaction with the treatment. Degree of satisfaction may be due to individual preferences and expectations and is difficult for the clinician to predict.
Conclusion
Within the limitations of the present clinical study, the following could be concluded: Despite the short bleaching time, almost all participants reported self-perceived side effects, no consideration taken to type or severity. Although the patient satisfaction with bleaching result and product ease of use were high for the OTC products, patients are likely to benefit from the regulations stated in the EU directive concerning cosmetic products. Prior to bleaching patients must be informed of the risk of negative side effects and major individual differences in both bleaching efficacy and types and intensity of side effects. Patient satisfaction with the bleaching result cannot be predicted.
Acknowledgement
Financial support was received from the Department of Prosthodontics and Dental Materials Science, Gothenburg University, Sweden. The authors acknowledge scientific discussions with associate professor Dr. Catarina Wallmann, Institute of Clinical Dentistry/Faculty of Medicine, University of Tromsø, Norway.
Disclaimer
The authors do not have any financial interest in the companies whose products are included in this study.
References
- Dahl JE, Pallesen U (2003) Tooth Bleaching - a Critical Review of the Biological Aspects. Crit Rev Oral Biol Med 14: 292-304.
- Hasson H, Ismail A, Neiva G (2008) Home-based chemically-induced whitening of teeth in adults (Review). Cochrane Database Syst Rev 4: 1-56.
- EU Directive 76/768/EEC (12899/11) (2011) Council of the European Union, Interinstitutional File: 2011/0164 (NLE), Brussels, Belgium.
- Tooth bleaching treatments. A review (2007) Association Dentaire Française (ADF) Dossiers, ADF, Paris, France.
- Burrows S (2009) A review of the efficacy of tooth bleaching. Dent Update 36: 537-551.
- Leonard RH, Sharma A, Haywood VB (1998) Use of different concentrations of carbamide peroxide for bleaching teeth: an in vitro study. Quintessence Int. 29: 503-507.
- Ziebolz D, Helms K, Hannig C, Attin T (2007) Efficacy and oral side effects of two highly concentrated tray-based bleaching systems. Clin Oral Investiq 11: 267-275.
- Browning WD, Blalock JS, Frazier KB, Downey MC, Myers ML (2007) Duration and timing of sensitivity related to bleaching. J Esteth Restor Dent. 19: 256-264.
- Tam L (1999) Clinical trial of three 10% carbamide peroxide bleaching products. J Can Dent Assoc 65: 201-205.
- Leonard RH, Haywood VB (1997) Risk factors for developing tooth sensitivity and gingival irritation associated with nightguard vital bleaching. Quintessence Int 28: 527-534.
- Bruzell EM, Pallesen U, Thoresen NR, Wallman C, Dahl JE (2013) Side effects of external tooth bleaching: a multi-centre practice-based prospective study. Br Dent J 215: E17.
- Burrows S (2009) A review of the safety of tooth bleaching. Dent Update 36: 604-614.
- World Medical Association (2013) Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. JAMA 310: 2191-2194.
- Gould D, Kelly D, Goldstone L, Gammon J (2001) Examining the validity of pressure ulcer risk assessment scales: developing and using illustrated patient simulations to collect the data. J Clin Nurs 10: 697-706.
- van der Burgt TP, ten Bosch JJ, Borsboom PCF, Kortsmit WJPM (1990) A comparison of new and conventional methods for quantification of tooth color. J Prosthet Dent 63: 155-162.
- Paul S, Peter A, Pietrobon N, Hämmerle CHF (2002) Visual and Spectrophotometric Shade Analysis of Human Teeth. J Dent Res 81: 578-582.
- Chu SJ (2003) Use of reflectance spectrophotometer in evaluating shade change resulting from tooth-whitening products. J Esthet Restor Dent 15: S42-48.
- Leonard RH Jr, Garland GE, Eagle JC, Caplan DJ (2002) Safety issues when using a 16% carbamide peroxide whitening solution. J Esthet Restor Dent 14: 358-367.
- Matis B, Mousa H, Cochran M, Eckert G (2000) Clinical evaluation of bleaching agent of different concentrations. Quintessence Int 31: 303-310.
- Schulte JR, Morrissette DB, Gasior EJ, Czajewski MV (1994) The effect of bleaching application time on the dental pulp. J Am Dent Assoc 125: 1330-1335.
- Li Y, Greenwall L (2013) Safety issues of tooth whitening using peroxide-based materials. Br Dent J 215: 29-34.
- Paul-Dauphin A, Guillemin F, Virion J-M, Briançon S (1999) Bias and precision in Visual Analogue Scales: A randomized controlled trial. Am J Epidemiol 150: 1117-1127.
- Auschill TM, Hellwig E, Schmidale S, Sculean A, Arweiler NB (2005) Efficacy, Side- effects and Patients’ Acceptance of Different Bleaching Techniques (OTC, in-office, at-home). Oper Dent 30: 156-163.
- Palé M, Mayoral JR, Llopis J, Vallès M, Basilio J, et al. (2014) Evaluation of the effectiveness of an in-office bleaching system and the effect of potassium nitrate as a desensitizing agent. Odontology 102: 203-210.
- Gurgan S, Cakir FY, Yazici E (2010) Different light-activated in-office bleaching systems: a clinical evaluation. Lasers Med Sci 25: 817-822.
- Wickholm S, Lahtinen A, Ainamo A, Rautalahti M (2012) [Adverse effects of Swedish smokeless tobacco “snus”]. Duodecim 128: 1089-96.
- Leonard RH Jr (1998) Efficacy, longevity, side effects, and patient perceptions of nightguard vital bleaching. Compend Contin Educ Dent 19: 766-781.
Appendix
Relevant Topics
- Advanced Bleeding Gums
- Advanced Receeding Gums
- Bleeding Gums
- Coronal Fracture
- Dental Anestheia and Sedation
- Dental Plaque
- Dental Radiology
- Dentistry and Diabetes
- Gum Cancer
- Gum Infection
- Occlusal Splint
- Oral Hygiene Blogs
- Oral Hygiene Case Reports
- Oral Hygiene Practice
- Oral Leukoplakia
- Oral Surgery Special Issue
- Orthodontistry
- Periodontistry
- Root Canal Treatment
Recommended Journals
Article Tools
Article Usage
- Total views: 17025
- [From(publication date):
May-2015 - Jul 17, 2024] - Breakdown by view type
- HTML page views : 12454
- PDF downloads : 4571