Pancreatic Squamous Cell Carcinoma: A Case Report and Review of the Literature
Received: 04-Jul-2022 / Manuscript No. roa-22-68381 / Editor assigned: 06-Jul-2022 / PreQC No. roa-22-68381 (PQ) / Reviewed: 20-Jul-2022 / QC No. roa-22-68381 / Revised: 22-Jul-2022 / Manuscript No. roa-22-68381 (R) / Published Date: 29-Jul-2022 DOI: 10.4172/2167-7964.1000390
Abstract
Background: Pancreatic squamous cell carcinoma is an exceptional disease. The pancreas is completely devoid of squamous cells, the development of squamous cell carcinoma at its level is controversial and of unknown origin. Squamous metaplasia of ductal cells has been observed in inflammatory episodes of pancreatic tissue. However, transformation to a squamous cell carcinoma remains exceptional. It is a very aggressive disease, most often locally advanced or metastatic at the time of diagnosis, and not very sensitive to the treatments offered.
Case Presentation: We describe here a case of a 64-year-old woman treated for a locally advanced pancreatic squamous cell carcinoma revealed by just diffuse abdominal pain. The diagnosis was confirmed by a peritoneal biopsy. She underwent palliative chemotherapy according to the protocol Capecitabine-Cisplatin, but the outcome was pejorative since she showed after 4 cures an enormous progression, preventing her from trying out another drug.
Conclusion: Due to the rarity of primary breast neuroendocrine tumors, no standard therapy exists.
We want to show through this case the pejorative prognosis of this rare tumor, and to motivate further prospective works in order to finally establish a better protocol to enhance our patients’ outcomes.
Keywords: Pancreas; Squamous cell Carcinoma; Metastasis; Prognosis
Introduction
Squamous cell carcinoma (SCC) of just pancreas is an exceptional primary malignant tumor. It accounts for 0.5 to 2% of all pancreatic tumors [1]. Its main differential diagnoses adenosquamous carcinoma and metastatic SCC of another primary origin. SCC has a much lower overall survival rate than adenocarcinoma, which is the most common histologic type [2]. To date, no therapeutic consensus has been established.
We describe here the case of a patient seen at the Mohamed VI Center for the treatment of cancers for a metastatic pancreatic SCC, and will also see through this case the clinicopathological characteristics of this tumor.
Case Presentation
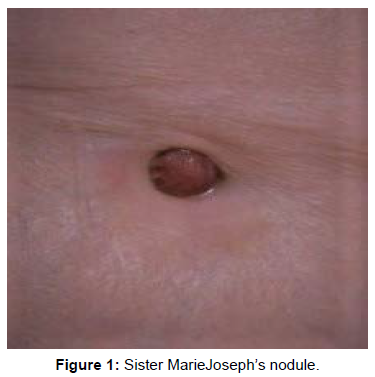
We describe the case of a 64-year-old patient whose symptoms go back 4 months before her consultation in our structure (08/24/2020). She reports an isolated abdominal meteorism, without other associated signs nor deterioration of the general condition. She consults with her general practitioner who performs an abdominal computed tomography (CT) scan revealing a tumor process in the body and tail of the pancreas measuring 8 cm on the long axis, with several areas of necrosis, with wide extension to the omentum, as well as scattered and necrotic peritoneal nodules. Metabolic imaging (Tep-scan) is requested as part of the extension assessment and reveals a locally advanced pancreatic tumor process with peritoneal and umbilical parietal extension corresponding to a Sister Marie Joseph’s nodule (Figure 1). Finally, a peritoneal biopsy with the puncture of the ascitic fluid is done. The anatomopathological examination finds a fibro-adipose tissue seat of a carcinomatous proliferation arranged in clusters and lobules. The cells are globularly provided with an elongated nucleus, monstrous in places, the seat of numerous abnormal mitosis, the cytoplasm is basophilic. The stroma is reactive and fibrous. It is seen in images of angio-invasion and an epidermoid contingent with keratin beads. Ascites fluid contains carcinoma cells of the same appearance as described above. The immunostaining complement found a diffuse positivity of the anti-P63 and anti-P40 antibodies, as well as an intense expression of the anti-pan-cytokeratin AE1 and AE3 antibodies, while the anti-CD117 or C-kit antibody was not expressed. The diagnosis of an SCC of pancreatic origin with diffuse peritoneal localization is confirmed. She then receives palliative chemotherapy according to the Capecitabine-Cisplatin protocol. At the end of 4 cures, she reports anorexia with grade 2 digestive intolerance which led to treatment being stopped for 4 days during the last cycle. An evaluation by thoraco-abdominal-pelvic CT (TAP CT) notes a clear progression of the process as well as peritoneal damage estimated at 76%, with the appearance of a highly suspicious hepatic lesion. Considering the poor response to the treatment, the side effects of this one and in agreement with the patient and her family it was decided to entrust her to our palliative care unit for specific treatments. She died one month later (Figure 1).
Discussion
Adenocarcinoma is the most common histological type and accounts for nearly 85% of all pancreatic cancers. Other rarer variants such as adenosquamous carcinoma and SCC are more rarely described. SCC alone represents 0.5 to 2% of all cases of pancreatic cancer [3].
The pancreas is lacking squamous epithelium. In case of inflammation, such as in pancreatitis and squamous cell carcinoma, these cells can be seen [4].
The origin of pancreatic SCC remains uncertain, but there are however some hypotheses [2, 4, 5].
• A primitive cell capable of differentiating both into squamous or glandular carcinoma and which undergoes a malignant modification.
• A squamous metaplasia of the ductal epithelium undergoing a malignant transformation following repeated episodes of pancreatitis [6].
• A variant of adenosquamous carcinoma in which the glandular component of cancer has disappeared while the squamous component has persisted [6].
• A pre-existing adenocarcinoma undergoes a squamous modification
• An atypical squamous cell that proliferates without undergoing any malignant modification.
• Squamous metaplasia of the pancreatic ducts in the presence of chronic inflammation, at the origin of squamous differentiation [7].
Current clinical data are limited to isolated cases like ours, or too small series. The English literature, for example, found only 61 cases of pure pancreatic SCC (meaning without glandular component) until 2004.
Due to its rarity, little work has been able to identify whether it was a distinct pathological entity, an under-sampled primary adenosquamous carcinoma, or metastasis from an occult squamous cell carcinoma [3]. This last case being the most frequently encountered, a paraclinical morphological and metabolic assessment makes it possible to eliminate a primary occult localization with squamous differentiation having metastasized to the pancreas.
Unlike the mutations of pancreatic ADK that are commonly known, data on SCC-specific mutations is limited. We note, for example, the hypothesis of a BRCA-2 exon 15 germline mutation in a patient with locally advanced SCC of the pancreas reported by Schultheis et al. [1] and in whom a BRCA-2 germline mutation was detected, given the history of ovarian and breast cancer in his parents in the first degree.
Besides this case, a family history of pancreatic cancer has rarely been reported. The clinical presentation of pancreatic SCC is no different from other pancreatic cancers. The average age of discovery is 61.9 years with a male predominance. Commonly reported symptoms include anorexia, weight loss, and abdominal pain with back radiation [8]. Depending on the anatomical site of the tumor, obstructive jaundice or pyloric stenosis may also be observed, but much more rarely [9].
Some clinical presentations are more unusual, sometimes with fever, fatigue, epigastric mass, hyperglycemia, or diabetes. Exceptionally, pancreatic SCC causes digestive hemorrhage (hematemesis or melena) responsible for an anemic syndrome, due to the contiguous invasion of the stomach and/or duodenum [10].
No tumor marker is specific for its diagnosis. However, tumor markers (CA-19.9 and ACE) are elevated in metastatic disease. In a few trials, the SCC antigen has been used to monitor response to treatment or for detection of recurrence [11]. Certain biological reactions can be observed, without being specific. Among them, there is a leukemoid reaction as reported by Erica et al. [9].
Pancreatic imaging, particularly computed tomography (CT), has an essential role in tumor diagnosis and staging. On imaging, pancreatic SCC has two specific characteristics: improvement in tumor contrast and angiographic blush pattern [7].
Regarding the location of the tumor, a pooled analysis published in 2016 reported that more than 50% of cases were located in the cephalic region, 21.6% in the caudal level, and 19.5% with multifocal involvement, and 5,9% of cases at the corporeal level [8].
Metabolic imaging has a primary interest in the diagnosis of this tumor. In fact, it has a dual role: it can detect metastatic locations earlier than standard imaging, and above all, it excludes the existence of a primary site other than the pancreas. Histological diagnosis with immunostaining can be performed with an imaging-guided biopsy. Optionally, fine-needle aspiration guided by endoscopic ultrasound may be proposed. Finally, a laparoscopic biopsy remains an alternative but is very little used.
Pancreatic SCC is usually diagnosed at a locally advanced or even metastatic stage [12]. As a result, curative resection is only possible in less than a third of patients, and in this case, overall survival (OS) is improved [8]. Pancreatic SCC is more aggressive than ADK and has a poorer prognosis.
Regarding the management of this tumor, no therapeutic consensus has been established [12]. We only have more or less large series and a few cases that have proposed different treatment protocols in order to identify the protocol providing the best benefit in OS [12]. The results were able to first identify the factors of poor prognosis, which are essentially age and stage of the disease. Indeed, an age over 70 years and stage IV disease is associated with a poor prognosis with a survival of 1.5 months in the absence of treatment. Surgical resection of the primary tumor is associated with longer survival in localized disease (stage I-II), (21 months for the surgery arm versus 5 months for the arm without surgery), regardless of the tumor site. Stage III presents a particularity because the surgery does not offer significant benefit in OS with a p=0.32.
However, postoperative chemotherapy/radiotherapy in the localized disease did not show a significant benefit in OS (21 vs 24 months) and this is probably because the main protocols proposed in the series are those proposed in current practice for the treatment of pancreatic ADK. Regarding locally advanced and metastatic disease, several multidrug therapy protocols in particular, based on gemcitabine or a 5-Fluoro-uracil combination and platinum salts have been proposed [13]. But with the poor results of OS ranging from 5 to 8 months depending on the series [14].
In our case, our patient received palliative chemotherapy according to the Capecitabine-Cisplatin protocol, with a very rapid progression of the disease and a deterioration of the general condition.
Conclusion
Pancreatic SCC is an extremely rare entity. Apart from a few morphological characteristics, its clinical presentation is the same as that of ADK.
Generally, the prognosis is poor when surgical excision is impossible. Currently, only stage I-II surgery appears to be an effective weapon, especially if combined with adjuvant radio chemotherapy. However, the diagnosis is too often made at an advanced stage, and curative treatment is rarely offered. The possibility of a hereditary mutation could be responsible for a familial form of this cancer.
Further research is needed to establish a mutational etiology in suspected familial cases. This last possibility would make it possible to diagnose the disease at an early stage, and with optimal management, would significantly improve OS.
Acknowledgement
None
Conflict of Interest
None
References
- Schultheis AM, Nguyen GP, Ortmann M, Kruis W, Büttner R, et al. (2014) Squamous cell carcinoma of the pancreas in a patient with germline BRCA2 mutation-response to neoadjuvant radiochemotherapy. Case Rep Oncol Med 2014: 860532.
- Makarova-Rusher OV, Ulahannan S, Greten TF, Duffy A (2016) Pancreatic squamous cell carcinoma: A population-based study of epidemiology, clinicopathologic characteristics and outcomes. Pancreas 45: 1432-1437.
- Brown HA, Dotto J, Robert M, Salem RR (2005) Squamous cell carcinoma of the pancreas. J Clin Gastroenterol 39: 915-919.
- Bixler HA, Castro MJ, Stewart J (2011) Cytologic differentiation of squamous elements in the pancreas. Diagn Cytopathol 39: 536-540.
- Abedi SH, Ahmadzadeh A, Alizadeh AHM (2017) Pancreatic Squamous Cell Carcinoma Case Rep Gastroenterol 11: 219-224.
- Bralet MP, Terris B, Brégeaud L, Ruszniewski P, Bernades P et al. (1999) Squamous cell carcinoma and lipomatous pseudohypertrophy of the pancreas. Virchows Archiv 434: 569-572.
- Al-Shehri A, Silverman S, King KM (2008) Squamous cell carcinoma of the pancreas. Curr Oncol 15: 293-297.
- Ntanasis-Stathopoulos I, Tsilimigras DI, Georgiadou D, Kanavidis P, Riccioni O (2017) Squamous cell carcinoma of the pancreas: a systematic review and pooled survival analysis. Eur J Cancer 79: 193-204.
- Lin E, Veeramachaneni H, Addissie B, Arora A (2018) Squamous cell carcinoma of the pancreas. Am J Med Sci 355: 94-96.
- Wahab A, Gonzalez JJ, Devarkonda V, Saint–Phard T, Singh T et al. (2019) Squamous cell carcinoma-A rare pancreatic exocrine malignancy. 20: 593-596.
- Minami T, Fukui K, Morita Y, Kondo S, Ohmori Y et al. (2001) A case of squamous cell carcinoma of the pancreas with an initial symptom of tarry stool. J Gastroenterol Hepatol 16: 1077-1079.
- Tella SH, Kommalapati A, Yadav S, Bergquist JR, Truty MJ et al. (2019) Survival and prognostic factors in patients with pancreatic squamous cell carcinoma. Eur J Surg Oncol 45: 1700-1705.
- Itani KM, Karni A, Green L (1999) Squamous cell carcinoma of the pancreas. J Gastrointest Surg 3: 512-515.
- Bideau K, Metges JP, Bayle S, André M, Robaszkiewicz M et al. (2006) Treatment of squamous cell carcinoma of the pancreas with gemcitabine. Clin Bio Gastroenterol 30: 1217-1220.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Oudad F, Tawfiq N, Bouchbika Z, Benchakroun N, Jouhadi H, et al. (2022) Pancreatic Squamous Cell Carcinoma: A Case Report and Review of the Literature. OMICS J Radiol 11: 390. DOI: 10.4172/2167-7964.1000390
Copyright: © 2022 Oudad F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 3173
- [From(publication date): 0-2022 - Mar 29, 2025]
- Breakdown by view type
- HTML page views: 2698
- PDF downloads: 475