Research Article Open Access
Pathological (Histological and Ultrastructural) Study in Stomach and Intestine of Heteropneustes fossilis (Bloch) to Excel Mera 71, a Glyphosate-Based Herbicide
Palas Samanta1,2, Sandipan Pal3, Aloke Kumar Mukherjee4 and Apurba Ratan Ghosh1*1Ecotoxicology Lab, Department of Environmental Science, The University of Burdwan, Golapbag, Burdwan, West Bengal, India
2Division of Environmental Science and Ecological Engineering, Korea University, Anam-dong, Sungbuk-gu, Seoul, Republic of Korea
3Department of Environmental Science, Aghorekamini Prakashchandra Mahavidyalaya, Subhasnagar, Bengai, Hooghly, West Bengal, India
4P.G. Department of Conservation Biology, Durgapur Govt. College, Durgapur, West Bengal, India
- Corresponding Author:
- Apurba Ratan Ghosh
Ecotoxicology Lab, Department of Environmental Science
The University of Burdwan, Golapbag
Burdwan, 713104, West Bengal, India
Tel: 91 342 2657938
E-mail: apurbaghosh2010@gmail.com
Received Date: September 15, 2016; Accepted Date: November 25, 2016; Published Date: December 02, 2016
Citation: Samanta P, Pal S, Mukherjee AK, Ghosh AR (2016) Pathological (Histological and Ultrastructural) Study in Stomach and Intestine of Heteropneustes fossilis (Bloch) to Excel Mera 71, a Glyphosate-Based Herbicide. J Gastrointest Dig Syst 6:479. doi: 10.4172/2161-069X.1000479
Copyright: © 2016 Samanta P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Effects of glyphosate-based commercial herbicide, Excel Mera 71 were performed to evaluate the pathological responses in stomach and intestine of Heteropneustes fossilis (Bloch) for duration of 30 days both under rice field and laboratory concentrations. Under light microscopy, stomach showed distortion in columnar epithelial cells (CEC), lamina propria (LP) and gastric glands under both conditions, but the severity of responses were more pronounced under laboratory condition. Severe fragmentation in mucosal folds (MF) and epithelial cells, and excessive mucus secretion were observed under scanning electron microscopic (SEM) study in laboratory study but alterations in stratified epithelial cells and microridges structure were not prominent under field; while deformation in mitochondria and endoplasmic reticulum and cytoplasmic vacuolations were seen under transmission electron microscopic (TEM) observation in both the conditions, but less severe in field study. Intestine showed distortion and fatty deposition in lamina propria, lifting of CEC and loss of brush border structures in laboratory study, while damage only at the tip of the mucosal villi and CEC were observed under field study through light microscopic observation. Under SEM study, degeneration in CEC and excessive mucus secretion over CEC were prominent, while under TEM study deformation and necrosis in mitochondria, severe cytoplasmic vacuolation, necrosis, cytosolic disorganization, and loss of cellular compartmentation were observed in laboratory study, but intestinal epithelium showed normal appearance under field observation. Therefore, present investigation depicted that long-term glyphosate exposure caused stronger pathological alterations under laboratory condition compared with field study and finally, displayed responses could be considered as indicators of herbicidal contamination in aquatic environment.
Keywords
Glyphosate (Excel Mera 71); Scanning electron microscopy; Transmission electron microscopy; Stomach; Intestine; Heteropneustes fossilis
Abbreviations
AMPA: Aminomethylphosphonic Acid; CEC: Columnar Epithelial Cells; DPX: Distyrene Plasticizer Xylene; GIT: Gastrointestinal Tract; H&E: Haematoxylin-Eosin; SEM: Scanning Electron Microscopy; TEM: Transmission Electron Microscopy
Introduction
Fish are considered as one of the most precious aquatic resources and are much more vulnerable to a large number of xenobiotic substances, which possess bioaccumulation properties and causing a serious risk to human life through food chain contamination. Fish constitute an important part of animal protein in rural and urban areas as well as top of the trophic levels in aquatic ecosystem. Therefore, alteration in constituent properties of natural aquatic environment due to contamination by hazardous substances is inevitable like pesticides and/or herbicides from agricultural fields. They usually affect the behaviours, biochemistry, and physiology of the non-target organisms like invertebrates and fish [1,2]. Simultaneously, due to widespread use of these chemical formulations to agricultural fields they ultimately reach to the water bodies through different pathways such as run-off, direct herbicidal applications, or by effluent discharge [3] and posing significant toxicological risks to the aquatic inhabitant such as fish, phytoplankton, zooplankton etc., [4] including human beings [5].
Herbicides are chemicals, used for controlling the unwanted plants or pests by intervening the biochemical pathways of plants like, photosynthesis, respiration, growth, cell and nucleus division, or synthesis of proteins, carotenoids or lipids [6]. Herbicide directly causes loss of macrophyte community and affects non-target aquatic organisms [7]. Glyphosate, a weak organic acid of the isopropylamine salt of glyphosate (N-phosphonomethyl glycine), is used as nonselective, post-emergent herbicide for controlling the weeds through inhibition of the enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), which block the aromatic amino acid biosynthesis via shikimate pathway [8,9]. Glyphosate is highly soluble in water but insoluble in organic solvents, and tends to bind tightly to sediment, suspended particulates, organic matter and soil within six inch of soil layers [10]. It is generally degraded by soil microbes to aminomethylphosphonic acid (AMPA) and carbon dioxide (CO2) [11]. Due to strong binding properties with soil particles, glyphosate and AMPA are not move to groundwater but they have potentiality to contaminate surface water due to its use patterns and erosion. However, relatively little information is available regarding the toxicity of this herbicide on freshwater fishes [12-14]. Heteropneustes fossilis (Bloch) an air-breathing freshwater carnivorous teleost with wide geographical distribution, easy availability, commercially important possessing easy maintenance and acclimatization to laboratory conditions ensures this species as an excellent test organism for aquatic ecotoxicology. Therefore, the present study is concerned with sublethal effects of the glyphosate-based commercial herbicide formulation, Excel Mera 71 on the stomach and intestine of H. fossilis (Bloch). Oral toxicity is best studied in the parts of alimentary canal especially in stomach and intestine due to absorption property. Therefore, the aim of the present study is to evaluate the cytopathological effects of Excel Mera 71 both under laboratory and field study on comparative basis through light and electron microscopic observations.
Materials and Methods
Experimental specimen
Heteropneustes fossilis (Bloch) with an average weight of 31.8 ± 3.44 g and total length of 16.6 ± 0.388 cm were purchased from nearby local market and were acclimatized for 15 days in 250 L aquaria. Both male and female fish were used for the experiment. Fish were provided with continuous aeration. A static-renewal system was used for the experiment with a natural photoperiod of 12 hour light and 12 hour dark. Fish maintenance and experiment was conducted based on the Animal Care and Use Committee of our University and was approved by Ethical Committee. Water parameters during the exposure period showed the following values: temperature, 26.5 ± 0.127°C; pH, 7.94 ± 0.040; electrical conductivity, 392 ± 0.62 μS/cm; total dissolved solids, 279 ± 0.69 mg/l; dissolved oxygen, 6.44 ± 0.05 mg/l; total alkalinity, 204 ± 7.30 mg/l as CaCO3; total hardness, 180 ± 3.74 mg/l (presented as CaCO3); sodium, 24.5 ± 0.56 mg/l; potassium, 5.33 ± 1.02 mg/l; orthophosphate, 0.03 ± 0.001 mg/l; ammoniacal-nitrogen, 1.66 ± 0.21 mg/l and nitrate-nitrogen, 0.21 ± 0.030 mg/l. At the end of acclimatization, fish were divided into two sets: first set of fish were transferred to field ponds at Crop Research and Seed Multiplication Farm and second set of fish brought to the laboratory aquarium. During acclimation and exposure period, fish were fed 32% commercial fish pellets (Tokyu).
Field experimental design
Field set of fishes were again grouped as follows: three control cages and three treated cages, each cage contains 10 fish. Dose (750 g/acre) recommended for rice culture was dissolved in water. It was applied on the first day of the experiment [15,16]. During the exposure period, glyphosate concentration was measured based on the method developed by Jan et al. [17] and it was recorded to be 1.20 mg/l. For fish rearing, a special type of cage was prepared based on Chattopadhyay et al. [18] with some modifications and installed at the middle of the ponds. Cages were rectangular in shape with the dimension of 2.5 × 1.22 m and height of the each cage was 1.83 m. The submerged height of the cage was 0.83 m. Cages were structured by strong bamboo. Four-sided wall, floor cage and cage cover was made with two nylon net (PVC nets): the inner mesh size and outer bearing mesh sizes was of 1.0 × 1.0 mm2 and 3.0 × 3.0 mm2, respectively. Water parameters measured as per APHA [19] during field study showed the following values: temperature, 24.03 ± 0.203°C; pH, 6.56 ± 0.087; electrical conductivity, 347 ± 1.15 μS/cm; total dissolved solids, 247 ± 1.45 mg/l; dissolved oxygen, 7.00 ± 0.157 mg/l; total alkalinity, 221 ± 3.53 mg/l as CaCO3; total hardness, 140 ± 2.31 mg/l as CaCO3; sodium, 63.4 ± 2.67 mg/l; potassium, 15.9 ± 2.10 mg/l; orthophosphate, 0.241 ± 0.026 mg/l; ammoniacal-nitrogen, 0.742 ± 0.111 mg/l and nitrate-nitrogen, 1.66 ± 0.035 mg/l.
Laboratory experimental design
In the laboratory, similar set up was maintained, three aquariums for control containing 10 fish and three for treatment. Sub-lethal dose of 17.20 mg/l was used for the experiment [13,14]. On every alternate day dose was applied. Measured glyphosate concentration in water was 16.88 mg/l during the study period. During exposure period, water parameters showed following values: temperature, 26.6 ± 0.120°C; pH, 7.93 ± 0.075; electrical conductivity, 426 ± 5.93 μS/cm, total dissolved solids, 303 ± 4.69 mg/l, dissolved oxygen, 5.06 ± 0.43 mg/l; total alkalinity, 210 ± 10.5 mg/l as CaCO3; total hardness, 163 ± 3.04 mg/l as CaCO3; sodium, 37.8 ± 1.02 mg/l; potassium, 7.26 ± 1.12 mg/l; orthophosphate, 0.04 ± 0.002 mg/l; ammoniacal-nitrogen, 7.09 ± 2.15 mg/l and nitrate-nitrogen 1.78 ± 0.263 mg/l.
Histological analysis
At the end of the exposure period, fish were anesthetized with tricaine methanesulphonate (MS 222) and finally, stomach and intestine were dissected out and fixed in respective fixatives for further study. For histological study, tissues were fixed in aqueous Bouin’s fluid solution for overnight and dehydrated through graded series of ethanol (70%, 90% and 100%) and finally, embedded in paraffin. Leica RM2125 microtome was used for paraffin sectioning at 3-4 μ. Finally sections were stained with haematoxylin-eosin (H&E) staining solutions and stained sections were observed under Leica DM2000 light microscope.
Ultrastructural analysis
For SEM study, stomach and intestine were fixed in 2.5% glutaraldehyde solution for overnight at 4°C followed by post-fixation with 1% osmium tetraoxide solution at 4°C for 2 h. After fixation, tissues were dehydrated through graded series of acetone followed by amyl acetate and finally, tissues were dried using critical point dryer (CPD) with liquid carbon dioxide. Then, tissues were mounted on metal stubs and sputter-coated with gold (thickness 20 nm). Finally, gold coated tissues were examined under scanning electron microscope (Hitachi S-530) installed at University Science Instrumentation Centre of the University of Burdwan, Burdwan, West Bengal, India. For TEM study, tissues were fixed in Karnovsky fixative for overnight at 4°C followed by post-fixation with 1% osmium tetraoxide at 4°C for 2 hour. After fixation, tissues were dehydrated by graded series of acetone followed by infiltration and finally, embedded in epoxy resin (araldite CY212). Then, ultrathin sectioning were done by using a glass knife (thickness 70 nm), and sections were collected on naked copper-meshed grids and dried. Finally, sections were stained with uranyl acetate and lead citrate and examined under TECHNAI G2 high resolution transmission electron microscope installed at Electron Microscope Facility, Department of Anatomy, AIIMS, New Delhi, India.
Results
Stomach
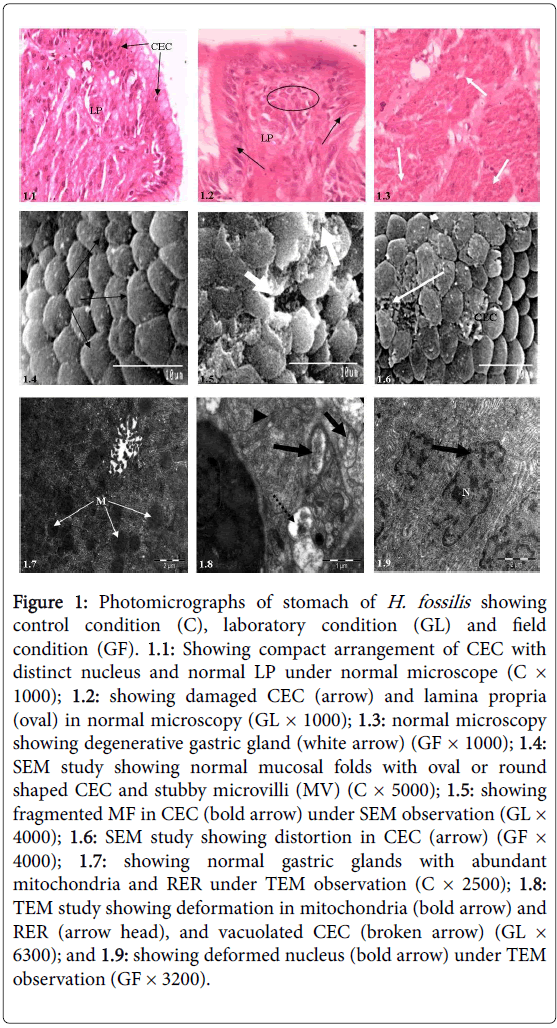
Histologically, stomach of the control fish is made up as usual four layers viz ., mucosa, submucosa, thick muscularis and serosa. Gastric mucosa is supported by columnar epithelial cells (CEC) with centrally placed nuclei. A thin layer of top plate externally covers the gastric columnar epithelium (Figure 1.1). Glyphosate exposure caused severe notable changes in CEC and lamina propria of stomach (Figure 1.2), while under field condition damage in the gastric epithelium was observed (Figure 1.3).
SEM observation displayed severe damage in CEC such as severe mucus secretion and fragmentation in CEC under laboratory study compared with control (Figure 1.5), but under field observation the responses were comparatively less than laboratory study, only mucin droplets over the epithelial surface were prominent (Figure 1.6).
Under TEM observation, when compared with control condition (Figure 1.7), stomach showed severe deformed mitochondria and rough endoplasmic reticulum (RER) and fatty deposition under laboratory study (Figure 1.8); but under field condition dilated mitochondria, irregular shaped nucleus and damaged endoplasmic reticulum were noticed (Figure 1.9).
Figure 1: Photomicrographs of stomach of H. fossilis showing control condition (C), laboratory condition (GL) and field condition (GF). 1.1: Showing compact arrangement of CEC with distinct nucleus and normal LP under normal microscope (C× 1000); 1.2: showing damaged CEC (arrow) and lamina propria (oval) in normal microscopy (GL × 1000); 1.3: normal microscopy showing degenerative gastric gland (white arrow) (GF × 1000); 1.4: SEM study showing normal mucosal folds with oval or round shaped CEC and stubby microvilli (MV) (C × 5000); 1.5: showing fragmented MF in CEC (bold arrow) under SEM observation (GL× 4000); 1.6: SEM study showing distortion in CEC (arrow) (GF× 4000); 1.7: showing normal gastric glands with abundant mitochondria and RER under TEM observation (C × 2500); 1.8: TEM study showing deformation in mitochondria (bold arrow) and RER (arrow head), and vacuolated CEC (broken arrow) (GL× 6300); and 1.9: showing deformed nucleus (bold arrow) under TEM observation (GF × 3200).
Intestine
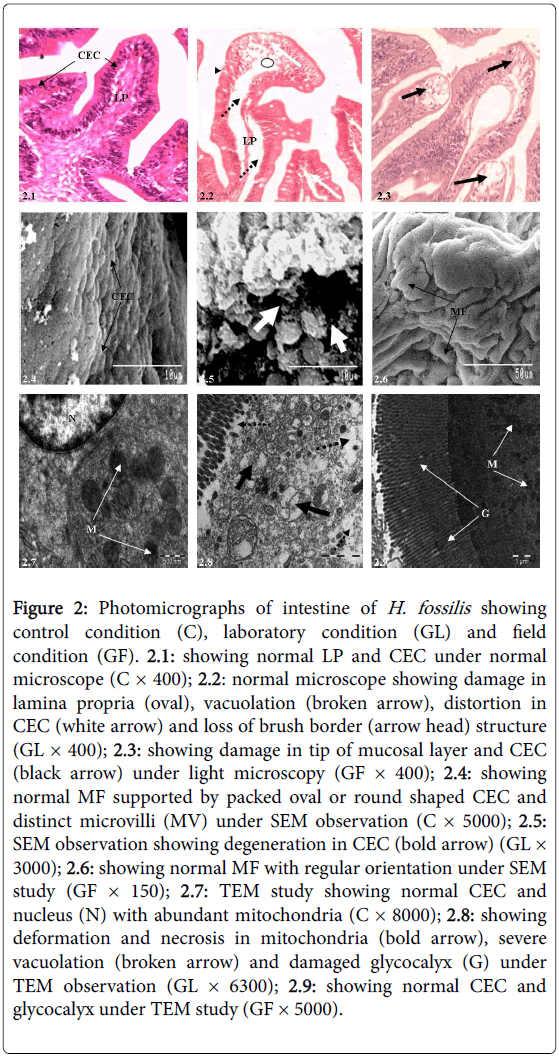
Likewise stomach, intestine of control fish possess four histological layers viz., mucosa, submucosa, muscularis and thin serosa. Mucosa is made up of simple and long finger like villi, which is supported by absorptive columnar epithelial cells with basally or centrally placed nucleus (Figure 2.1). A thin top plate of brush borders embraces the villi. Intestinal mucosa is supported by several mucous cells. Lamina propria of submucosa is formed by loose connective tissues and serosa layer is provided with compact network of blood vessels.
Most conspicuous pathological alterations seen under light microscopy after glyphosate exposure were distortion and vacuolation in connective tissues of lamina propria, severe degeneration in CEC which included epithelial lifting and complete disappearance of brush border (Figure 2.2) compared with the control condition (Figure 2.1). The extent of damage in field condition was comparatively less, except distortion in the tip of villi and some epithelial cells (Figure 2.3).
Under SEM observation, when compared with control condition (Figure 2.4) intestine displayed severe degeneration in the topology of CEC, excessive secretion of mucus over the epithelial cells (Figure 2.5) under laboratory study, but under field study, mucosal folds and CEC showed almost normal natural appearances (Figure 2.6). The transmission electron microscopic analysis when compared with controlled condition (Figure 2.7) displayed deformed and necrosed mitochondria and severe vacuolations. Presence of necrosis and cytoplasmic vacuolation, cytosolic disorganization and loss of cellular compartmentation in the laboratory condition were seen under both light and electron microscopy (Figure 2.8), while under field study intestinal epithelium showed less deformity (Figure 2.9).
Figure 2: Photomicrographs of intestine of H. fossilis showing control condition (C), laboratory condition (GL) and field condition (GF). 2.1: showing normal LP and CEC under normal microscope (C × 400); 2.2: normal microscope showing damage in lamina propria (oval), vacuolation (broken arrow), distortion in CEC (white arrow) and loss of brush border (arrow head) structure (GL × 400); 2.3: showing damage in tip of mucosal layer and CEC (black arrow) under light microscopy (GF × 400); 2.4: showing normal MF supported by packed oval or round shaped CEC and distinct microvilli (MV) under SEM observation (C × 5000); 2.5: SEM observation showing degeneration in CEC (bold arrow) (GL× 3000); 2.6: showing normal MF with regular orientation under SEM study (GF × 150); 2.7: TEM study showing normal CEC and nucleus (N) with abundant mitochondria (C × 8000); 2.8: showing deformation and necrosis in mitochondria (bold arrow), severe vacuolation (broken arrow) and damaged glycocalyx (G) under TEM observation (GL × 6300); 2.9: showing normal CEC and glycocalyx under TEM study (GF × 5000).
Discussion
Present study is first time reporting the toxicity of glyphosate-based commercial herbicide formulation, Excel Mera 71 in freshwater fish, H. fossilis through pathological observations under light, scanning and transmission electron microscopic study, although Senapati et al. [12] noticed histopathological alterations in gill, oesophagus, stomach and intestine of Channa punctatus induced by Excel Mera 71 under laboratory study and Samanta et al. [13,14] demonstrated biochemical alterations in different teleostean fish species including H. fossilis.
The orally administered contaminated food particles especially by herbicidal contamination after entering through the GI tract first stored and digested in the stomach and then pass to the intestine for absorption. So, the histological and cytopathological changes observed under present investigation might be due to formation of organochloride acid secreted by cardiac stomach part, which ultimately helps to induce the lesions in respective regions. Histological responses to Excel Mera 71 exposure in stomach observed under light microscopy included damage to the CEC and lamina propria and gastric glands caused destruction of the mucous membrane of the intestine, which ultimately alters the absorption of food materials [20]. Present findings are in agreement with the findings of Olaley et al. [21], who also demonstrated these alterations due to alteration of oxidative metabolism in the stomach. Therefore, these histological alterations observed in gastric mucosa could be considered as first warning signs caused by exposition of xenobiotics. The most notable ultrastructural alterations observed in stomach under laboratory study were severe fragmentation in epithelial cells, and excessive secretion of mucus under SEM study.
Results can be correlated with the findings of Senapati et al. [12], who reported similar lesions in Channa punctatus after glyphosate exposure under laboratory study. Comparatively less pathological lesions in epithelial cells under field study might be due to inhabitant of natural pond ecosystem and dilution effects. Excess secretions of mucus under field condition indicate higher protection against the xenobiotic exposure. TEM observation showed severe deformation in mitochondria and RER and fatty deposition in stomach of H. fossilis both under laboratory and field conditions. Rebolledo and Vial [22] also demonstrated deformation in nucleus and mitochondria, distortion in RER, necrosis and vacuolation in cytoplasm, appearance of vast amount of RER and damage in tubular network, dilated mitochondria in stomach of Halaelurus chilensis. Similarly, Carrassón et al. [23] noticed vast amount of smooth ER, RER and mitochondria, presence of tubule-vascular network and heterochromatic nuclei in Dentex dentex. Therefore, these pathological alterations in the gastric epithelium observed in stomach could lead to functional alterations and interfere with the fundamental process such as digestive physiology.
Intestine is the second most important organ after stomach and experienced to come into contact with food-borne contaminants [24]. Present histological examination exhibited variability in intestinal lesions and higher damage in laboratory study which included distortion and vacuolation in lamina propria, severe damage in absorptive CEC, lifting of columnar epithelial cells and complete loss of brush border. Additionally, damage at the mucosal tip and intestinal villi were also observed under field study. Epithelial degeneration observed in intestine indicated increased formation of free radicals and if not removed by free radical scavengers caused damage to the intestinal tract including gastric mucosa and finally, inhibit the normal function of several enzymatic activities [25,26]. Kumari and Kumar [4] also noticed degeneration in serosa, mucosa and submucosal layers, and necrosis, proliferation and desquamation of the superficial parts of intestinal villi in Channa striatus and Heteropneustes fossilis collected from polluted water. Braunbeck and Appelbaum [24] also observed epithelial lining in intestine of Cyprinus carpio after endosulfan exposure and indicated that necrosed epithelial lining is responsible for intestinal malabsorption. Additionally, Cengiz et al. [27] noticed oedema, degeneration, accumulation of lymphocytes and eosinophils in intestine of G. affinis to deltamethtin exposure. SEM observations showed severe degeneration in CEC and excessive secretion of mucus to glyphosate exposure. Damages in CEC of H. fossilis observed under present investigation were also demonstrated in C. punctatus after glyphosate exposure by Senapati et al. [12]. Excessive mucus secretion indicates that fish are under stress and trying to cope with this toxic stress imposed by herbicidal exposure. TEM observation showed that chronic exposure to glyphosate in H. fossilis caused several cytopathological alterations in intestinal epithelium i.e., deformation and necrosis in mitochondria and severe fatty deposition under laboratory study. Presence of necrosis and cytoplasmic vacuolation were seen on both under light and electron microscopy representing focal tissue and cytosolic disorganization and loss of cellular compartmentation. Under field observation, intestine showed no such marked pathological lesions in epithelial cells and this might be due to natural aquatic condition. However, Sastry and Siddiqui [28] reported damages in intestinal mucosa of Channa punctatus after endosulfan exposure. Therefore, these alterations might alter intestinal transportation processes as a first mode of toxic action and represents compensatory responses against herbicidal attack.
In conclusion, present findings demonstrated that chronic exposure of glyphosate induces histopathological and ultrastructural changes in stomach and intestine. The cytopathological lesions in the gastrointestinal tract differed significantly to different exposure conditions and representing an integration of cumulative effects of physiological and biochemical contaminants. Generally, these responses were pronounced in laboratory-exposed fish than fieldexposed fish which ultimately indicating higher disturbances of the cellular metabolism as well as stronger structural alterations under laboratory condition. Therefore, these histopathological signs including ultrastructural lesions reflecting different environmental conditions for fish in aquatic ecosystem and could be considered as sensitive biomarkers of xenobiotic exposure and finally may be interpreted as metabolic disorders stimulated by the xenobiotic substances.
Acknowledgements
The authors like to thank the Department of Science and Technology, Govt. of India for the financial assistance through INSPIRE Fellowship Program (DST/INSPIRE Fellowship/2011/164, Dt. 29.09.2011). We also like to thank the Head, Department of Environmental Science, The University of Burdwan, Burdwan, West Bengal, India for providing the laboratory facilities and library facilities during the course of this work. We are also thankful to the respective reviewers for improving our manuscript.
References
- Pandey AC (1988) Impact of Endosulfan (Thiodan EC 35) on behavior and dynamics of oocyte development in the teleostean fish Colisafasciatus. Ecotoxicol Environ Saf15: 221-225.
- Vosyliene MZ, Kazlauskiene N (1997) Alterations in fish health status parameters after exposure to different stressors. ActaZoologicaLituanica Hydrobiology9: 82-95.
- Daabees AY, El-Domiatty NA, Soliman, SA, El-Toweissy MY (1992) Comparative action of three synthetic pesticides on serum liver and brain enzymes of the freshwater Clariaslazera. J Egypt GerSocZool 10: 105-119.
- Kumari AS, Kumar NSR (1997) Effects of water pollution on histology of intestine of two fresh water fishes from Hussainsagar Lake (AP). Indian J Environ Toxicol7: 68-70.
- Dutta HM, Maxwell LB (2003) Histological examination of sublethal effects of diazinon on ovary of bluegill, Lepomismacrochirus. Environ Pollut121: 95-102.
- Ecobichon DJ (1991) Toxic effects of pesticides in Casarett and Doull's Toxicology-The Basic Science of Poisons (Amdur MO, Doull J, Klaassen DC edn), McGraw-Hill Inc, New York, pp: 565-622.
- Ervnest H (2004) A Textbook of Modern Toxicology (3rd edn), John Wiley & Sons Hoboken, New Jersey, pp: 557.
- SteinrückenHC, Amrhein N (1980) The herbicide glyphosate is a potent inhibitor of 5-enolpyruvylshikimic acid-3-phosphate synthase. BiochemBiophys Res Commun94: 1207-1212.
- Cavas T, Könen S (2007) Detection of cytogenetic and DNA damage in peripheral erythrocytes of goldfish (Carrassiumauratus) exposed to a glyphosate formulation using micronucleus test and comet assay. Mutagenesis22: 263-268.
- Duke SO (1988) Glyphosate in Herbicides: Chemistry, Degradation and Mode of Action (Kearney PC and Kaufman DD edn) Marcel Dekker, New York, pp: 1-70.
- Ruppel ML, Brightwell BB, Schaefer J, Marvel JT (1977) Metabolism and degradation of glyphosate in soil and water. J Agric Food Chem25: 517-528.
- Senapati T, Mukherjee AK, Ghosh AR (2009) Observations on the effect of glyphosate based herbicide on ultrastructure (SEM) and enzymatic activity in different regions of alimentary canal and gill of Channapunctatus (Bloch). J Crop and Weed 5: 236-245.
- Samanta P, Pal S, Mukherjee AK, Ghosh AR (2014) Evaluation of metabolic enzymes in response to Excel Mera 71, a glyphosate-based herbicide, and recovery pattern in freshwater teleostean fishes. BioMed Research Interanational.
- Samant P, Pal S, Mukherjee AK, Ghosh AR (2014) Biochemical effects of glyphosate based herbicide, Excel Mera 71 on enzyme activities of acetylcholinesterase (AChE), lipid peroxidation (LPO), catalase (CAT), glutathione-S-transferase (GST) and protein content on teleostean fishes. Ecotoxicol Environ Saf107: 120-125.
- Samanta P, Pal S, Mukherjee AK, Kole D, Ghosh AR (2016) Toxic effects of glyphosate-based herbicide, excel mera 71 on gill, liver and kidney of Heteropneustesfossilis under laboratory and field conditions. Journal of Microscopy and Ultrastructure 4: 147-155.
- Samanta P, Pal S, Mukherjee AK, Senapati T, Ghosh AR (2016) Histopathological and ultrastructural alterations in Anabas testudineus exposed to glyphosate-based herbicide, excel mera 71 under field and laboratory conditions. J Aquac Res Development 7: 436
- Jan MR, Shah J, Muhammad M, Ara B (2009) Glyphosate herbicide residue determination in samples of environmental importance using spectrophotometric method. J Hazard Mater 169: 742-745.
- ChattopadhyayDN, Mohapatra BC, Adhikari S, Pani PC, Jena JK, et al. (2013) Effects of stocking density of Labeorohita on survival, growth and production in cages. AquacultInt 21: 19-29.
- APHA, AWWA, WPCF (2005) Standard Methods for the Examination of Water and Wastewater (21st edn), Washington, DC.
- Anderson W, Hickey JJ, Risebrough RW, Hughes DF, Christensen RE (1969) Significance of chlorinated hydrocarbon residues to breeding pelicans and cormorants. Can Field Nat83: 91-112.
- Olaley SB, Adaramoye OA, Erigbali PP, AdeniyiOS (2007) Lead exposure increases oxidative stress in the gastric mucosa of HCl/ethanol exposed rats. World J Gastroenterol13: 5121- 5216.
- Rebolledo IM, Vial JD (1979) Fine structure of the oxynticopeptic cell in the gastric glands of Elasmobranch species (Halaeluruschilensis). Anat Rec 193: 805-822.
- Carrassón M, Grau A, Dopazo LR, Crespo S (2006) A histological, histochemical and ultrastructural study of the digestive tract of Dentexdentex (Pisces, Sparidae). HistolHistopathol 21: 579-593.
- Braunbeck T, Appellbaum S (1999) Ultrastructure alternation in the liver and intestine of carp Cyprinuscarpio induced orally by ultra low doses of enodsulfan. Dis Aquat Organ 36: 183-200.
- Dai W, Du H, Fu L, Jin C, Xu Z, Liu H (2009) Effects of dietary Pb on accumulation, histopathology, and digestive enzyme activities in the digestive system of tilapia (Oreochromisniloticus). Biol Trace Elem Res 127: 124-231.
- Abdallah GM, El-Sayed SM, AboSalem OM (2010) Effect of lead toxicity on coenzyme Q levels in rat tissues. Food ChemToxicol48: 1753-1756.
- Cengiz EL, Unlü E, Balci K (2001) The histopathological effects of Thiodan® on the liver and gut of mosquito fish, Gambusiaaffinis. J Environ SciHealth B36: 75-85.
- Sastry KV, Siddiqui AA (1982) Effect of endosulfan and quinalphos on intestinal absorption of glucose in the freshwater murrelChannapunctatus. ToxicolLett 12: 289-293.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 5198
- [From(publication date):
December-2016 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 4273
- PDF downloads : 925