Parkinsons Disease Treatment Using Cell Transplantation
Received: 04-May-2020 / Accepted Date: 18-Jul-2020 / Published Date: 25-Jul-2020 DOI: 10.4172/2161-0460.1000491
Abstract
The testing of human fetus mesencephalic tissues with intrastriatal transplantation clinically,that are rich in dopamine producing neurons. Parkinson’s disease patient showed cell transplantation works and in many cases produces impervious improvements. Due to the poor availability of tissues, this method could only be administered in fewer number of patients and acclimitization was very difficult,leads to immense deflection in operative outcomes. For transplantation, undifferentiated (stem) cells and special (reprogrammed) cells could be availed potentially to emoulument dopamine producing neurons.From human embryonic stem cells dopamine producing neurons that will be of the appropriate substantia nigra phenotype can be emolumented in larger numbers and soon will be ready for application in patients.Dopamine producing neurons obtained from pluripotent stem cells of human are supposed to be used for clinical transplantation. In a controlled clinical studies,the present data justifies leading in a way with these dopamine producing neurons, that should be tested by choosing desirable patients, anticipation of cell and methods of transplantation.
Keywords: Human fetus mesencephalic tissues; Dopamine producing neurons; Human pluripotent stem cells; Stem cells transplantation; Human embryonic stem cells; Reinnervation, Denervated striatum; Substantial nigra
Introduction
Till 1970s, it was thought that the re-establishment of central nervous system in human beings which was impossible in past will never ever be accessible in future. Two articles with attainable association had been broadcasted in 1979, showed the intrastriatal implants of fetus mesencephalic dopamine rich tissues in rodents could improve indications of experimental Parkinson’s disease. That deep-rooted neurodegenerative shambles, in humans, is personalized by damage of movement, malfunction and death of vital nerve cells in the brain, called neurons [1,2]. Motor symptoms could be treated auspiciously by dopamine producing drugs for many years but as the time passed, these drugs had low denouement and could show involuntary movements as side effects. Through transplantation, the animals model developed the peradventure of an innovative therapeutic avenue for Parkinson’s disease patients that is depraved on reestablishing of the cadaverous dopamine producing neurons by hygienic ones [3].
The first clinical transplantation was not done by fetus mesencephalic tissue of human in Parkinson’s disease patients. Autologous adrenal medulla cells were implanted into striatum of four Parkinson’s disease patients to provide a local catecholamine source, in early work that was done by Seiger and Backland and their co-workers, but the beneficial outcomes were very insufficient. The first intrastriatal implantations of fetus mesencephalic tissue from human were performed, in the Parkinson’s disease patient, rich in dopamine producing neuroblasts, in 1987. Until late 1990s, clinical studies were continued. In open-label studies and improvements were reported, but there were two doubleblind tests authenticated no compelling alterations as compared to sham-operated controls.
Now the therapy of cell research for Parkinson’s disease has accessed a new astonishing stage and current progress in this field give reason for anticipation [4]. There are three main fountain head of dopamine producing neurons that are being planned for clinical applications. Such as
• Human fetal mesencephalic tissue
• Human embryonic stem cells
• Human induced pluripotent stem cells
These clinical trials has ambited for these different types of fountainhead and the main challenges, type of stem cells that are being accounted for transplantation in mammalian models of parkinson’s disease will be characterized here [4-7].
Human Fetal Mesencephalic Tissue Transplantation
Human mesencephalic tissue transplantation had not progressed into a clinical emulous treatment for the Parkinson’s disease patients, but the studies had provided us with a beneficial acumen for the basic principle of cell remedial in Parkinson’s disease patients. Dopaminergic neurons derived from the human fetus dopamine-rich mesencephalic tissue are cogitated gold standard as compared with those dopaminergic neurons which are obtained from the other sources towards clinical applications.
The fetal dopaminergic neurons, from many studies, can be survived and augmented after intrastriatal transplantation in the parkinson’s disease patient’s brains [8]. Uptake of F-DOPA in the implanted putamen and histopathological studies had granted the adaptations of implanted dopamine producing neurons and Reinnervation of the striatum had exhibited with the help of Positron Emission Tomography (PET) [9,10]. Four patients were observed with major clinical improvements at 7-10 years after transplantation, in those patients uptake of F-DOPA codified in the implanted putamen after surgery of 10-16 years showed the accustom absolution of dopamine that was avenue by C-raclopride binding. Mesencephalic fetus implants conversed the deficiencies in the movement-related cortical activation similar to that of the clinical improvements, providing the attestations for the functional melding of dopamine into the host neuronal circuitry.
In several open-lab trials, the clinical benefits had been examined and for many years the best auspicious cases with L-DOPA treatment checked out. Two patients were enslaved to reciprocal intrastriatal transplantation of human fetus mesencephalic tissue displayed that the human fetus dopaminergic grafts could give rise to clinically competitive betterments [8]. After grafting of up to 18 years the motor improvements in these two patients were continued. Due to the expiration of the striatal dopaminergic functions, the improvements in these two patients are averment with the help of establishment of putaminal uptake of F-DOPA and binding of C-raclopride [4].
When two sham surgeries controlled clinical studies with the reciprocal intraputaminal implants had not approved the affirmative effects in the open-label tests, at the same time a major complication for the field appeared. But the modern improvements of motor functions at 12 months were determined by Freed et al. in the first trial [11]. Others, examined clinical benefits and enduring grafts upto four years after transplantation in the open-label repetition of those two patients. At 24 months, Parkinson’s disease symptoms were not different between the grafted and sham groups in the second trials. During the first 6 to 9 months after transplantations, improvements were observed. As recommended the adulterate after outcome of immunosuppression at the six months, the function of the graft had been blunted in response to the immune reaction. However, it is contrary that the advanced Parkinson’s disease patients with the wide-spread denervation will show efficacious results by intraputaminal transplantation. In less acutely disabled patients improvements were seen according to Olanow et al. [12].
In human beings, the long term survived grafts had been briefed to show the minimum asseveration of The Dopamine Transporter (DAT), which showed that alpha-syncline pathology is accomplices defy the synaptic dysfunction. Dopamine transporter binding ,in one such case, increased after grafting and continued pedestrian at fourteen years of post-transplantation.at 4-14 years after transplantation, few narrated the flourishing expression of dopamine transporter and normal mitochondrial localization in the implanted dopamine producing neurons in 5 parkinson’s disease patients and the degree of graft pathology assorted around these patients. Dopamine producing cell therapy is applicable therapeutic choice due to the following reasons:
• Slowness of disease advancement
• Majority of the grafted neurons didn’t have effect after a decade
• Improvement is long-term that patients experienced
Through DBS, that is deep brain stimulation, of the Globus pallidus, dyskinesias which referred as involuntary movements of muscles which had progressed in a subset of the implanted patient were treated. Several mechanism in the animal models had been identified which were underlie the grafted-induced dyskinesias, like as postsynaptic super sensitivity that was constituted by the chronic treatment of L-DOPA before the transplantation. Dyskinesias caused by the implant-derived serotonergic hyper innervation of the striatum after transplantation accommodated strong evidence during the clinical ascertainments. Three patients, in which moderately severe graftinduced dyskinesias with major clinical improvement was established, showed that exorbitant serotonergic innervation in the grafted striatum. Through the administration of 5-HT1A receptor agonist, that subdue transmitter absolution from serotonergic neurons dyskinesia were abrogated.
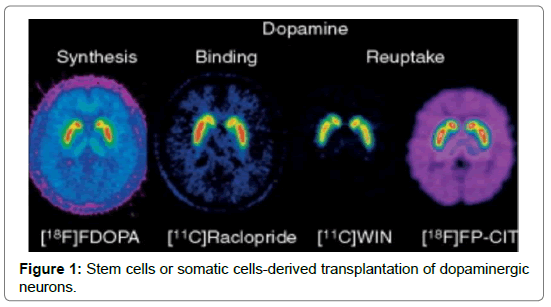
Human fetal mesencephalic tissue will be implanted into striatum with the optimized patient selection and tissue preparation procedures, in the ongoing EU-sponsored TRANSEURO clinical trial (www.transeuro.org.uk). As compared with the antecedent trials the patients were younger and earlier in their disease and they had not developed any cogent L-DOPA induced dyskinesias [13]. Human fetal mesencephalic tissue, however, could be get solely in a limited amount and it is difficult to standardize, but it would become very useful in a larger numbers of patients for transplantation. So, new sources of dopaminergic neurons are needed. Dopaminergic neurons’ generation from the stem cells or progenitor cells or by reprogramming of somatic cells (Figure 1).
Conventional aspects
There are some conventional aspects that are very important.
ӹӹ For the production of transplantable dopamine producing neurons in the parkinson’s disease ,many potential antecedents of cells had been adduced, including the pluripotent cells such as Embryonic Stem Cells (ES), neural stem cells, Induced Pluripotent Cells (IPS) and somatic cells which directly converted into the dopaminergic neurons.
ӹӹ A clinical competitor cell should be originated from human and should be the characteristics of substantial nigra neurons to be produced and able to perform paramount function [14]. Other types of cells produced from the stem cells or reprogrammed cells need to be known that is serotonin neurons, the number of non-nigral dopaminergic neurons, other non-dopaminergic neurons, undifferentiated precursors/stem cells, glial elements and non-neural cells.
ӹӹ Methods for cell grouping would most considerably of major concernment. These methods would grant:
• Removal of unwanted cells
• Adjunction of dopamine producing neurons of the appropriate mesencephalic phenotype along with the favorable phase of differentiation
ӹӹ Requirements for efficacy should be emphasized for a cell based therapy for Parkinson’s disease.
ӹӹ A cell-based therapy to be clinically competitive should give rise to long lasting major improvement >60-70% of mobility and suppression of dyskinesia’s and the improvement of symptoms resistant to other reinforcements and treatments of disease progression [15].
Dopamine producing neurons obtained from stem cells and reprogrammed cells haven’t been performed scientifically abominable clinical studies with transplantation.
Characteristics of candidate/competitive cells
• The candidate/competitive cells that is used for the transplantation must have following characteristics so that the graft might survive
• Competitive cell must be originated from human that are going to be used in clinically tests.
• Clinical candidate cell must have the characteristics of substantial nigra neurons to be able to abet maximal recovery of functions.
• Clinical competitive cell must be adroit to survive long term
• Clinical competitive cell must be differentiated into the appurtenant neuronal phenotype, that is, nigral dopamine producing neurons after transplantation into Parkinson’s disease model.
• Advancement in cell division must not be beyond 1-2 months used for transplantation.
Human Embryonic Stem Cells-Derived Dopaminergic Grafts
The survival and Behavioural ameliorations of grafts were first announced a decade ago, proceeded the intrastriatal transplantation of human embryonic stem cells obtained from dopamine producing neurons in a rodent parkinson’s disease model. Large numbers of dopamine producing neurons of substantial nigra phenotype were shown by the grafts but there were potentially tumor producing and mitotic undifferentiated Neuro-epithelial cells too. In 2011, kriks and coworkers explicated a novel protocol, in which human embryonic stem cells were disciple efficiently into dopamine producing neurons. Floor plate cells were collected using SMAD signaling inhibition and the high level of sonic hedgehog in vitro. By the activation of the signaling, a midbrain floor plate deity was induced and the cells were antithesized into the dopamine producing neurons precursors. High number of substantial nigra dopamine producing neurons was survived long lasting in rodents after the transplantation of intrastriatal and surprisingly there was no formation of tumors [16]. In larger nonhuman primate brain, a major portion of striatum could reinnervate. Steinbeck and coworkers exposed the motor recovery which is induced dopamine producing neurons obtained from human embryonic stem cells which was grafted into the dopamine-denervated mouse striatum with the help of electro-physiological, pharmacological and opt genetics accessions. That graft modulated glutamatergic transmission analogous to endogenous substantial nigra neurons in the host striatum. Dopamine producing neurons that obtained from human embryonic stem cell had been implanted into the rat model of parkinson’s disease had the aptitude of axonal growth and long term adaptations and the functional adequacy as that of the human mesencephalic dopamine producing neurons.
Advantages
• By using the novel protocol, which was developed by kriks and coworkers, there are many advantages of dopamine producing neurons from stem cells of human embryo.
• High numbers of dopamine producing neurons derived from human were obtained and the cells survived transplantation.
• Reinnervate the denervated striatum and these neurons were of the correct phenotype that means of correct substantial nigra.
• These cells improved the clinically relevant behavioural deficiencies.
• The efficacy of the stem cells that were obtained from human embryo was commensurable with the efficacy of fetal dopamine producing neurons that had activated long term symptomatic relief in Parkinson’s disease patients.
• Dopamine producing neurons that were obtained from stem cells of human embryo has the capability for axonal growth and long-term survival.
Human Induced Pluripotent Stem Cells
By the reprogramming of fibroblast through pluripotent stage, called Induced Pluripotent Stem Cells (IPS), Human dopaminergic neurons are produced. The patients’ specific tissues and cells can be emolumented and accounted for the transplantation with the help of this technology. Furthermore, ethical issues and immune responses that were related to human embryonic stem cells can be avoided. There are some potential problems like tumourgenesis and variability of reprogramming. The risk of increased vulnerability in the patient’s specific cells of Parkinson’s disease also existed.
The outgrowth of the boundless axons that were derived from the human induced pluripotent stem cells implanted in the striatum of the denervated rodents had not been conclusively reported and the capability to improve the behavioral deficiencies were incompletely known [10]. After implantation of induced pluripotent stem cells derived dopamine producing neurons, the functional recovery had been reported in the striatum of rodents. The compactness of fibers which were extending from the grafts was low, it was broadcasted that dopamine had not acted through synaptic release but via diffuse volume transmission. There was a question that if it could work in a smaller rodents then would it work in a larger human brain? So the answer is here, recently Hall, briefed that the autologous dopamine producing neurons obtained from induced pluripotent stem cells could survive in larger number in non-human primate for 2 years after implantation into striatum and gave rise to Reinnervation and improvement in motor functions. Out of three grafted monkeys, the findings were contemplated only in one and these findings provided preclinical evidences followed by clinical translation of dopamine producing neurons obtained from the induced pluripotent stem cells in Parkinson’s disease for transplantation.
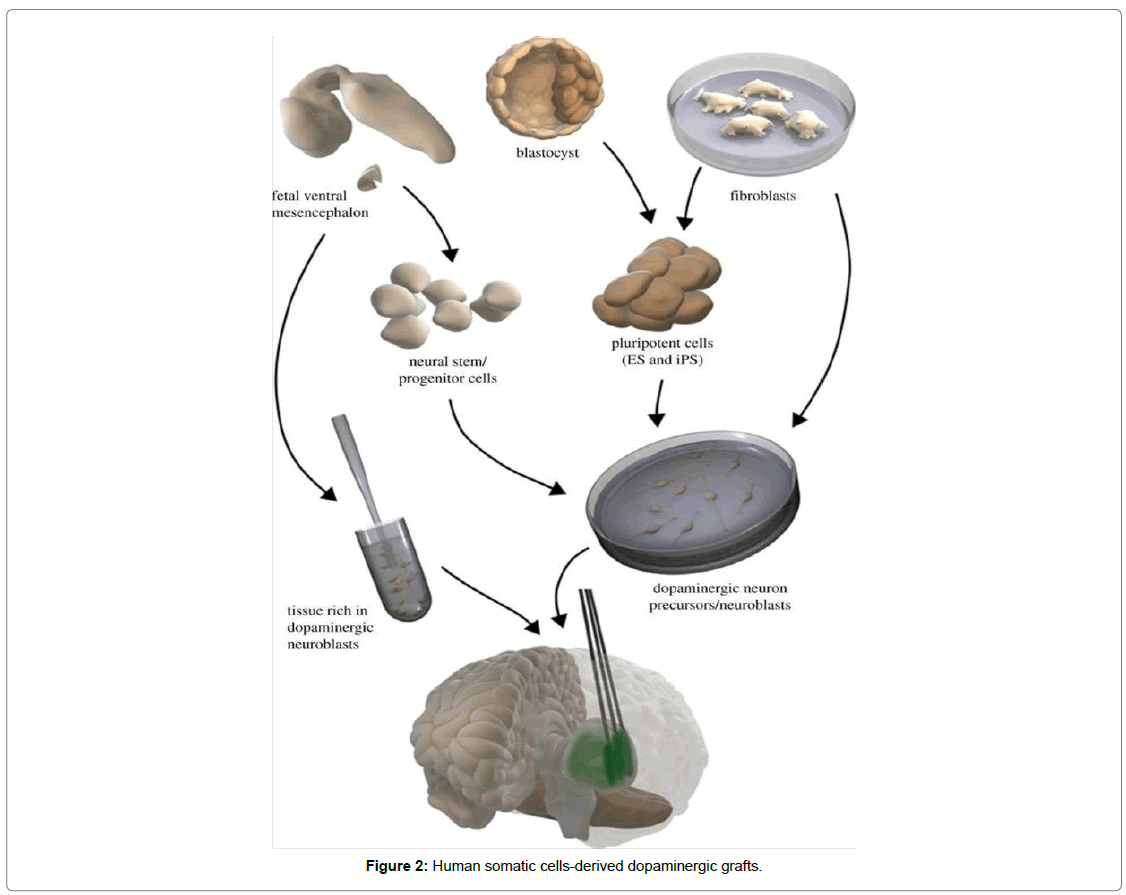
The production of functional dopamine producing neurons could also be done by the direct conversion of human fibroblast that was of potential interest in the clinical contexts. Fibroblasts that were obtained from mouse, directly disciple into dopaminergic neurons showed survival in transplantation. More capacious axonal outgrowth of dopaminergic neurons derived from mouse fibroblast were implanted into striatum of denervated rat was recently reported. Betterments in stepping and deficiency in Apo morphine-induced rotational asymmetry were shown. By pharmacological activation of dopamine producing neurons performance was adorned. Before clinical practices of dopamine producing neurons much work is needed to be done when direct conversion can be considered. Such cells originated from human could survive in larger numbers, give rise to substantial improvement depicted to behaviorism deficiencies in animal Parkinson’s disease model and the Reinnervation should be demonstrated as a first step (Figure 2).
Challenges
For auspicious clinical application of stem cells, there are following three major challenges.
Potency
In an animal Parkinson’s disease model, the first challenge is the affirmation of capability of the generated dopamine producing neurons after transplantation. In order to abet clinically competitive improvement, the fresh cells have to be worked efficiently as human fetal dopamine producing neurons. In order to actuate the counts of cells for transplantation and implants’ counts in patients, potency should be known which includes growth capability ,axonal growth and dopamine release.
Safety
It is the second major challenge to establishment of safety in Parkinson’s disease patients for transplantation. Tumour formation and graft-induced dyskinesias’ risk should be minimized that had been examined for fetal mesencephalic tissue implantation. The integrity of all types of cells in the grafts is very important to be determined. For the elimination of tumors forming cells, cell sorting is used.
Selection
The third major challenge in the clinical trial is the selection of most condign patient. Patient must have in an approximately earlier stage of disease but there are chances of therapeutic benefits too.
Clinical Trials
Treating Parkinson’s disease, stem cell-based therapies are moving towards the clinical testings. In October 2016, capitalization of pluripotent stem cells to treat Parkinson’s disease was approved. In many centers, clinical trials of stem cell-based therapies are abutting. In these clinical trials that were done in 2014, implantation of human fetus mesencephalic tissue showed proofs for cell replacement strategy. Now in clinical application, human embryonic stem cells and induced pluripotent stem cells are adopted. Capitalization of optimal patient selection, transplantation methods and preparation of cells, development of beneficial treatment for Parkinson’s disease are done in well-controlled clinical studies.
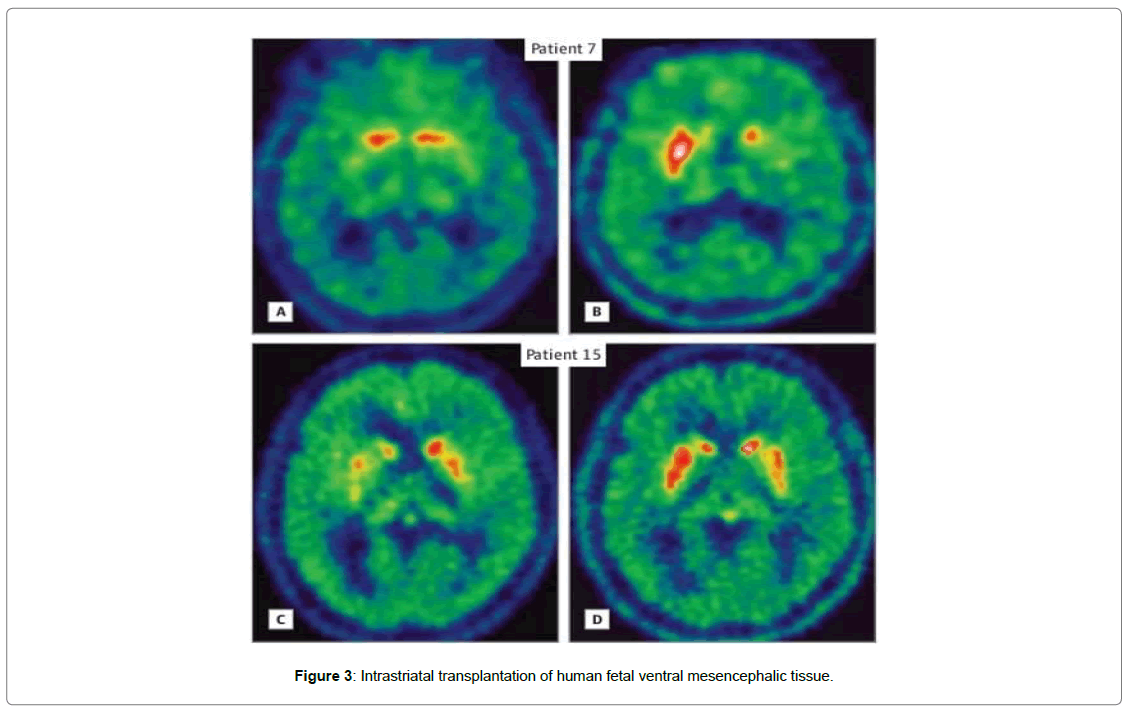
Two patients (patients 7 and 15) were taken that apprehended intrastriatal transplantation of human fetal ventral mesencephalic tissue that were rich in dopamine producing neuroblasts as experimental treatment for Parkinson’s disease. Both of them, throughout their disease channels experienced excellent responses to levodopa treatment. For treating putamen of patient 7 and putamen and caudate nucleus of patient 15, a magnetic resonance imaging guided stereotactic method had been adopted, at the age of 49 and 54. By capitalization of Unified Parkinson’s Disease Rating Scale (UPDRS), motor function was measured. Preoperative and postoperative clinical scores were compared with current scoring as shown in the table below (Table 1) (Figure 3).
| Characters | Patient 7 | Patient 15 |
|---|---|---|
| Sex | Male | Male |
| Age/disease duration | 39/10 | 42/12 |
| Disease phenotype | Akinetic rigid | Akinetic rigid |
| Mean UPDRS part III score in practically defined ‘off’/best ‘on’ conditions (range 0-108) | 38/11 | 23/3.4 |
| Parkinson’s disease related medication | Levodopa 300 mg/d | Levodopa 900 mg/d |
| Pergolide 1.5 mg/d | Pergolide 1.5 mg/d | |
| Selegiline 10 mg/d | Amantadine 200 mg/d | |
| Amantadine 300 mg/d | 2-3 apomorphine injections (5 mg/0.5 mL)/d | |
| Date/location of transplantation/no. of trajectories | Apr 1993/left putamen/5 | Nov 1996/left putamen+caudate/5+2 |
| Sept 1994/right putamen/5 | Nov 1996/right putamen+caudate /5+2 | |
| No. per side/age of donors, week post conception (embryos) | 5/6-8 | 4/6-9 |
| Transplantation preparation | Fresh tissue; cell suspension | Fresh tissue; cell suspension |
| Immunosuppressant | Cyclosporine, azathioprine, prednisolone (2 day before to 48 months after grafting) | Cyclosporine, azathioprine, prednisolone (2 day before to 20 months after grafting) |
Table 1: Comparison of preoperative and postoperative clinical scores with current scoring.
Conclusion
Transplantation of patient no. 7 experienced motor improvements that became visible over the course of four years. After first transplantation, 26 months later the patient stopped levodopa treatment. All dopamine producing agents had been departed while the patient’s motor status continued to be better by the fifth postoperative year. The patient established sustained motor improvements at the last assessment of 18 years post grafting. No anomaly in motor examination and free of any pharmacological dopamine stand-in therapy was reported. Patient’s swallowing was normal and falls and freeze had not appeared as a problem and all the activities of daily living was continued as independently.
In patient no. 15, there was no improvement shown during first two years after transplantation. However, motor function became evident from the fourth year after grafting ,at the end of which the patient was able to stop all dopaminergic medications. Motor improvements were perpetuated after transplantation of 15 years of assessment. The patient had minor rigidity, bradykinesia, normal gait and intact postural reflexes during motor examination. The patient was free of any dopaminergic medications.
In the basal ganglia, dopaminergic Reinnervation was normal. Anyhow symptoms like dullness, apprehensions, mood swings and sleep cycle problems were appeared in patients. There are several therapeutic options for Parkinson’s disease patients which are in advance stage. In the field of cell therapy for Parkinson’s disease, there has been a steady scientific progress.
References
- Brkund A, Stenevi U (1979) Reconstrucyon of the nigrostriatal dopamine pathway by intraceberal nigral transplants. Brain Res 177: 55-560.
- Perlow MJ, Freed WJ, Hoffer BJ, Seiger A, Olson L, Wyatt RJ (1979) Brain grafts reduce motor abnormalities produced by dstruction of nigrostriatal dopamine system. Science 204: 643-647.
- Backlund EO, Granberg PO, Hamberger B, Knutsson E, Martensson A, et al. (1985) Transplantation of adrenal medullary tissue to striatum in parkinsonism.First clinical trials. J Neurosurg 62: 169-173.
- Lindvall O, Backlund EO, Frade L, Sedvall G, Freedman R, et al. (1987) Transplantation in Parkinson’s disease: Two major cases of adrenal medullary grafts to the putamen. Ann Neurol 22: 457-468.
- Lindvall O (2013) Developing dopaminergic cell therapy for parkinson’s disease-give up or move forward? Mov Disord 28: 268-273.
- International Stem Cell Corporation (2015) International stem cell corporation receives authorization to initiate phase I/IIa clinical trial of ISC-hpNSC for the treatment of parkinson’s disease. Press Release.
- Brundin P, Strecker RE, Clarke DJ, Widner H, Nilsson OG, et al. (1988) Can human fetal dopamine neuron grafts provide a therapy for Parkinson’s disease? Prog Brain Res 78: 441-448.
- Brundin P, Barker RA, Parmar M (2010) Neural grafting in Parkinson’s disease problems and possibilities. Prog Brain Res 184: 265-294.
- Thompson L, Bjorklund A (2012) Survival, differentiation and connectivity of ventral mesencephalic dopamine neurons following transplantation. Prog Brain Res 200: 61-95.
- Barker RA, Drouin-Ouellet J, Parmar M (2015) Cell- based therapies for Parkinson disease-past insights and future potential. Nat Rev Neurol 11: 492-503.
- Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, et al. (2001) Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med 344: 710-719.
- Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, et al. (2003) A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol 54: 403-414.
- BarkerRA, BarrettJ, MasonSL, Björklund A (2013) Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. Lancet Neurol 12: 84-91.
- Hagell P, Schrag A, Piccini P, Jahanshahi M, Brown R, et al. (1999) Sequential bilateral transplantation in Parkinson’s disease effects of the second graft. Brain 122: 1121-1132.
- BrundinP, Pogarell O, Hagell P, Piccini P, Widner H, et al. (2000) Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson’s disease. Brain 123: 1380-1390.
- Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, et al. (1992) Core assessment program for intracerebral transplantations (CAPIT). Mov Disord 7: 2-13.
Citation: Khalid S (2020) Parkinson’s Disease Treatment Using Cell Transplantation. J Alzheimers Dis Parkinsonism 10: 491. DOI: 10.4172/2161-0460.1000491
Copyright: © 2020 Khalid S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2259
- [From(publication date): 0-2020 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 1510
- PDF downloads: 749