Research Article Open Access
Parent and Provider Decision-Making for Infants with Hypoxic-Ischemic Encephalopathy
| Allen KA1*, Brandon DH2,3, Holditch-Davis D2, Cotten CM3, Docherty SL2,3 | |
| 1Department of Biobehavioral Nursing and Health Science, University of Washington, School of Nursing, USA | |
| 2Duke University School of Nursing, Duke University, Department of Pediatrics, School of Medicine, USA | |
| 3Department of Pediatrics, Duke University, School of Medicine, USA | |
| Corresponding Author : | Kimberly A. Allen University of Washington School of Nursing, USA Tel: 312-355-5182 E-mail: kimberly.allen2@gmail.com |
| Received July 14, 2014; Accepted August 14, 2014; Published August 20, 2014 | |
| Citation: Allen KA, Brandon DH, Holditch-Davis D, Cotten CM, Docherty SL (2014) Parent and Provider Decision-Making for Infants with Hypoxic-Ischemic Encephalopathy. J Preg Child Health 1:106. doi: 10.4172/2376-127X.1000106 | |
| Copyright: © 2014 Allen KA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
Background: Hypoxic ischemic encephalopathy (HIE) is one of the most serious complications of full term birth that can lead to long-term neurological consequences or death. Parents and providers are faced with making complex decisions about which therapies to pursue within hours of birth.
Purpose: The purpose of this study was to describe the decisions made for infants with HIE, who participated in the decision-making process, and what factors influenced the decision-making process.
Design: A longitudinal, prospective, multiple case study design was used to study infant illness trajectories and parental responses (parental distress and hope) associated with caring for infants with HIE.
Results: Two groups of parent decision-making emerged: standard care and experimental care. The decisionmaking groups appear to be dictated by the treatment the infants received within hours of birth. Parents within each group shared specific responses including similar hope and distress, which continued over the first 2 postnatal months.
Conclusions: The results indicate that the type of medical therapy the infant receives determines the level of parental participation in decision-making. In the group of parents, in which providers obtained consent for study interventions parents had less hope for their infant even though the infant had a lower severity of illness compared to the infants receiving standard therapies. The reverse was true for parents of infants receiving standard therapy, parents were more hopeful even though their infants had a higher severity of illness.
| Keywords |
| Hypoxic ischemic encephalopathy; Parents; Decision making; Distress; Case study; Mixed methods; moderate hypothermia |
| Introduction |
| Hypoxic ischemic encephalopathy (HIE) is a brain injury that occurs because of a hypoxic and ischemic event during the prenatal, intrapartum or postnatal periods that prevents adequate blood flow and oxygen delivery to the infant’s brain [1]. HIE is one of the most serious complications of full term birth [2], occurring in up to 2.5 per 1000 live births [3-5]. Advances in technology and complex interventions to support cardiopulmonary function and prevent secondary consequences of HIE in infants has increased survival but has created more complex medical decision-making for parents and providers. Critical decisions aimed at preventing further injury to the infant with HIE must be made within minutes to several hours following birth; decisions centered on issues of long-term support, such as sustaining nutritional support and therapy to assist meeting the infant’s developmental milestones [6-8]. |
| Parents of newborn infants have expectations about the infant’s future, but those hopes are dramatically changed when parents learn their infant has HIE [7,8]. Parents of infants in the intensive care unit report that hope was a major influence in their decisions surrounding resuscitation at delivery and life-sustaining measures [9,10]. Parents of infants with HIE vacillated between hope and despair based on the changes in their infant’s illness [7]. Generally, parents of critically ill infants feel that despite bad news or a poor prognosis, physicians should maintain hope but not provide false hope [9]. Parent and provider differences in views of long-term outcomes and hope for a full recovery is a common source of stress of the decision-making process and goalsetting, which can lead to conflict between parents and providers [11]. Understanding the alignment between parent and provider hope for a ‘normal’ long-term neurological outcome for the infant is critical to determine how the response affects decision-making. |
| Parents experience intense distress and shock after giving birth and learning that their ‘normal’ infant has suffered brain damage [8,12,13]. Some parents report sadness, guilt, and powerlessness over the intensive care environment within the first hours and days of delivery [7]. Research has shown that parents have difficulty processing information under such stress [9,14-16]. This is made more complex for parents of infants with HIE because their newborn often has a healthy, well-developed appearance. Yet these parents are forced to make decisions about treatments within 6-24 hours of birth because this time is when therapies are thought to be most effective [17-20]. The stress parents experience does not end once the infant is discharged because many infants require follow-up care including physical and occupation therapy and continued assessments from specialists [8]. Causes of distress and anxiety after discharge include lack of understanding of the disease, lack of information on how to access services for their infant, and difficulty communicating with healthcare providers [8,21,22]. Parents continue to make decisions under high levels of stress and anxiety throughout the infants’ lives, but how stress and anxiety affect their decision-making remains unknown. Therefore, the purpose of this study was to describe the decisions made for infants with HIE, who participated in the decision-making process, and what factors influenced the decision-making process. |
| Methods |
| A longitudinal, prospective, multiple case study design was used to study parental decision-making and parental responses (e.g., hope, distress) to illness in infants with HIE. Case study design was chosen to allow for the context and complexity of decision-making to be explored through multiple sources and types of data [23]. This approach allowed for an in-depth exploration of how changes in the infant’s illness affected parental distress and hope during the first 2 months of the infant’s life. Exploring the convergence of multiple sources of data related to infant illness and parental responses (distress and hope) allowed for the search for patterns to identify possible explanations of why changes in hope and distress occurred [24]. |
| Setting and Sample |
| The setting for the study was a tertiary academic medical center in the Southeastern United States. Infants in the study were cared for in a Level IV neonatal intensive care unit (NICU), pediatric intensive care unit, and step-down areas. The protocol for treatment of HIE at this hospital was based on the timing of the symptoms when the infant presented to the facility, whether the infant met inclusion criteria for any experimental therapies, and the severity of the HIE. See Table 1 for severity of hypoxic-ischemic encephalopathy [25]. The standard therapy available at the hospital for infants with HIES presenting with moderate or severe symptoms were whole-body moderate hypothermia therapy and supportive care [25]. The two experimental interventions were late whole-body moderate hypothermia [26] and volume and red-blood cell reduced umbilical cord cells + whole-body moderate hypothermia [27]. See Table 2 for medical inclusion and exclusion criteria for the therapies [25-27]. |
| Eleven cases were studied across the first two postnatal months. Each case consisted of the infant, mother and/or father, and at least two health care providers. The providers for each case included physician, nurse practitioner, or registered nurse. Ten of the 11 cases studied are included in this analysis cases were enrolled concurrently. The remaining case was not included because the infant had congenital abnormalities and a genetic syndrome, which led to different types of decisions and a more complex illness course. |
| Measures |
| Infant illness and treatment characteristics |
| Illness and treatments were collected daily from the infant’s medical record while the infant was hospitalized and then at each visit for follow-up care. Following discharge, information about infant health status was collected in monthly parent interviews. The Technology Dependence Scale (TDS) was utilized to quantify the infant’s severity of illness. The scale included 12 items, with higher scores indicating more technology dependence and increased severity of illness. Items include the care environment, invasive lines, nutrition, blood draws, monitors, respiratory assistance, skin care, specialized oral care, external drains/ catheters, colloid administration, mobility, and medications. Previous studies have shown that scores on the TDS significantly decreased over time in premature infants and children with cancer. Interclass correlations ranged from 0.8 to 0.98, in previous samples [28]. |
| Parent demographics |
| Parents completed a demographic questionnaire upon enrollment with information on age, race, marital status, education level, income, and number of children. |
| Parent interviews |
| Narrative-style interviews were conducted at study entry and monthly for the first two months of the infant’s life. Parents were interviewed individually and interviews were digitally recorded. Narrative-style interviews were used to encourage the participants to tell their story about making decisions for their infant and to give them some control over the direction of the interview [29]. The two main foci of the monthly interviews were how the infant was progressing through the illness and the parental experience of making decisions for the infant. Interviews lasted about 15 minutes (range 4-54 minutes). See Table 3 for sample interview questions. |
| Provider interviews |
| Narrative interviews were conducted with providers (physician and nurse) at study entry, and following hospital discharge. Interviews were digitally recorded. Interviews focused the providers story of treating the infant and the decisions made for the infant with HIE. Additional foci included the HCP’s perception of parental understanding of the infant’s condition, the way decisions were made, the provider’s feelings about the decisions, and factors that influenced the provider’s decisions. HCP were interviewed individually. Interviews lasted an average of 15 minutes (range 5-37 minutes). |
| Impact of events scale-revised |
| The 22-item Impact of Events Scale-Revised (IES-R) was used to assess parental distress in response to HIE in the infant [30]. Items reflect posttraumatic stress symptoms including intrusion, avoidance and hyper arousal [31]. Participants rated each item on a scale from 0 (not at all) to 4 (extremely) for the past 7 days. Scores range from 0 to 88. Criterion, content, and construct validity have been established [30]. Internal consistencies for the total scale are 0.95 to 0.96; for the intrusion scale 0.90 to 0.94; for the avoidance scale 0.86 to 0.87; and for the hyper arousal scale 0.85 to 0.91 [31,32]. |
| Parental and provider hope |
| A 0 to 100 numeric rating scale was used to assess participant’s level of hope for the infant [33]. The scale was administered during each interview while the parent and provider described how they felt the infant was doing from a medical standpoint. Thus, in addition to providing a quantification of their level of hope, the rating scale also served as an elicitation tool to encourage participants to talk more about their hope for the infant. The rating scale had five anchors (no hope, hopeful, somewhat hopeful, very hopeful, extremely hopeful), evenly spaced across a horizontal line. Participants marked the line with an X and dated their rating. The same scale, with prior ratings, was presented to the participants at each data collection time point so participants could describe how their view of hope changed as the infant’s illness course progressed. |
| Data Collection Procedures |
| The study was approved by the institutional review board for the protection of human subjects. All data were collected by the first author. Infant admissions to the NICU were screened 3 times per week. Parents were approach for study consent shortly after admission or within the first postnatal week. Providers were consented once parents had consented. Parent data collection began within 1 week of study enrollment and data collection measures continued monthly until the infant was 2 months of age. Providers were enrolled at study entry, after any life-threatening event, and at discharge of the infant. Interview data were collected at a private location chosen by the parents or providers or via telephone. |
| Data Preparation |
| The digitally recorded interviews were transcribed verbatim. Each written transcript was reviewed to ensure congruence with the digital record. Participants were given pseudonyms to ensure confidentiality. |
| Data Analysis |
| The goal of the analysis was to describe influences on parent decision-making across the first 2 months of the infant’s life. Initially data from each quantitative measure, medical records and themes from the interviews was analyzed separately. Then the individual pieces of the data within each case was merged and displayed graphically. A visual analysis procedure was used to search for patterns within each of the 10 cases, then between similar cases (e.g., all moderate HIE), and finally across all 10 cases. |
| Conventional content analysis was used to analyze the interview data to allow for identification of themes and related patterns [34]. Interview transcripts were approached systematically, extracting the main storyline to identify key events in the infant’s illness and any associated decisions. Then individuals involved in specific decisions were identified [35]. Interrater reliability was established by coding all of the transcripts from 5 of the 10 cases by KAA and SLD who then compared coding. The coding, along with definitions were then presented to DHB and discussed until agreement was reached among KAA, SLD, and DHB. Factors that affected decision-making were grouped together under broad categories such as ‘communication’, ‘hope’, and ‘stressors’. Definitions for categories were developed from the data and refined as the analysis progressed [34]. Once data were separated into broad categories, subcategories emerged allowing similar ideas to be clustered together. Data analysis occurred concurrently with data collection to inform future data collection. |
| The Impact of Events Scale-Revised, parental hope, and provider hope scores were all totaled and scored per protocol. Each of the scores was graphed for each of the three time points for each case. |
| To categorize all of the infant’s daily illness and treatment data, a technique described by Docherty, Sandelowski and Brandon [36] was modified for use with infants with HIE. Using this technique, interview data, self-report-data, and medical record data were assimilated to categorize the illness and treatment characteristics into five mutually exclusive treatment categories that could then be compared across infants: initiation of treatment, experimental interventions, maintenance of treatment, escalation of treatment, or withdrawing/ withholding treatment. Table 4 provides the definitions for each decision type and examples from the illness course of infants with HIE. |
| Once all of the data for a case was analyzed separately, the data was combined for visual analysis, which included a search for patterns or trends in varied configurations of the integrated data displayed across the infant’s illness Data were then discussed among all authors until a consensus was reached. Cases were first examined separately (within case analysis) and then compared to every other case (between case analysis) to gain a better understanding of the typical infant illness trajectories and parental responses, and finally cases were compared on a specific set of variables (e.g., all mothers, mothers with low hope) across all cases [37]. Ultimately, the goal of the analysis was to identify influences on the decision making process across the first 2 months of the infants’ illness. |
| Results |
| Two groups of cases emerged from the analysis. The groups appeared to be related to the treatment the infants received and the parent responses to the environment and decision-making process. The first group had infants who received experimental therapy and the second group included infants who received standard care. The results are presented based on the groupings. |
| Infant and parent characteristics |
| In total, 10 cases consisting of 10 infants and 17 parents were studied. Four cases made up the experimental therapy group and six of the cases were in the standard therapy group. The experimental therapy group (two infants received late moderate hypothermia and two infants received volume and red-blood cell reduced umbilical cord cells) was made up of infants with moderate HIE. These infants had better Apgar scores at birth and had less ventilator dependent days than the standard therapy group. One infant was diagnosed with seizures by electroencephalography (EEG). No infant required placement of a gastrostomy tube or required intensive follow-up care by specialist. Most of these parents were married, had at least some college education, and made $15,000 to $25,000 annually. |
| The cases classified as standard therapy included infants who had moderate or severe HIE. Three infants had seizure activity diagnosed on EEG. Infants in this group also needed placement of a gastrostomy tube because of poor feeding, in-home physical and speech therapy due to poor muscle tone, and intensive follow-up care by specialists. Most parents in this group were single, had a high school education, and earned $15,000 to $25,000 annually. Although descriptive differences existed between groups, no statistically significant differences were found. Table 5 provides a description of the infant and parent characteristics. |
| Provider demographics |
| A total of 33 physicians and nurses participated. Of the 15 physicians who participated, 10 were male, 10 were Caucasian and not Hispanic and 4 were Caucasian, Hispanic and 1 was Asian. All nurses were female, 12 were Caucasian, 5 were African-American and 1 was American Indian. |
| Infant illness |
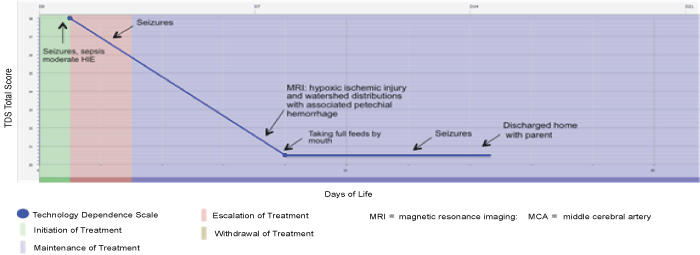
| The typical illness course of an infant within the experimental group during the initial hospitalization period is displayed in Figure 1. All four infants in this group had moderate HIE upon admission to the hospital. Three infants required intubation during the course of hospitalization, but none required a prolonged stay. These infants averaged 2.3 days in initiation of treatment (e.g., initiation of cardiopulmonary resuscitation and initiation of physical therapy), 1 day in experimental treatment (e.g., volume and red-blood cell reduced umbilical cord cells), 1.5 days in escalation of treatment (e.g., addition of a second medication to manage seizure activity), 10 days in maintenance of treatment (e.g., managing settings on the ventilator to maintain oxygenation and ventilation), and 0 days in withdrawing/withholding treatment. None were discharged home with advanced technology such as gastronomy tubes or tracheotomy tubes. Some infants displayed mildly delayed motor skills during the hospitalization. The average length of stay for these infants was 13.5 days. |
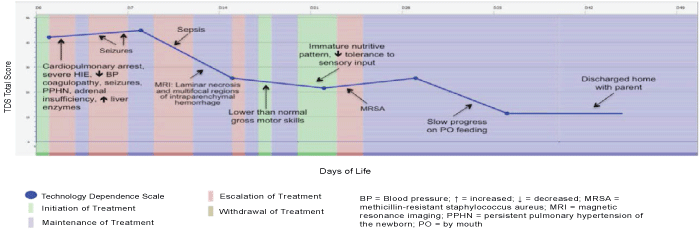
| Figure 2 displays a typical illness course of an infant in the standard care group during the initial hospitalization. Most of these infants were admitted with severe HIE (n=4) and most required cardiopulmonary resuscitation in the delivery room and intubation (n=5). The treatment categories were similar to those of the infants who received experimental therapy, except that the standard care infants had more days of maintenance treatment: initiation of treatment 3.2 days on average (initiation of whole-body moderate hypothermia, initiation of physical and occupation therapy), 0 days in experimental treatment, 4.3 days in escalation of treatment (addition of second medication to manage seizure activity, additional need to technology to meet nutritional needs with placement of gastronomy tube), 31.7 days in maintenance (management of nutritional needs, balancing of oxygenation and ventilation settings, continuation of physical and occupational therapy), and 0 days in withdrawal/withholding treatment. The limited number of escalation of treatment days indicates that there were relatively few points that decisions could be made to withdraw support, even if the infant appeared to be suffering or only had limited brain activity. Once infants were stable, delayed motor skills and difficulty in establishing a nutritive sucking pattern were common morbidities. A prolonged hospital stay was required for most infants with an average stay of 39.7 days. Half of the infants were discharged home with gastronomy tubes because they were unable to establish nutritive sucking. |
| Participation in HIE care decisions |
| Parents were asked to explain their role in making decisions about care of their infant. The two main decisions for parents were presented to them by providers, who decided based on whether the infant with HIE met treatment criteria based on protocols. The main decision for parents in the experimental group was whether to participate in the experimental intervention, either late whole-body moderate hypothermia or volume and red-blood cell reduced umbilical cord cells. Most parents in the standard care group reported not participating in the decision to transfer the infant to the tertiary hospital for HIE therapy, only one parent reported involvement in the decision-making process. |
| Experimental group parents participated more in the decisionmaking process and had greater understanding than standard care parents. One parent in the experimental care group was not medically able to participate due to anesthesia for a cesarean section. Six of the seven parents in the experimental group reported participating in the decision for experimental care. Parents consented for participation in research because the study intervention offered potential benefit and risks that while unknown could be outweighed by the potential for benefit. The father of an infant receiving volume and red-blood cell reduced umbilical cord cells explained he and his wife chose to consent to this experimental treatment because: |
| • My wife and I debated for a long time and talked to people that we knew, and a friend that had medical knowledge far and away above what we had. And the conclusion that I came to is that it appeared that there is limited downside to participating in the study. And it was not because of the potential upside that was kind of framed to us, it was because in my mind that was entirely unproven. |
| Overall, the experimental group parents understood the consent process, the intervention their infant was receiving, and the reasons why they needed the care. The mother of an infant receiving late moderate hypothermia described the process: |
| • They had been doing research and if there was any kind of brain damage or brain cells that were destroyed due to the lack of oxygen that the cooling process could ultimately help revitalize or rejuvenate those cells. She would be on a cool mattress and would drop her temperature down to 33.3°C, I think. She would be watched and her brain function would be watch as well. It was a 50/50 chance she would be cooled because you have to have a control as well as a test person. |
| Standard care parents were less involved in the decision to transfer the infant and proceed with moderate hypothermia. Most of the mothers were overwhelmed and drowsy from the cesarean section when their infant was transferred to the tertiary hospital. One parent did work with the physician at the outlying facility to make the decision to transfer the infant and continue with moderate hypothermia. The reason the parent decided to consent was because the tertiary hospital offered a higher level of care. Four of 10 parents reported that physicians made the decision for the infant to be transferred to receive moderate hypothermia. Five of the 10 reported no involvement in the decision but did not know who made the decision. Because moderate hypothermia within 6 hours of birth is a standard protocol within the tertiary hospital, most providers did not include parents in the decisions. One mother was distraught and confused when discussing how she learned her infant needed to be transferred to the tertiary hospital: |
| • They just stuck some papers in my face and told me that my baby wasn’t going to make it and I had to sign them. I was so drugged up. They gave me so many drugs. So I signed the papers and I got to see my son kick his little leg. They told me he was a very, very sick child, but my son was fine like the whole nine months I’ve been pregnant. He was fine. |
| The standard care parents appeared to have less understanding of HIE and the moderate hypothermia intervention than the parents in the experimental care group. The mother of an infant with severe HIE, who had a continuous EEGs consistent with severe diffuse brain dysfunction, multiple seizures, and diffuse cerebral edema was unclear about how her son was progressing: |
| • At first I got discouraged and I was very upset. But when I walked in here today and saw that he was drinking a bottle that helped a lot because that’s something that they said that he probably wouldn’t be able to do and he’s doing it. I have something to look forward to. |
| Given that none of the parents had prior knowledge of HIE or the care needed within the first 72 hours of life, parents had to rely on providers to supply information on the infant’s condition and care decisions. Providers approached the care of the infant based on the clinical condition and the treatment protocols available. |
| Level of participation after initial HIE treatment |
| The decision to participate in experimental therapy or standard therapy for initial HIE treatment is made within 72 hours of the infant’s birth. However, parents needed to make other decisions after this time. Experimental group and standard care group parents had similar experiences with other decisions. Parents described participating in decisions related to the administration of blood products, placement of a g-tube(standard care), transferring back to the birth hospital, seeking medical treatment for suspected viral or bacterial infections after hospital discharge, and changes in the infant’s formula. Each of these decisions required parents to either sign a consent form or make a choice to seek care or change care. This may have clearly delineated to the parents that they were making a choice and the providers were not making the decision for them. |
| Only the standard care parents were asked to make a decision about placement of a g-tube. Parents chose to have the g-tube placed because they wanted the infant to continue to gain weight and meet the milestones necessary for discharge from the hospital. A mother of an infant with severe HIE who received a g-tube explained her decisionmaking process as: |
| • There was no question about it. It was something he needed to help him in the long run. The boy needs to eat so we needed to do what was best for him. It was difficult at first seeing it and seeing him in pain and stuff, but we knew in the long run that it would be the best thing for him. |
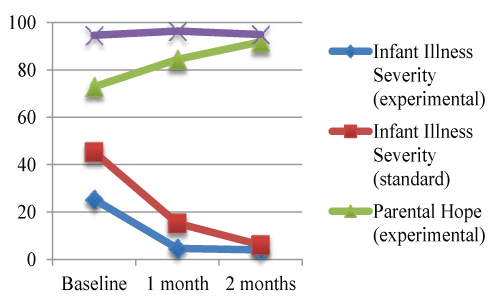
| Illness severity compared to hope |
| Experimental care infants all had moderate HIE and lower illness severity and dependence on technology compared to infants in the standard care group. However, as shown in Figure 3, experimental group parents had lower hope than standard care parents from birth to 1 month. When the infants reached 2 months of age, experimental care and standard care parents had similar hope levels and their infants had similar illness severity. |
| While the hope ratings differed between the groups, what parents were hopeful for were very similar. Throughout the first 2 months, parents were hopeful that their infants would survive and have no long-term consequences from the HIE. The father of an infant with meconium aspiration requiring 24 days of ECMO and discharge with a g-tube and intensive physical and occupational therapy, continued to hope his infant would have normal development. The father stated, “he’s going to come home and, you know, just normal as every other baby”. |
| Parent and provider hope comparison |
| Differences between parent and provider hope can lead to difficulty in communication and conflict, thus we compared the groups on hope. Providers caring for infants receiving experimental care were significantly more hopeful than providers caring for the standard care infants, t(18)=4.32, p=0.0004. Interestingly, parents of infants in the experimental group were significantly less hopeful, t(13)=1.90, p=0.08,than the providers in the same group. The opposite was true for parents whose infants received standard care with parents being significantly more hopeful than the providers, t (20)=5.32, p<0.0001. |
| Even though parents and providers differed on their ratings of hope for infants with HIE, their descriptions of hope in the interviews were very similar. Parents hoped that the infants would have minimal effect from the hypoxic-ischemic injury, be able the take formula through a bottle, meet developmental milestones, and have normal development. Providers hoped that the infants would have a normal, healthy life. When the infant had a significant injury, providers still wanted the infant to meet the milestones, but they were guarded in their hopefulness. For example, a physician explains his hope for an infant with moderate HIE who had seizures in the first few days of life: |
| • I think that it’s a favorable but guarded prognosis. It is one where if I had a child with no seizures and came through this pretty unscathed then I would be pretty optimistic that we’re going to do alright. Children who may have had some seizures associated in their course, I think you have to have a little more guarded view as to what might be the outcome. |
| Parents and providers did report having conflict as defined as a disagreement about the treatment plan requiring a change in care or additional discussion between the parents and providers. Within the interview data, parents reported that conflict occurred most often in the experimental group (three of the four cases). The conflict was related to the monitoring the infant was receiving in the ICU, the timing of infant discharge, and the location where the infant received care. Only one case in the standard care group experienced conflict between the parents and providers due to the infant receiving the wrong medication. No conflict was identified because of differences in parental and provider hope. |
| Parental distress |
| Parental distress differed between the experimental therapy and standard care groups. Parents in the experimental group had lower distress than standard care parents. This pattern continued throughout the first 2 months of the infants’ life (Figure 4). Both groups of parents reported similar stressors including seeing the infant with monitors and tubes, being separated from family and other children, not knowing what is happening, lack of sleep, miscommunication with providers, mothers still having pain from cesarean section, witnessing infants in pain or having seizures, and struggling with the cost of being far from home. |
| Discussion |
| Two patterns of parental decision-making emerged for caring for infants with HIE: experimental care and standard care. The groups were derived from cases that were made up of an infant, parents, and providers (physicians and nurses). Parents in the experimental therapy group reported participating in more decisions for their infants. The only two studies on parents of infants who sustained a hypoxic-ischemic injury are contradictory with one finding parents felt involved in the process of selecting therapy for their infant [7] and the other finding some parents reported being semi-conscious and having a hard time understanding the implications of care because of the medications they had received [8]. Parents faced with signing experimental consent forms and being directly asked by providers to choose study interventions for their infant could lead to parents being more involved in their infants compared to parents of infants who did not participate in the decision about the intervention for hypoxic-ischemic encephalopathy. The majority of parents of infants who received standard care reported the physician making the decision or not knowing who made the decision transfer the infant to the tertiary hospital. Most parents did not receive an in-depth discussion about hypoxic-ischemic encephalopathy and the risks and benefits of treatments and the short and long-term outcomes. The differences in participation and responses to the infant’s illness course between the experimental and standard care groups may be explained by these early discussions between the parents and providers. |
| Experimental group parents had lower distress levels than standard therapy parents throughout the first 2 months. Two potential reasons for this were decreased severity of infant illness and more information about their infant from the providers. The infants in the experimental group had higher Apgar scores at 5 minutes, had less ventilator days, had less hospital days, and were able to breast/bottle feed sooner than standard care infants. We did not ask parents to rate how sick they thought their infants were, but in their interviews, most parents were concerned their infant could die or suffer significant brain damage. Exactly how much illness severity contributed to parent distress is not clear. The experimental group of parents might have been provided more information about HIE and the prognosis during the consent process for participation in the experimental treatments. The experimental care parents and providers were together more in the beginning because time was spent discussing why the infant qualified for an experimental therapy and the risks and benefits associated with the study. The stressors identified by the parents in both groups were similar. The majority of these stressors were identified in previous studies that focused on parents of critically ill infants and infants with HIE [7,8]. |
| Initially, experimental group parents were less hopeful than providers. Potential explanations for this response were that the parents were presented with a grim prognosis for their infants who did not receive treatment for HIE, fear that the infant needed experimental therapy to survive, or uncertainty about whether the infant would survive. Future research needs to include asking parents more about their hopefulness in order to understand these differences and to ascertain the impact of the infant’s illness severity on their hopefulness. |
| As the goal of this study was to obtain a rich and deep description of decision-making for infants with HIE, we studied a small number of cases. A larger, multi-center study that allows for variation on key attributes (e.g., race, age, socioeconomic status) is needed to better understand how parents of infants with HIE participate in decisionmaking and experience hope and distress across the infant illness course. Better descriptions are needed of why parents have low hope, parental view of illness severity, and the fears parents have in order to understand the complexities of decision-making. Parents who chose to participate in this study may be a selective group of parents who were less distressed than non-participants (two sets of parents of two infants approached chose not to participate in the study). There were certain demographic groups of parents that were excluded from participation, such as parents who did not speak English and adoptive parents. While our protocol excluded individuals who could not speak English, only one mother was unable to participate because of this exclusion while the father was able to participate. Including parents of varying cultural backgrounds is essential for a fuller understanding of that it means to have a child with cognitive deficits changes. |
| Conclusion |
| The complex decision making process experienced by parents of infants with HIE appears to be heavily weighted by factors related to the therapy the infant required and the decision and consent process to participate in a clinical trial related to HIE. While, parents and providers within each group shared key responses and made similar decisions, several findings remain unexplained. How infant illness severity affects parents, and especially their hope and distress, is unclear and requires additional exploration. Interestingly, the parents of infants receiving experiment therapies had lower hope than the providers in the same group. This may indicate that parents initially understood HIE and the need for experimental treatment to indicate the infant to have a poor prognosis for meaningful recovery. Another unexplained finding is why conflict did not emerge between parents and providers about the prognosis of the infants. Future research must address how parents determine illness severity, why parent hope was low, and increase the number of cases recruited to potentially capture conflict to try to understand how those cases differ from cases without conflict. |
| Acknowledgements |
| This study was supported in part by NIH Roadmap Scholarship TL1 RR 024126-01 to the first author; NIH/NINR 1F31NR012083-01 to the first author; NIH/NINR T32NR007106 to the first author; and NIH/NINR R01NR010548 to the second and last authors. |
References
- Long M, Brandon DH (2007) Induced hypothermia for neonates with hypoxic-ischemic encephalopathy. J Obstet Gynecol Neonatal Nurs 36: 293-298.
- Schiariti V, Klassen AF, Houbé JS, Synnes A, Lisonkova S, et al. (2008) Perinatal characteristics and parents' perspective of health status of NICU graduates born at term. J Perinatol 28: 368-376.
- Martinez-Biarge M, Madero R, González A, Quero J, García-Alix A (2012) Perinatal morbidity and risk of hypoxic-ischemic encephalopathy associated with intrapartum sentinel events. Am J Obstet Gynecol 206: 148.
- Kurinczuk JJ, White-Koning M, Badawi N (2010) Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev 86: 329-338.
- Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE (2008) A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol 199: 587-595.
- Allen KA, Brandon DH (2011) Hypoxic Ischemic Encephalopathy: Pathophysiology and Experimental Treatments. Newborn Infant Nurs Rev 11: 125-133.
- Nassef SK, Blennow M, Jirwe M (2013) Experiences of parents whose newborns undergo hypothermia treatment following perinatal asphyxia. J Obstet Gynecol Neonatal Nurs 42: 38-47.
- Heringhaus A, Blom MD, Wigert H (2013) Becoming a parent to a child with birth asphyxia-From a traumatic delivery to living with the experience at home. Int J Qual Stud Health Well-being 8: 1-13.
- Kavanaugh K, Savage T, Kilpatrick S, Kimura R, Hershberger P (2005) Life support decisions for extremely premature infants: report of a pilot study. J Pediatr Nurs 20: 347-359.
- Boss RD, Hutton N, Sulpar LJ, West AM, Donohue PK (2008) Values parents apply to decision-making regarding delivery room resuscitation for high-risk newborns. Pediatrics 122: 583-589.
- Verhagen AA, de Vos M, Dorscheidt JH, Engels B, Hubben JH, et al. (2009) Conflicts about end-of-life decisions in NICUs in the Netherlands. Pediatrics 124: e112-119.
- Wilson SM, Miles MS (2001) Spirituality in African-American mothers coping with a seriously ill infant. J Soc Pediatr Nurs 6: 116-122.
- Rosenthal ET, Biesecker LG, Biesecker BB (2001) Parental attitudes toward a diagnosis in children with unidentified multiple congenital anomaly syndromes. Am J Med Genet 103: 106-114.
- Pector EA (2004) Views of bereaved multiple-birth parents on life support decisions, the dying process, and discussions surrounding death. J Perinatol 24: 4-10.
- Yee W, Ross S (2006) Communicating with parents of high-risk infants in neonatal intensive care. Paediatr Child Health 11: 291-294.
- Alderson P, Hawthorne J, Killen M (2006) Parents' experiences of sharing neonatal information and decisions: consent, cost and risk. Soc Sci Med 62: 1319-1329.
- Stola A, Perlman J (2008) Post-resuscitation strategies to avoid ongoing injury following intrapartum hypoxia-ischemia. Seminars in Fetal & Neonatal Medicine 13: 424-31.
- Shalak L, Perlman JM (2004) Hypoxic-ischemic brain injury in the term infant-current concepts. Early Hum Dev 80: 125-141.
- Hoehn T, Hansmann G, Buhrer C,(2008) Therapeutic hypothermia in neonates. Review of current clinical data, ILCOR recommendations and suggestions for implementation in neonatal intensive care units. Resuscitation 78: 7-12.
- Shankaran S (2009) Neonatal encephalopathy: treatment with hypothermia. J Neurotrauma 26: 437-443.
- Minnes P, Steiner K (2009) Parent views on enhancing the quality of health care for their children with fragile X syndrome, autism or Down syndrome. Child Care Health Dev 35: 250-256.
- Buelow JM, McNelis A, Shore CP, Austin JK (2006) Stressors of parents of children with epilepsy and intellectual disability J Neurosci Nurs 38: 147-154, 176.
- Edwards D, Dattilio F, Bromley D (2004) Developing evidence-based practice: The role of case-based research. Professional Psychology: Research and Practice 35: 589-97.
- Gerring J (2007). Case study research: Principles and practices.Cambridge University Press, New York, USA
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, et al. (2005) Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 353: 1574-1584.
- Laptook A, Higgins RD (2014) Evaluation of Systemic Hypothermia Initiated After 6 Hours of Age in Infants =36 Weeks Gestation With Hypoxic-Ischemic Encephalopathy: A Bayesian Evaluation. A Protocol for the NICHD Neonatal Research Network, National Institutes of Health.
- Cotten CM, Murtha AP, Goldberg RN, Grotegut CA, Smith PB, et al. (2014) Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J Pediatr 164: 973-979.
- Docherty SL, Brandon DH, Allen KA, Silva S, Miles MS (2011). Development and validation of an instrument to measure technology dependence in medically fragile children. Southern Nursing Research Society. Jacksonville, Fl2011.
- Sandelowski M (1991) Telling stories: narrative approaches in qualitative research. Image J Nurs Sch 23: 161-166.
- Weiss D, Marmar C (1997) The impact of event scale-revised. In: Wilson J and Keane T, (Eds.). Assessing psychological trauma and PTSD. The Guilford Press, New York, USA
- Beck JG1, Grant DM, Read JP, Clapp JD, Coffey SF, et al. (2008) The impact of event scale-revised: psychometric properties in a sample of motor vehicle accident survivors. J Anxiety Disord 22: 187-198.
- Creamer M1, Bell R, Failla S (2003) Psychometric properties of the Impact of Event Scale - Revised. Behav Res Ther 41: 1489-1496.
- Docherty SL (2008) Decision-Making for Infants with Complex Life-Threatening Conditions. Duke University: NINR, USA
- Hsieh HF, Shannon SE (2005). Three approaches to qualitative content analysis. Qual Health Res 15: 1277-1288.
- Sandelowski M (1995) Qualitative analysis: what it is and how to begin. Res Nurs Health 18: 371-375.
- Docherty SL, Sandelowski M and Brandon DH. Visualization methods for organizing, analyzing, and integrating diverse data. Journal of Mixed Methods Research. under review
- Stake R (2006) Mulitple case study analysis. The Guilford Press, New York, USA
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
Figures at a glance
 |
 |
 |
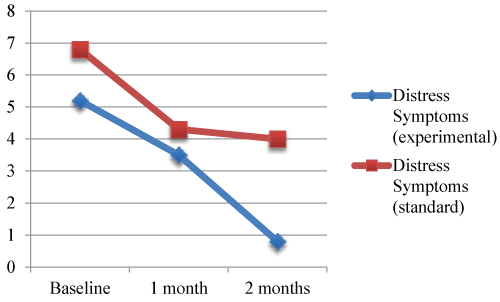 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15840
- [From(publication date):
October-2014 - Jul 11, 2025] - Breakdown by view type
- HTML page views : 10859
- PDF downloads : 4981
